Abstract
Background and purpose:
Macrophage migration inhibitory factor (MIF) is now known to be a pro-inflammatory cytokine associated with insulin resistance. Our aim was to investigate whether angiotensin converting enzyme 2 (ACE2) could modulate the expression of MIF and the insulin/Akt-endothelial nitric oxide (NO) synthase (eNOS) signalling in a human endothelial cell line (EAhy926).
Experimental approach:
A recombinant plasmid encompassing human ACE2 gene was constructed and transfected into the EAhy926 cells. The mRNA, phosphorylation and protein levels of p22phox, MIF, Akt and eNOS in endothelial cells were determined by real-time PCR and Western blot analysis, respectively.
Key results:
Gene transfer of ACE2 suppressed the expression of p22phox and MIF induced by angiotensin (Ang) II and Ang IV, accompanied by a decreased level of malondialdehyde in cells. In addition, Ang II diminished insulin-stimulated phosphorylation of Akt (at Ser473) and eNOS (at Ser1177) and NO generation, effects which were reversed by ACE2 gene transfer and anti-MIF treatment in endothelial cells.
Conclusions and implications:
The results reveal that gene transfer of ACE2 regulated Ang II-mediated impairment of insulin signalling and involved the Akt-eNOS phosphorylation pathway. These beneficial effects of ACE2 overexpression appear to result mainly from blocking MIF expression in endothelial cells, suggesting that the ACE2 gene may be a novel therapeutic target for diseases related to inflammation and insulin resistance.
Keywords: angiotensin-converting enzyme 2 (ACE2), angiotensin II, macrophage migration inhibitory factor, nitric oxide, insulin resistance
Introduction
Insulin resistance is a pro-inflammatory state associated with enhanced oxidative stress, which has been closely linked to abnormalities in the renin–angiotensin system (RAS) (Dandona et al., 2005; Kim et al., 2006; Zhong et al., 2007). Within the RAS, the octapeptide angiotensin (Ang) II and the hexapeptide angiotensin IV (Ang IV) (angiotensin 3–8) exert various deleterious effects by promoting the production of pro-inflammatory cytokines and reactive oxygen species (ROS), causing vessel inflammation and oxidative excess (Crackower et al., 2002; Esteban et al., 2005; Zhong et al., 2007). Angiotensin II (Ang II) can induce the expression of p22phox, a key subunit of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which is increased in inflammation and insulin resistance (Chabrashvili et al., 2003; Dandona et al., 2005). Furthermore, Ang II regulates insulin signalling via a pathway involving impairment of phosphatidylinositol 3-kinase (PI3K)-dependent activation of Akt–endothelial NOS (eNOS) phosphorylation (Kim et al., 2006; Zhong et al., 2007). Ang IV may act as a potentiator of Ang II signalling and play a key role in the inflammatory events (Esteban et al., 2005). However, little is known about the regulatory roles of Ang IV in oxidative stress and insulin signalling. The first aim of this study was to evaluate the effects of Ang II and Ang IV on p22phox expression and the insulin/Akt signalling pathway.
In addition, Ang II and Ang IV can activate nuclear factor-κB (NF-κB), which in turn stimulates transcription of pro-inflammatory factors such as macrophage migration inhibitory factor (MIF) and tumour necrosis factor-α (TNF-α) (Esteban et al., 2005; Kim et al., 2006). MIF is now known as a widely expressed pro-inflammatory cytokine and associated with insulin resistance through specific actions that block transduction of insulin signalling (Lin et al., 2000; Busche et al., 2001; Dandona et al., 2004, 2005; Herder et al., 2006). We previously demonstrated that MIF is implicated in atherosclerosis linked to inflammation and insulin resistance and anti-MIF treatment produces anti-inflammatory effects in endothelial cells (Lin et al., 2000). More recently, the prevention of myocardial MIF expression was paralleled by activation of the PI3K/Akt signalling pathway that controls NO production in response to insulin (Ha et al., 2006). These observations have given strong support to the concept that MIF blockade may potentially contribute to diminution of inflammation and improvement of insulin signalling.
Interestingly, Ang II and Ang IV share signalling and degradation pathways, and are substrates for the angiotensin-converting enzyme 2 (ACE2) (Warner et al., 2004; Der Sarkissian et al., 2006). ACE2 seems to act as a negative regulator of the RAS and has become an important therapeutic target in the control of cardiovascular diseases and diabetes (Crackower et al., 2002; Warner et al., 2004; Der Sarkissian et al., 2006; Ingelfinger, 2006). We previously reported that ACE2 overexpression evokes beneficial effects of lowering blood pressure and attenuating myocardial damage in the spontaneously hypertensive rat, in which hypertension is often accompanied by insulin resistance (Zhong et al., 2004). In the present study, we investigated further the regulatory roles of ACE2 gene in the expression of MIF and insulin signalling involving the Akt–eNOS phosphorylation pathway in EAhy926 human endothelial cells. We found that gene transfer of ACE2 reversed Ang II-induced impairment of the insulin/Akt–eNOS signalling pathway and was paralleled by a decrease of MIF expression in endothelial cells.
Materials and methods
Cloning of ACE2 gene
Gene-specific primers were designed on the basis of published human ACE2 sequence (GenBank no. AF241254) with the primers (sense: 5′-CACCATGTCAAGCTCTTCCTG-3′; antisense: 5′-AAAGGAGGTCTGAACATCATCAGTG-3′). These primers were used to amplify the full-length cDNA from a human fetal heart cDNA library (Gibco, Carlsbad, CA, USA). This was accomplished by using a two-step PCR protocol using PfxUltima DNA polymerase (Invitrogen, Carlsbad, CA, USA).
Transfection of recombinant ACE2 gene in endothelial cells
The purified PCR product with a full length of 2419 bp was ligated into the pcDNA3.1-D/V5-His-TOPO vector (Invitrogen) and transformed into One Shot TOP10 Competent Cells according to the manufacturer's instructions. Positive colonies were randomly picked up and then verified by PCR amplification and DNA sequence. Recombinant plasmid DNA containing the ACE2 gene (pACE2) was prepared with the ChargeSwitch Plasmid ER (endotoxin reduced) Mini Kit (Invitrogen) and used to transfect EAhy926 endothelial cells with Lipofectamine 2000 Reagent (Invitrogen). Stable-transfected cell lines were selected using 200 μg ml−1 G418 (Merck; Darmstadt, Germany).
Cell culture and measurement of MDA and NO levels
For experiments, EAhy926 endothelial cells were serum starved for at least 18 h in serum-deprived medium and plated into 12-well plates at a density of 5 × 105 cells per ml. Time-dependent responses to Ang II or Ang IV (100 nM, 0.5–24 h) were tested in endothelial cells. In protocols with the recombinant pACE2 transfection, pACE2-treated or vehicle-treated cells were stimulated by 100 nM Ang II or Ang IV for 6 h. The angiotensin type 1 receptor (AT1) antagonist losartan (10 μM), AT2 receptor antagonist PD123319 (1 μM) and AT4 receptor antagonist divalinal-Ang IV (300 nM) were added to cells for 30 min before exposure to 100 nM Ang II and Ang IV. Furthermore, the PI3K inhibitor LY294002 (10 μM), the eNOS inhibitor NG-nitro-L-arginine methyl ester (L-NAME, 100 μM) and the anti-MIF neutralizing antibody (100 μg ml−1) were used to treat cells for 30 min in the presence or absence of Ang II and Ang IV followed by stimulation with insulin (100 nM).
The malondialdehyde (MDA) in the cell lysate was determined as thiobarbituric acid reactive substances and the NO concentration with the Griess reagent as described previously (Chabrashvili et al., 2003; Zhong et al., 2005). The resulting supernatants of protein sample mixture with medium were measured at 532 nm (for MDA) and 550 nm (for NO) with a Beckman DU650 spectrophotometer. All procedures were carried out according to the manufacturer's protocol. Analyses were performed in duplicates.
Isolation of total RNA and real-time PCR
The mRNA expression of human ACE2, p22phox and MIF in extracts from cells was determined using real-time quantitative PCR as reported previously (Zhong et al., 2004, 2005). Total RNA was isolated from endothelial cells using TRIzol reagent. cDNA was then synthesized with a standard reverse transcription-PCR (RT-PCR) technique performed with human gene-specific primers: MIF sense 5′-CTCTCCGAGCTCACCCAGCAG-3′, antisense 5′-CGCGTTCATGTCGTAATAGTT-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) sense 5′-ACCACAGTCCATGCCATCAC-3′, antisense 5′-TCCACCACCCTGTTGCTGTA-3′. The primers and TaqMan probes (Table 1) were designed with the Primer Express Software. In the present study, we successfully amplified known amount of plasmids (pGEM-T easy vector; Promega, Madison, WI, USA) encompassing human ACE2, p22phox, MIF and GAPDH cDNA to serve as gene standards after DNA sequence analysis. Real-time PCR was carried out in a 25-μl reaction mixture prepared with a TaqMan PCR core reagent kit (ABI; Foster, CA, USA). These results were normalized using human GAPDH as the internal control. Each sample was run and analysed in triplicate.
Table 1.
Primers and probes used for real-time PCR reactions in cells extracts
| Gene | GenBank no. | Probe (FAM-5′–3′-TRAMA) | Sense primer (5′–3′) | Antisense primer (5′–3′) |
|---|---|---|---|---|
| ACE2 | AF241254 | ATGCCTCCCTGCTCATTTGCTTGGT | ATCCTTCCTATATCAGTCCAATTG | TCCAAAATCTACCCCACATAT |
| MIF | BC013976 | AGCCTGCACAGCATCGGCAAGAT | ACCAGCTCATGGCCTTCG | CTTGCTGTAGGAGCGGTT |
| p22phox | NM_000101 | AAGAGGAAGAAGGGCTCCACCATGGA | TGGCGGGCGTGTTTGTGT | CCACGGCGGTCATGTACTTC |
| GAPDH | BC083511 | CTGCACCACCAACTGCTTAGCACCC | CCCATGTTCGTCATGGGTGT | TGGTCATGAGTCCTTCCACGATA |
Abbreviations: ACE2, angiotensin-converting enzyme 2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MIF, macrophage migration inhibitory factor.
Western blotting
Lysates from cultured endothelial cells were subjected to western blot analysis as described previously (Zhong et al., 2004, 2005). Protein concentration was determined using the BCA Protein Array Kit (Pierce, Rockford, IL, USA). Protein samples were separated by 8–15% sodium dodecyl sulphate-polyacrylamide gel electrophoresis, transferred onto a nitrocellulose membrane (Hybond ECL, Amersham, Piscataway, NJ, USA), and then blocked with 1% Blot-Qualified BSA (Promega) for 90 min. The membranes were incubated overnight at 4 °C with primary antibodies against ACE2, 6 × His, p22phox, MIF, Akt, eNOS, Ser473-Akt, Ser1177-eNOS and β-actin (1:200∼1:1000) and then incubated with alkaline phosphatase-labelled secondary antibody (Santa Cruz, Biotechnology, Santa Cruz, CA, USA; 1:5000) for 60 min at room temperature. The positive bands corresponding to aim proteins were developed with the Western Blue stabilized substrate for alkaline phosphatase (ProtoBlot II AP System, Promega).
Statistical analysis
All results are expressed as means±s.d. Statistical analysis was performed either by Student's t-test or by ANOVA followed by Bonferroni's test, if appropriate. Statistical significance was accepted when P<0.05.
Materials
All reagents were purchased from Sigma Chemical Company (St Louis, MO, USA) unless otherwise stated. EAhy926 is an immortalized endothelial cell line produced from human umbilical vein endothelial cells and was generously provided by Dr SH Lin from the Baker Institute, Melbourne, Australia. Antibodies against ACE2, p22phox, MIF, Akt, eNOS, phospho-Akt (Ser473), phospho-eNOS (Ser1177) and β-actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA), R&D Systems (Minneapolis, MN, USA) and Cell Signaling Technology (Beverly, MA, USA), respectively. TRIzol and anti-His antibody were obtained from Invitrogen. The kits for measurement of NO and MDA were purchased from Nanjing Jiancheng Bioengineering Institute (Jiangsu, China).
Results
The cloning, transfection and expression of recombinant ACE2 in cells
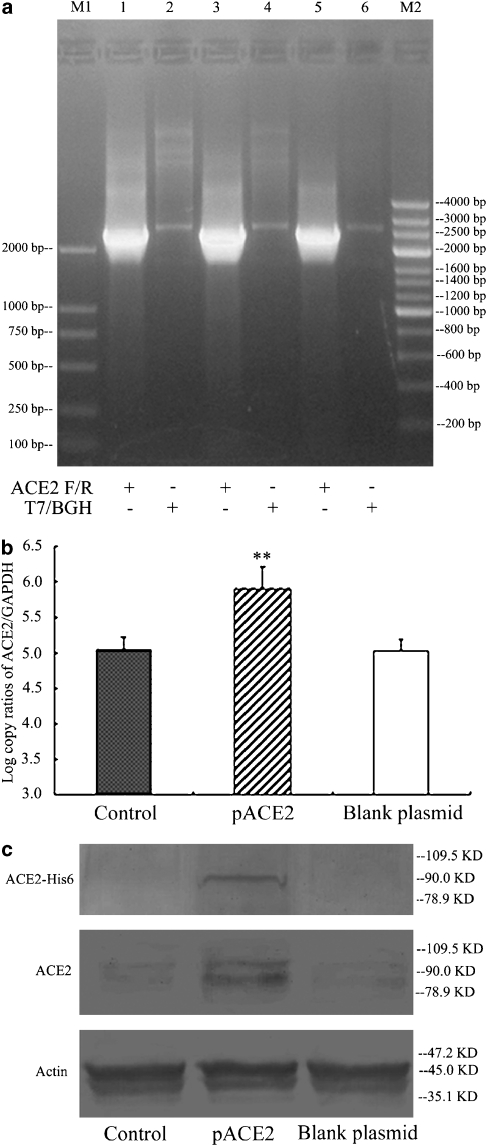
The full-length cDNA of the ACE2 gene was successfully amplified and inserted into the pcDNA 3.1 Directional TOPO vector. The positive recombinant transformants were analysed by PCR amplification with the use of ACE2 gene-specific primers and the T7/BGH priming site in the vector, respectively (Figure 1a). Real-time PCR and western blot analyses showed that the ACE2 mRNA and protein were overexpressed in pACE2-transfected cells compared with the controls (n=5, P<0.01), but not in blank plasmid-transfected cells (Figures 1b and c). Furthermore, the recombinant 6 × His-pACE2 fusion protein of 90 kDa was detected in pACE2-transfected cells, whereas it did not appear in controls and blank plasmid-transfected endothelial cells (Figure 1c).
Figure 1.
The identification and expression of recombinant pACE2 in endothelial cells. (a) The recombinant pACE2 was analysed by PCR amplification using ACE2 and T7/BGH primers with the product size being 2419 and 2775 bp, respectively. M1, M2 indicate two different DNA markers; (b) The mRNA expression of ACE2 was determined by real-time PCR in cells treated with pACE2, vehicle or blank plasmid (mean±s.d.; n=5). **P<0.01 vs control; (c) The protein expression of ACE2 was determined by western blotting with antibodies against 6 × His and ACE2 (90 kDa) in cells pretreated with pACE2, vehicle or blank plasmid. ACE2, angiotensin-converting enzyme 2.
Effects of pACE2 gene transfer on the p22phox expression and MDA levels
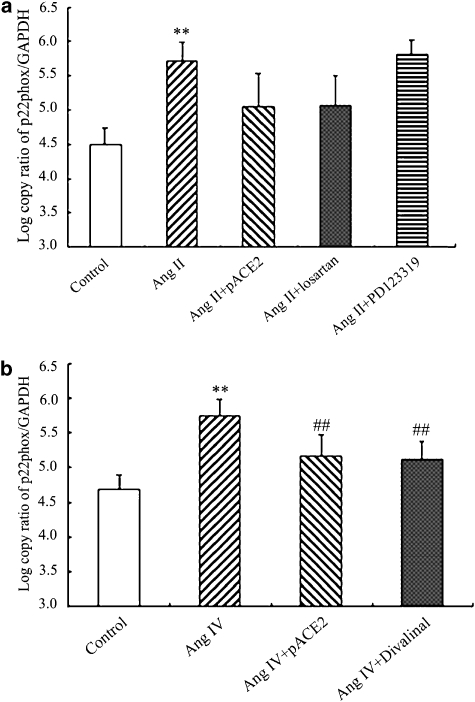
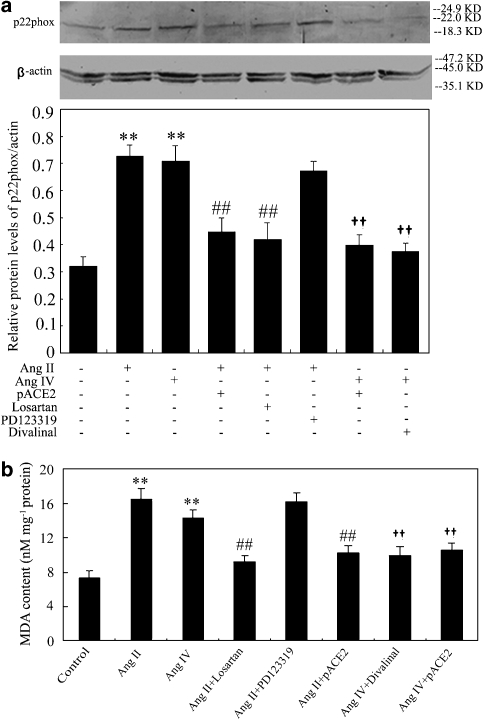
As shown in Figures 2 and 3, the mRNA and protein expression of p22phox and the generation of MDA were increased in endothelial cells after treatment with Ang II or Ang IV. However, in cells transfected with pACE2, Ang II was not able to raise p22phox protein or to increase MDA (Figure 3a). The effects of gene transfer of pACE2 on p22phox mRNA were less clear, Ang II now inducing a level that was neither less than its effect in non-transfected cells and not greater than that in control unstimulated cells (Figure 2a). Pretreatment of non-transfected cells with the AT1 receptor antagonist, losartan, before stimulation with Ang II produced a similar profile of effects—no clear change on p22phox mRNA (Figure 2a) but a significant inhibition of changes in p22phox protein (Figure 3a) and in MDA levels (Figure 3b). Pretreatment with the AT2 receptor antagonist, PD 123319, did not affect any of the responses to Ang II in non-transfected EAhy926 cells (Figures 2a and 3).
Figure 2.
Effects of Ang II, Ang IV, pACE2 gene transfer and angiotensin antagonists on mRNA for p22phox in endothelial cells. The mRNA was analysed by real-time PCR and is expressed as log copy ratio of p22phox/GAPDH. The stimulatory effects of Ang II or Ang IV on the mRNA for p22phox expression was prevented by pACE2. The AT1 selective antagonist losartan, but not the AT2 antagonist PD123369, also prevented the stimulation by Ang II (a) whereas that of Ang IV was prevented by the selective AT4 antagonist, divalinal-Ang IV. (b) Data shown are mean±s.d. (n=4–6). **P<0.01 vs control; ##P<0.01 vs Ang IV alone. ACE2, angiotensin-converting enzyme 2; Ang II, angiotensin II; Ang IV, angiotensin IV;AT1, angiotensin type 1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Figure 3.
Effects of Ang II, Ang IV, pACE2 gene transfer and angiotensin antagonists on p22phox protein expression and MDA levels in endothelial cells. The p22phox protein expression and MDA levels were analysed by western blots and normalized to β-actin (a) or by TBA assays (b), respectively. Both variables were stimulated by Ang II or Ang IV and this stimulation prevented by pACE2. Stimulation was also inhibited by the corresponding receptor antagonists. Data shown are mean±s.d. (n=4–6). **P<0.01 vs control; ##P<0.01 vs Ang II or Ang IV alone; ††P<0.01 vs Ang IV alone. ACE2, angiotensin-converting enzyme 2; Ang II, angiotensin II; Ang IV, angiotensin IV; MDA, malondialdehyde; TBA, thiobarbituric acid.
The hexapeptide Ang IV, at the same concentration as Ang II (100 nM), also stimulated p22phox mRNA (Figure 2b) and protein (Figure 3a) and MDA production (Figure 3b) in EAhy926 cells. Either transfection with pACE2 or pretreatment with the Ang IV antagonist, divalinal-Ang IV, prevented these responses to Ang IV (Figures 2 and 3).
Effects of pACE2 gene transfer on Ang II- and IV-stimulated MIF expression
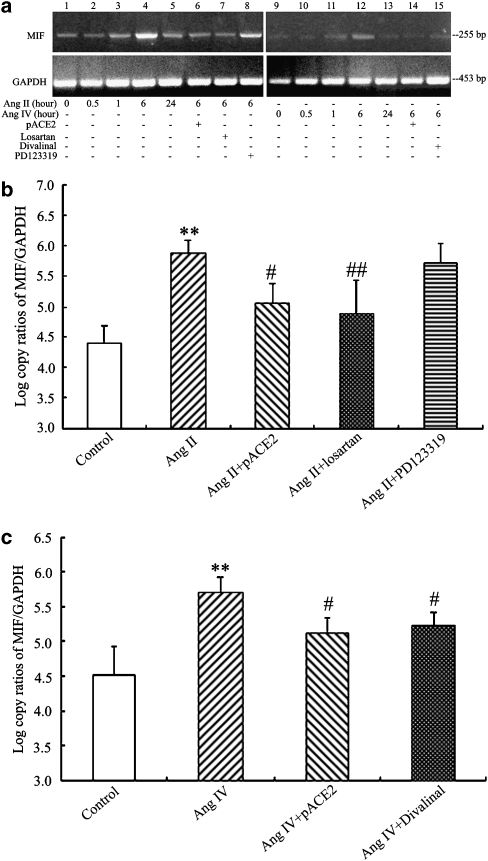
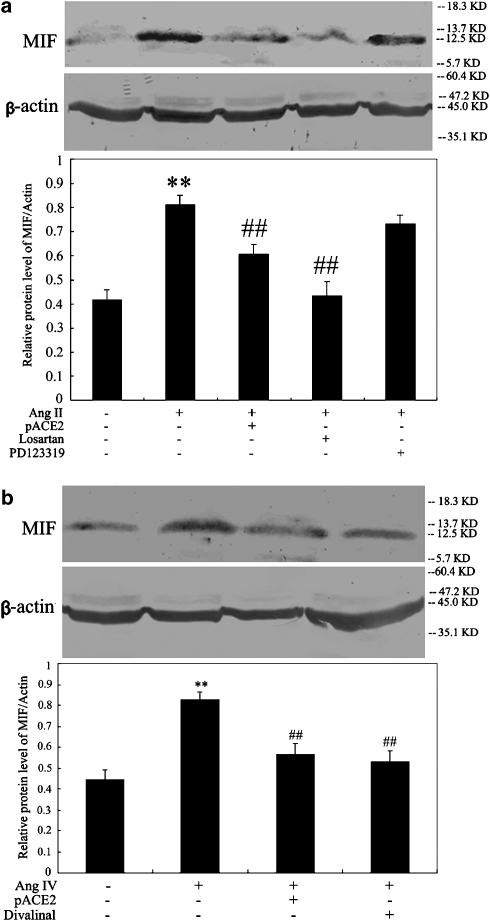
As illustrated by Figure 4, exposure of endothelial cells to Ang II or Ang IV resulted in a time-dependent increase in MIF mRNA expression, with maximal effect occurring after 6 h of incubation, as assessed by semiquantitative RT-PCR (n=4, P<0.05, respectively; Figure 4a) and real-time PCR (n=5–6, P<0.05, respectively) (Figures 4b and c). Moreover, the protein expression of MIF was clearly enhanced in cells stimulated by Ang II or Ang IV for 6 h, as shown by western blot analysis (n=4–5, P<0.01, respectively; Figures 5a and b). The Ang II-induced upregulation of mRNA and protein expression of MIF was suppressed by pACE2 gene transfer and losartan treatment whereas treatment with PD123319 had no effect (Figures 4a, b and 5a). In addition, the Ang IV-evoked enhancement of mRNA and protein expression of MIF was reversed by pACE2 gene transfer and divalinal-Ang IV treatment in endothelial cells (Figures 4a, c and 5b).
Figure 4.
Effects of pACE2 gene transfer on MIF mRNA (normalized to human GAPDH) in endothelial cells, stimulated with Ang II or Ang IV. Regular RT-PCR (a, n=4), real-time PCR analysis (b and c, n=5–6) disclosed the stimulatory effects of Ang II or Ang IV on the mRNA expression of MIF. In cells pretreated with pACE2 or the antagonists, losartan or divalinal-Ang IV, this stimulation was prevented. Values are mean±s.d. **P<0.01 vs control; #P<0.05 ##P<0.01 vs Ang II or Ang IV alone. ACE2, angiotensin-converting enzyme 2; Ang II, angiotensin II; Ang IV, angiotensin IV; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MIF, macrophage migration inhibitory factor; RT, reverse transcription.
Figure 5.
Effects of pACE2 gene transfer on MIF protein (normalized to β-actin) in endothelial cells, stimulated with Ang II or Ang IV. Western blots showed stimulation of MIF protein by Ang II (a) or by Ang IV (b) (n=4–5) and the inhibitory effects of pACE2 gene transfer or the corresponding antagonists. Values are mean±s.d. **P<0.01 vs control; ##P<0.01 vs Ang II or Ang IV alone. ACE2, angiotensin-converting enzyme 2; Ang II, angiotensin II; Ang IV, angiotensin IV; MIF, macrophage migration inhibitory factor.
Effects of pACE2 gene transfer on the insulin/Akt–eNOS signalling pathway
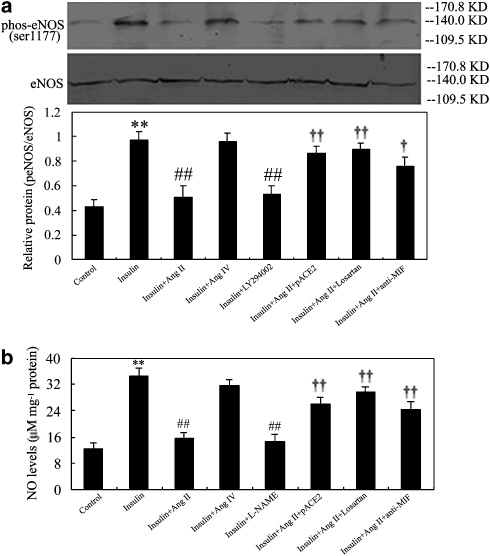
As shown in Figures 6 and 7, Ang II stimulation had no effect on the phosphorylation of Ser473-Akt and Ser1177-eNOS in EAhy926 cells (n=5). In contrast, insulin stimulated the phosphorylation of Ser473-Akt (Figure 4) and Ser1177-eNOS (Figure 7a) in these cells, accompanied by the enhancement of NO generation (Figure 7b). Treatment with Ang II diminished insulin-stimulated Ser473-Akt (Figure 6) and Ser1177-eNOS (Figure 7a) activation and NO generation (Figure 7b), which were strikingly reversed by pACE2 gene transfer or by losartan or by treatment with anti-MIF neutralizing antibody. Furthermore, the PI3K inhibitor LY294002 also prevented insulin-stimulated phosphorylation at Ser473-Akt (Figure 6) and Ser1177-eNOS (Figure 7a) activation. The eNOS inhibitor L-NAME significantly inhibited insulin-stimulated NO production (Figure 7b). However, exposure of cells to Ang IV did not influence insulin-stimulated phosphorylation of Ser473-Akt and Ser1177-eNOS and NO generation (n=5, P>0.05, respectively; Figures 6 and 7).
Figure 6.
Effects of pACE2 gene transfer on the phosphorylation of Akt in endothelial cells. Representative western blots exhibited the phosphorylation of Ser473-Akt (60 kDa) in cells pretreated with or without pACE2, in which responded to Ang II or Ang IV in the presence or absence of insulin, losartan, LY294002 and anti-MIF neutralizing antibody. Each bar shows mean±s.d. (n=5–6). **P<0.01 vs control; ##P<0.01 vs insulin alone; †P<0.05 ††P<0.01 vs insulin plus Ang II. ACE2, angiotensin-converting enzyme 2; Ang II, angiotensin II; Ang IV, angiotensin IV; MIF, macrophage migration inhibitory factor.
Figure 7.
Effects of pACE2 gene transfer on the phosphorylation of eNOS and generation of NO in endothelial cells. Representative western blots and bars exhibited the phosphorylation of Ser1177-eNOS (a, 140 kDa) and the levels of NO (b) in cells pretreated with or without pACE2, in which responded to Ang II or Ang IV in the presence or absence of insulin, losartan, LY294002, L-NAME and anti-MIF neutralizing antibody. Each bar shows mean±s.d. (n=5–6). **P<0.01 vs control; ##P<0.01 vs insulin alone; †P<0.05 ††P<0.01 vs insulin plus Ang II. ACE2, angiotensin-converting enzyme 2; Ang II, angiotensin II; Ang IV, angiotensin IV; eNOS, endothelial NOS; L-NAME; NG-nitro-L-arginine methyl ester; MIF, macrophage migration inhibitory factor.
Discussion
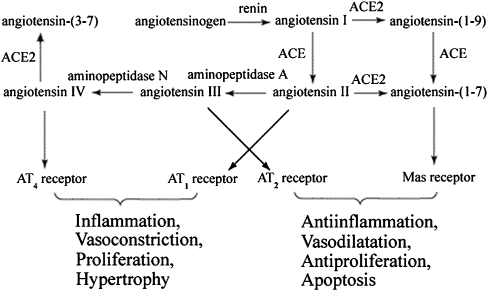
ACE2 is a multifunctional enzyme that plays a central role (Figure 8) in balancing the levels of Ang II and Ang IV with protective peptides such as angiotensin (1–7) (Ang (1–7)), which may attenuate oxidative excess and inflammation through antagonism of Ang II actions and potentiation of NO release (Oudit et al., 2003, 2007; Warner et al., 2004; Der Sarkissian et al., 2006; Ingelfinger, 2006; Hamming et al., 2007; Keidar et al., 2007; Krum and Gilbert, 2007). Mice with targeted disruption of ACE2 develop abnormal cardiac function, decreased levels of Ang (1–7) and enhanced production of Ang II. This would result in enhancement of ROS, induction of inflammation and insulin resistance (Crackower et al., 2002; Oudit et al., 2003, 2007; Marrero et al., 2004; Der Sarkissian et al., 2006; Kim et al., 2006). In contrast, overexpression of ACE2 may tip the balance of the RAS towards levels of these protective peptides and thereby exert some beneficial effects on inflammation and on the damage caused to insulin signalling (Der Sarkissian et al., 2006; Krum and Gilbert, 2007). In the current study, we constructed a recombinant plasmid encompassing the ACE2 gene and successfully transfected it into cultured human endothelial cells.
Figure 8.
Interactions between angiotensin peptides and peptidases. Note that ACE2 is involved in the metabolism of two pro-inflammatory peptides, Ang II and Ang IV, and thus can influence the balance between pro- and anti-inflammatory consequences of activating the RAS. ACE2, angiotensin-converting enzyme 2; Ang II, angiotensin II; Ang IV, angiotensin IV; RAS, renin–angiotensin system.
The most striking observation here is that exposure of endothelial cells to Ang II and Ang IV resulted in increases in the expression of MIF. Most studies have identified MIF to be a pleiotropic cytokine that regulates a broad spectrum of inflammatory reactions by enhancing the production of cytokines, including TNF-α, interleukin (IL)-1β and IL-6 (Fingerle-Rowson and Bucala, 2001; Dandona et al., 2004; Kong et al., 2005; Schmeisser et al., 2005; Burger-Kentischer et al., 2006). Ang II and Ang IV participate in the oxidative stress response via activation of endothelial NADPH oxidase and NF-κB, leading to increased ROS, inflammation and insulin resistance (Chabrashvili et al., 2003; Oudit et al., 2003, 2007; Marrero et al., 2004; Esteban et al., 2005; Kim et al., 2006). MDA is a breakdown product of the oxidative degradation of cell membrane lipids and is generally considered biochemical marker for lipid peroxidation and oxidative stress (Chabrashvili et al., 2003). In states of oxidative stress, myocardial MIF production was increased in a time- and dose-dependent manner, which was completely abolished in the presence of oxidoreductases (Takahashi et al., 2001). As shown in our study, the expression of NADPH oxidase subunit p22phox and the generation of MDA were augmented in endothelial cells pretreated with Ang II or Ang IV. These peptides facilitate oxidative stress and contribute to the activation of NF-κB, subsequently potentiating the transcription of pro-inflammatory genes such as MIF, TNF-α and IL-6 (Chabrashvili et al., 2003; Oudit et al., 2003; Dandona et al., 2005; Esteban et al., 2005). On the basis of the data presented here, enhanced p22phox and MDA levels may be, at least in part, responsible for Ang II- and IV-induced increases in MIF expression in endothelial cells (Figure 6). Blockade of the AT4 receptor with divalinal-Ang IV inhibited the Ang IV-induced expression of p22phox and MIF in endothelial cells. Our findings indicate that Ang IV, a degradation product of Ang II, could play an active role in the oxidative stress and inflammatory responses via AT4 receptors, thus increasing the contribution of the RAS in the inflammatory progress associated with insulin resistance. Gene transfer of ACE2 also strikingly suppressed the expression of p22phox and MIF in endothelial cells, stimulated by Ang II and Ang IV. To our knowledge, these results provide the first description of an important role for ACE2 in counteracting the pro-oxidative and pro-inflammatory effects of Ang II and Ang IV.
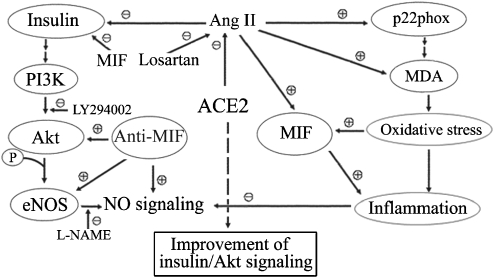
There is increasing evidence that the vascular endothelium is a tissue responsive to insulin and that this response involves the PI3K/Akt–eNOS signalling pathway (Kim et al., 2006; Zhong et al., 2007). In the present study, insulin stimulated the phosphorylation of Akt at Ser473 and of eNOS at Ser1177, resulting in the augmentation of NO production in the cultured endothelial cells. Treatment with Ang II or the PI3K inhibitor LY294002 inhibited insulin-stimulated Ser473-Akt and Ser1177-eNOS activation and NO generation, whereas Ang IV did not alter any of these responses to insulin. Thus Ang II, but not Ang IV, served to dampen insulin signalling through impairment of the PI3K–Akt–eNOS pathway, thereby promoting insulin resistance (Marrero et al., 2004; Kim et al., 2006). The inhibitory actions of Ang II were also reversed by treatment with anti-MIF antibody in endothelial cells. Since pro-inflammatory mechanisms may contribute to the pathogenesis of insulin resistance through interference with insulin signalling, it is possible that Ang II-induced MIF expression may represent a novel mechanism that contributes to the pathophysiological changes leading to insulin resistance (Dandona et al., 2004, 2005; Herder et al., 2006). MIF stimulates the secretion of insulin induced by glucose from β-cells in pancreatic islets, which may result in hyperinsulinaemia in pro-inflammatory insulin-resistant states (Dandona et al., 2004; Herder et al., 2006). Moreover, MIF also functions as an enzyme that reduces sulphhydryl linkages and breaks them. This action could potentially reduce the biological activity of insulin and the efficiency of insulin receptors and contribute to insulin resistance (Dandona et al., 2004). MIF blockade would therefore produce a protective effect on inflammation and insulin resistance in endothelial cells. Blockade of MIF is known to depress production of pro-inflammatory mediators such as TNF-α, IL-1β and IL-6, which induce both inflammation and insulin resistance (Dandona et al., 2005; Burger-Kentischer et al., 2006; Herder et al., 2006). Metformin, an insulin sensitizer, exerts an anti-inflammatory effect by suppressing plasma MIF concentrations, in addition to its glucose-lowering effect, in patients with obesity and diabetes (Dandona et al., 2004). As shown in our study, gene transfer of ACE2 significantly prevented MIF expression stimulated by Ang II. This protective effect of ACE2 was paralleled by restoration of insulin-stimulated phosphorylation of Ser473-Akt and Ser1177-eNOS and production of NO in endothelial cells exposed to Ang II. Similarly, anti-MIF antibody treatment strikingly reversed the inhibitory effects of Ang II on the insulin/Akt–eNOS signalling pathway in endothelial cells. This beneficial effect of MIF blockade may be due to inhibition of crosstalk between Ang II-AT1 signalling and insulin signalling. Thus, a reduction in MIF expression induced by ACE2 gene transfer may potentially contribute to a decrease in insulin breakdown and improvement of insulin signalling transduction in endothelial cells (Figure 9). Generalizing the results, ACE2 may act as a protective enzyme in insulin-resistant states, serving to reverse the Ang II-induced impairment of insulin signalling, through the Akt–eNOS phosphorylation pathway.
Figure 9.
Putative protective mechanisms of ACE2 against Ang II-mediated impairment of insulin/Akt signalling pathway. On one hand, Ang II induces the expression of the NADPH oxidase subunit p22phox, contributing to the enhancement of MDA production and oxidative status and MIF expression. On the other hand, Ang II regulates insulin signalling via a pathway involving impairment of PI3K-dependent activation of Akt–eNOS phosphorylation and NO generation. These inhibitory effects of Ang II can be reversed by ACE2 gene transfer and anti-MIF treatment in endothelial cells. These beneficial effects of ACE2 are thought to result mainly from blocking the expression of MIF. ACE2, angiotensin-converting enzyme 2; Ang II, angiotensin II; e NOS, endothelial NOS; MDA, malondialdehyde; MIF, macrophage migration inhibitory factor; NADPH, nicotinamide adenine dinucleotide phosphate; PI3K, phosphatidylinositol 3-kinase.
In summary, the present study defines for the first time a regulatory role for the ACE2 gene in the oxidative stress, inflammation and insulin resistance induced by Ang II and its hexapeptide fragment, Ang IV. ACE2 gene transfer suppressed the expression of p22phox and MIF and the production of MDA caused by Ang II and Ang IV in human endothelial cells. Ang II, but not Ang IV, might serve to dampen insulin signalling pathway involved in impairment of Akt and eNOS phosphorylation, which were reversed by ACE2 gene transfer and anti-MIF treatment. These beneficial effects of ACE2 overexpression were thought to result mainly from blocking expression of MIF. Thus, the ACE2 gene may be a novel therapeutic target for the inflammation- and insulin resistance-related diseases, such as diabetes. Further investigation focusing on the interactions among ACE2, Ang II and MIF in the endothelium would enable us to better understand the regulatory roles of ACE2 in the pathophysiological mechanisms involved in the inflammatory response and the insulin/Akt–eNOS signalling pathways.
Acknowledgments
This project was supported by the 39th China Postdoctoral Science Foundation (Grant no. 20060390195), National Natural Science Foundation of China (Grant nos. 30772142, 30700328), Natural Science Foundation of Guangdong Province (Grant nos.015015, 7300041) and Guangdong Provincial Medical Science Foundation (Grant no. A2006047).
Abbreviations
- ACE2
angiotensin-converting enzyme 2
- Ang II
angiotensin II
- Ang IV
angiotensin IV
- Ang (1-7)
angiotensin-(1-7)
- AT1
angiotensin type 1 receptor
- eNOS
endothelial NOS
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- L-NAME
NG-nitro-L-arginine methyl ester
- MDA
malondialdehyde
- MIF
macrophage migration inhibitory factor
- NADPH
nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor-κB
- PI3K
phosphatidylinositol 3-kinase
- RAS
renin–angiotensin system
- ROS
reactive oxygen species
- TNF-α
tumour necrosis factor-α
Conflict of interest
The authors state no conflict of interest.
References
- Burger-Kentischer A, Gobel H, Kleemann R, Zernecke A, Bucala R, Leng L, et al. Reduction of the aortic inflammatory response in spontaneous atherosclerosis by blockade of macrophage migration inhibitory factor (MIF) Atherosclerosis. 2006;184:28–38. doi: 10.1016/j.atherosclerosis.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Busche S, Gallinat S, Fleegal MA, Raizada MK, Sumners C. Novel role of macrophage migration inhibitory factor in angiotensin II regulation of neuromodulation in rat brain. Endocrinology. 2001;142:4623–4630. doi: 10.1210/endo.142.11.8502. [DOI] [PubMed] [Google Scholar]
- Chabrashvili T, Kitiyakara C, Blau J, Karber A, Aslam S, Welch WJ, et al. Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. Am J Physiol Regul Integr Comp Physiol. 2003;285:R117–R124. doi: 10.1152/ajpregu.00476.2002. [DOI] [PubMed] [Google Scholar]
- Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111:1448–1454. doi: 10.1161/01.CIR.0000158483.13093.9D. [DOI] [PubMed] [Google Scholar]
- Dandona P, Aljada A, Ghanim H, Mohanty P, Tripathy C, Hofmeyer D, et al. Increased plasma concentration of macrophage migration inhibitory factor (MIF) and MIF mRNA in mononuclear cells in the obese and the suppressive action of metformin. J Clin Endocrinol Metab. 2004;89:5043–5047. doi: 10.1210/jc.2004-0436. [DOI] [PubMed] [Google Scholar]
- Der Sarkissian S, Huentelman MJ, Stewart J, Katovich MJ, Raizada MK. ACE2: A novel therapeutic target for cardiovascular diseases. Prog Biophys Mol Biol. 2006;91:163–198. doi: 10.1016/j.pbiomolbio.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Esteban V, Ruperez M, Sanchez-Lopez E, Rodriguez-Vita J, Lorenzo O, Demaegdt H, et al. Angiotensin IV activates the nuclear transcription factor-kappaB and related proinflammatory genes in vascular smooth muscle cells. Circ Res. 2005;96:965–973. doi: 10.1161/01.RES.0000166326.91395.74. [DOI] [PubMed] [Google Scholar]
- Fingerle-Rowson GR, Bucala R. Neuroendocrine properties of macrophage migration inhibitory factor (MIF) Immunol Cell Biol. 2001;79:368–375. doi: 10.1046/j.1440-1711.2001.01024.x. [DOI] [PubMed] [Google Scholar]
- Ha T, Hua F, Grant D, Xia Y, Ma J, Gao X, et al. Glucan phosphate attenuates cardiac dysfunction and inhibits cardiac MIF expression and apoptosis in septic mice. Am J Physiol Heart Circ Physiol. 2006;291:H1910–H1918. doi: 10.1152/ajpheart.01264.2005. [DOI] [PubMed] [Google Scholar]
- Hamming I, Cooper ME, Haagmans BL, Hooper NM, Korstanje R, Osterhaus AD, et al. The emerging role of ACE2 in physiology and disease. J Pathol. 2007;212:1–11. doi: 10.1002/path.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herder C, Kolb H, Koenig W, Haastert B, Muller-Scholze S, Rathmann W, et al. Association of systemic concentrations of macrophage migration inhibitory factor with impaired glucose tolerance and type 2 diabetes: results from the Cooperative Health Research in the Region of Augsburg, Survey 4 (KORA S4) Diabetes Care. 2006;29:368–371. doi: 10.2337/diacare.29.02.06.dc05-1474. [DOI] [PubMed] [Google Scholar]
- Ingelfinger JR. ACE2: A new target for prevention of diabetic nephropathy. J Am Soc Nephrol. 2006;17:2957–2959. doi: 10.1681/ASN.2006090986. [DOI] [PubMed] [Google Scholar]
- Keidar S, Kaplan M, Gamliel-Lazarovich A. ACE2 of the heart: from angiotensin I to angiotensin (1–7) Cardiovasc Res. 2007;73:463–469. doi: 10.1016/j.cardiores.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–1904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- Kong YZ, Huang XR, Ouyang X, Tan JJ, Fingerle-Rowson G, Bacher M, et al. Evidence for vascular macrophage migration inhibitory factor in destabilization of human atherosclerotic plaques. Cardiovasc Res. 2005;65:272–282. doi: 10.1016/j.cardiores.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Krum H, Gilbert RE. Novel therapies blocking the renin–angiotensin–aldosterone system in the management of hypertension and related disorders. J Hypertens. 2007;25:25–35. doi: 10.1097/HJH.0b013e3280113950. [DOI] [PubMed] [Google Scholar]
- Lin SG, Yu XY, Chen YX, Huang XR, Metz C, Bucala R, et al. De novo expression of macrophage migration inhibitory factor in atherogenesis in rabbits. Circ Res. 2000;87:1202–1208. doi: 10.1161/01.res.87.12.1202. [DOI] [PubMed] [Google Scholar]
- Marrero MB, Fulton D, Stepp D, Stern DM. Angiotensin II-induced insulin resistance and protein tyrosine phosphatases. Arterioscler Thromb Vasc Biol. 2004;24:2009–2013. doi: 10.1161/01.ATV.0000140059.04717.f3. [DOI] [PubMed] [Google Scholar]
- Oudit GY, Crackower MA, Backx PH, Penninger JM. The role of ACE2 in cardiovascular physiology. Trends Cardiovasc Med. 2003;13:93–101. doi: 10.1016/s1050-1738(02)00233-5. [DOI] [PubMed] [Google Scholar]
- Oudit GY, Kassiri Z, Patel MP, Chappell M, Butany J, Backx PH, et al. Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovasc Res. 2007;75:29–39. doi: 10.1016/j.cardiores.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Schmeisser A, Marquetant R, Illmer T, Graffy C, Garlichs CD, Bockler D, et al. The expression of macrophage migration inhibitory factor 1alpha (MIF 1alpha) in human atherosclerotic plaques is induced by different proatherogenic stimuli and associated with plaque instability. Atherosclerosis. 2005;178:83–94. doi: 10.1016/j.atherosclerosis.2004.08.038. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Nishihira J, Shimpo M, Mizue Y, Ueno S, Mano H, et al. Macrophage migration inhibitory factor as a redox-sensitive cytokine in cardiac myocytes. Cardiovasc Res. 2001;52:438–445. doi: 10.1016/s0008-6363(01)00408-4. [DOI] [PubMed] [Google Scholar]
- Warner FJ, Smith AI, Hooper NM, Turner AJ. Angiotensin-converting enzyme-2: a molecular and cellular perspective. Cell Mol Life Sci. 2004;61:2704–2713. doi: 10.1007/s00018-004-4240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong JC, Huang DY, Liu GF, Jin HY, Yang YM, Li YF, et al. Effects of all-trans retinoic acid on orphan receptor APJ signaling in spontaneously hypertensive rats. Cardiovasc Res. 2005;65:743–750. doi: 10.1016/j.cardiores.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Zhong JC, Huang DY, Yang YM, Li YF, Liu GF, Song XH, et al. Upregulation of angiotensin-converting enzyme 2 by all-trans retinoic acid in spontaneously hypertensive rats. Hypertension. 2004;44:907–912. doi: 10.1161/01.HYP.0000146400.57221.74. [DOI] [PubMed] [Google Scholar]
- Zhong JC, Yu XY, Huang Y, Yung LM, Lau CW, Lin SG. Apelin modulates aortic vascular tone via endothelial nitric oxide synthase phosphorylation pathway in diabetic mice. Cardiovasc Res. 2007;74:388–395. doi: 10.1016/j.cardiores.2007.02.002. [DOI] [PubMed] [Google Scholar]