Abstract
Background and purposes:
Erythropoietin (EPO) has been shown to protect against myocardial infarction in animal studies by activating phosphatidylinositol-3 kinase (PI3K)/Akt and ERK1/2. However these pro-survival pathways are impaired in the diabetic heart. We investigated the ability of EPO to protect human atrial trabeculae from non-diabetic and diabetic patients undergoing coronary artery bypass surgery, against hypoxia-reoxygenation injury.
Experimental approach:
Human atrial trabeculae were exposed to 90min hypoxia and 120min reoxygenation. EPO was administered throughout reoxygenation. The developed force of contraction, calculated as a percentage of baseline force of contraction, was continuously monitored. The involvement of PI3K and ERK1/2 and the levels of activated caspase 3(AC3) were assessed.
Key results:
EPO improved the force of contraction in tissue from non-diabetic patients (46.7+/-1.7% vs. 30.2+/-2.2% in control, p<0.001). These beneficial effects were prevented by the PI3K inhibitor, LY294002 and the ERK1/2 inhibitor, U0126. EPO also significantly improved the force of contraction in the diabetic tissue, although to a lesser degree. The levels of activated caspase 3 were significantly reduced in EPO treated trabeculae from both non-diabetic and diabetic patients, relative to their respective untreated controls.
Conclusions and implications:
EPO administered at reoxygenation protected human myocardial muscle by activating PI3K and ERK1/2 and reducing the level of activated caspase 3. This cardioprotection was also observed in the diabetic group. This data supports the potential of EPO being used as a novel cardioprotective strategy either alone or as an adjunct in the clinical setting alongside existing reperfusion therapies.
Keywords: hypoxia, reoxygenation, signal transduction, diabetes, MAP kinase, PI3K/Akt, caspase 3, human myocardium
Introduction
The vast majority of cardioprotective therapies following acute myocardial infarction (AMI) have focused on the rapid restoration of coronary artery blood flow, either by thrombolysis, percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG). Although rapid reperfusion has achieved significant success in terms of large-scale reductions in patient mortality and morbidity, reperfusion of ischaemic myocardium can paradoxically still result in further loss of myocytes, a phenomenon termed lethal reperfusion-induced injury (Braunwald and Kloner, 1985). Future therapies will need to be focused on limiting reperfusion-induced injury following an AMI resulting in increased salvage of ischaemic myocardium, and subsequently improved mortality and morbidity.
Erythropoietin (EPO) is a kidney cytokine which regulates the production of blood cells (haematopoiesis) used in clinical practice to treat anaemia in patients with chronic renal and heart failure. Initial studies in the late 1990s suggested that EPO may protect against ischaemic brain injury by preventing apoptotic cell death (Sadamoto et al., 1998; Sakanaka et al., 1998). Subsequently, a number of studies have linked EPO with protection against myocardial ischaemia–reperfusion injury. In this regard Calvillo et al. (2003) reported that EPO could protect rat cardiomyocytes against apoptotic cell death induced by chronic hypoxia. In addition, Tramontano et al. (2003) reported similar findings in neonatal cardiomyocytes with EPO-induced protection being mediated by Akt (protein kinase B) activation. Moon et al. (2003) further demonstrated that intravenous administration of EPO (3000 IU kg−1) given at the time of myocardial ischaemia in a model of permanent coronary ligation, reduced subsequent myocardial infarct size 24 h later and at 8 weeks. Other studies (Cai et al., 2003) have also demonstrated that intraperitoneal administration of EPO improved the recovery of left ventricular function and decreased apoptotic cardiomyocyte death in isolated perfused rat hearts 24 h later. It has also been reported that EPO protects H9C2 cells from apoptotic cell death by upregulating Akt, the extracellular signal-regulated kinases (ERK1/2) and JAK1-STAT, and reduces myocardial infarct size as well as activating both Akt and ERK1/2 in rabbits when given prior to ischaemia (Parsa et al., 2003). Crucially, this cardioprotective effect of EPO was dissociated from its haemopoietic effect in this study. In support of a direct effect of EPO on cardiomyocytes, both EPO and the EPO receptor (a cytokine receptor) have been reported in myocardial tissue of different species including man (Wright et al., 2004; Deeping et al., 2005). Importantly, we have recently demonstrated that EPO administered at the time of myocardial reperfusion reduces myocardial infarct size in both perfused and in situ rat hearts through the activation of Akt and ERK1/2, members of the RISK pathway (Bullard et al., 2005), a finding which has been confirmed in the canine heart (Hirata et al., 2005).
However, despite the growing body of evidence reaffirming the cardioprotective effects of EPO in animal tissue, no studies to date have focussed on the direct effect of EPO in human myocardial tissue. The work presented in this paper investigated the effect of EPO when administered at the point of reoxygenation following severe hypoxia using human atrial muscle taken from both non-diabetic and from type II diabetic patients undergoing coronary artery bypass surgery.
Methods
Tissue donors
Informed consent was obtained from each patient prior to inclusion in the study. These documents are kept in our archives. Prior ethical approval for the study was obtained from the Joint University College London/University College London Hospital Committees on the Ethics of Human Research; reference number 00/0275. The investigation conforms to the principles outlined in the Declaration of Helsinki (1997) for use of human tissue or subjects.
Right atrial appendages were harvested from 58 patients (46 male and 12 female patients), who were diagnosed with stable coronary artery disease and admitted for elective coronary bypass surgery. Of these patients, 16 were suffering from type II diabetes and were undergoing treatment for this condition. The age range of patients was between 36 and 80 (mean 62.4 years for non-diabetic patients and 62.6 for the diabetic ones). The sampled patient population were prescribed a range of medications. All patients having been diagnosed with coronary artery disease were on the following medications: aspirin, angiotensin-converting enzyme inhibitors, β-blockers and statins. Duration of type II diabetes in the diabetic patients ranged from 2 to 17 years with median duration of about 7 years.
Exclusion criteria: patients with a prior history of atrial arrhythmias, right ventricular failure and those receiving antiarrhythmic treatment were not included in the study.
Human atrial model of hypoxia/reoxygenation
Harvesting and preparing the samples; exclusion criteria
Right atrial appendages were harvested from patients undergoing coronary artery bypass grafting and immediately placed in ice-cold Tyrode's buffer. The Tyrode's buffer had been oxygenated with 95% O2, and 5% CO2. The pH of the transport medium was carefully checked prior to transport to ensure that it was between 7.35 and 7.45, the partial pressure of O2 was between 50 and 60 kPa and the partial pressure of CO2 between 4.0 and 6.0 kPa. Temperature was kept below 4 °C. The atrial appendage was fixed to a rubber-based dissection dish and secured in place with small pins. The dish was perfused with ice-cold pre-oxygenated Tyrode's buffer and the appendage was dissected. Once suitable trabeculae were identified, a 5.0 silk tie was placed at either end of the trabeculae, which was then excised from the atrial tissue. One end of the trabeculae was attached to a fixed point in the perfusion chamber and the other to a force transducer.
Trabeculae were excluded if they were less than 1.2 mm in diameter and 2 mm in length and/or if the time from surgery to mounting in the organ bath was longer than 20 min. Some atrial appendages were technically very difficult to dissect resulting in mechanical trauma to the trabeculae which subsequently either contract very poorly or not at all, therefore they were also excluded. The characteristics of the trabeculae used in the following experiments are presented in Table 1.
Table 1.
Morphometric characteristics of the human atrial trabeculae used in the studies
| Experimental condition | Control | Precond | EPO 25 | EPO 50 | EPO 150 | EPO+LY | LY | EPO+ U0 | U0 | Diab Control | Diab+EPO |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight (mg) | 0.46 | 0.41 | 0.61 | 0.56 | 0.57 | 0.88 | 0.89 | 0.51 | 0.52 | 0.55 | 0.77 |
| s.e. | 0.10 | 0.06 | 0.09 | 0.11 | 0.10 | 0.33 | 0.15 | 0.06 | 0.05 | 0.12 | 0.08 |
| Diameter (mm) | 0.90 | 0.78 | 0.82 | 0.98 | 0.92 | 1.00 | 0.96 | 0.93 | 0.85 | 0.76 | 0.9 |
| s.e. | 0.06 | 0.08 | 0.06 | 0.06 | 0.03 | 0.05 | 0.07 | 0.06 | 0.07 | 0.09 | 0.06 |
| Length (mm) | 3.80 | 3.70 | 5.30 | 3.80 | 4.80 | 6.60 | 6.10 | 5.64 | 5.53 | 5.73 | 5.10 |
| s.e. | 0.35 | 0.15 | 0.31 | 0.22 | 0.70 | 0.74 | 0.35 | 0.49 | 0.85 | 0.63 | 0.64 |
| Cross section (mm2) | 0.71 | 0.61 | 0.56 | 0.78 | 0.50 | 0.82 | 0.76 | 0.69 | 0.59 | 0.49 | 0.72 |
| s.e. | 0.09 | 0.08 | 0.08 | 0.09 | 0.46 | 0.09 | 0.10 | 0.09 | 0.08 | 0.12 | 0.09 |
| Sample number | 8 | 7 | 9 | 10 | 6 | 7 | 8 | 8 | 6 | 7 | 8 |
The values are represented as means with s.e., from the number of samples given at the bottom of each column. All samples were from non-diabetic patients, except for the last two columns. Experimental conditions were as follows: All trabeculae were subjected to hypoxia-reoxygenation and randomized to the following groups: Control: untreated trabeculae; Precond: with preconditioning hypoxia; EPO 25, 50, 150: treated with EPO 25, 50 or 150 ng ml−1; EPO+LY: EPO 50 ng ml−1+ the PI3K inhibitor LY294002; LY: LY294002 alone; EPO+UO: EPO 50 ng ml−1+ the ERK 1/2 inhibitor U0126; UO: U0126 alone; Diab control: untreated trabeculae from diabetic patients; Diab+EPO: diabetic trabeculae+EPO 50 ng ml−1.
Experimental protocols
After dissection, the suitable atrial trabeculae were suspended horizontally in an organ bath and superfused with a modified Tyrode buffer comprising (in mM) 118.5 NaCl, 4.8. KCl, 24.8 NaHCO3, 1.2 KH2PO4, 1.44 MgSO4·7H2O, 1.8 CaCl2·2H2O, 10.0 glucose and 10.0 pyruvic acid, oxygenated with a 95% O2–5% CO2 mixture. pH was maintained between 7.35 and 7.45 with a partial pressure of O2 between 50 and 60 kPa and CO2 between 4.0 and 6.0 kPa. Constant temperature of 37 oC was maintained with a heat exchanger (Techne Circulators C 85-A, Cambridge, UK). The trabeculae were connected to force transducers and the developed contractile forces were amplified and recorded with a Powerlab/8sp instrument (AD Instruments).
The atrial trabeculae were stimulated at 1 Hz and allowed to stabilize for a total of 75 min. After 30 min of stabilization, the trabeculae were gradually stretched over a 15 min period to reach their maximum force of contraction, followed by a further 30 min of stabilization. If the developed force of contraction was less than 0.5 g after stretching, these trabeculae were excluded.
The trabeculae were then exposed to a period of simulated ischaemia (SI) which comprised of 90 min superfusion with a glucose-free hypoxic Tyrode buffer containing (in mM) 118.5 NaCl, 4.8 KCl, 24.8 NaHCO3, 1.2 KH2PO4, 1.44 MgSO4·7H2O, 1.8 CaCl2·2H2O, 7.0 choline chloride and 10.0 pyruvic acid and field stimulation at 3 Hz. The hypoxic buffer was bubbled with 95% N2–5% CO2 in order to lower the partial pressure of O2 of the buffer in the organ bath to <7 kPa (pH 7.24–7.35). After 90 min SI, the trabeculae were reoxygenated with superfusion of oxygenated Tyrode buffer and a field stimulation of 1 Hz.
Human trabeculae were randomized to various experimental groups (Figure 1). The control group (n=8) atrial trabeculae were superfused with a normoxic Tyrode buffer. In an hypoxic preconditioning group (n=7), used as a positive control to demonstrate that cardioprotection could be achieved in this human atrial model of hypoxia/reoxygenation (Walker et al., 1995), the atrial trabeculae were subjected to 5 min of superfusion with hypoxic, substrate-free buffer and paced at 3 Hz prior to the 90 min of sub-lethal hypoxia. This was followed by 20 min of reoxygenation with normoxic buffer and pacing at 1 Hz. A dose–response study was carried out using 25 ng ml−1 (n=9), 50 ng ml−1 (n=10) and 150 ng ml−1 (n=6) EPO at reperfusion. All subsequent experiments were done using 50 ng ml−1 EPO. In the EPO at reoxygenation group (n=10) EPO (50 ng ml−1) was administered at the point of reoxygenation and throughout the 2 h reoxygenation period. EPO with LY294002 (15 μM) (n=7) or EPO with U0126 (10 μM) (n=8) were administered at the point of reoxygenation and throughout the reoxygenation period. LY294002 (n=8) or U0126 (n=6) alone were also administered at the start of and throughout reoxygenation as negative controls. The concentrations of EPO, LY294002 and U0126 used have all been demonstrated to be effective in rat heart studies, both in vitro and in vivo (Bullard et al., 2005). For the diabetic subgroup, atrial trabeculae from type II diabetic patients were harvested and exposed to the same protocol with EPO being added in the buffer at reoxygenation as for the non-diabetic trabeculae (n=7 in control group and n=8 in EPO-treated group).
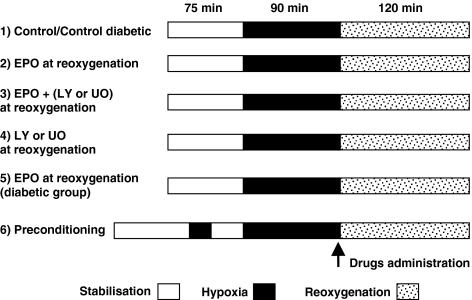
Figure 1.
Experimental protocols. All groups were stabilized with stimulation at 1 Hz for 75 min. The groups then were exposed to 90 min of hypoxia (pacing at 3 Hz during superfusion with a hypoxic, glucose-free buffer) followed by 120 min of reoxygenation (reperfusion) with buffer (control) or different drugs added in the control buffer. Group 5 involved the administration of EPO throughout reoxygenation, to myocardium from diabetic patients. Group 6 was treated with a preconditioning exposure to hypoxia (see Methods) and was used as a positive control group.
Contractile function was recorded at 15 min intervals during reoxygenation and expressed as a percentage of the baseline (stabilization) force of contraction for each individual human trabecula. In addition, each trabecula was weighed and measured in terms of its diameter and length. The cross-sectional area was calculated from the measured diameter of the atrial trabeculae (Figure 1 illustrates the various experimental protocols used). These morphometric data are presented in Table 1.
Western blot analysis
At the end of the hypoxia/reoxygenation period some trabeculae were snap-frozen for further analysis. The samples were prepared for western blot analysis as follows: following sonication in suspension buffer (3 times for 5 s on ice) and centrifugation for 10 min at 10 000 g, a small volume of the supernatant was used for measuring the protein concentration (Pierce BSC kit), and the rest was boiled with an equal volume of sample buffer. For gel electrophoresis, 30 μg of protein were loaded in each well, followed by transfer onto nitrocellulose paper. The membranes were probed with caspase-3 antibody (Cell Signalling) in order to assess its degree of cleavage (activation). All buffers are routinely used as previously described (Efthymiou et al., 2005). The efficacy of the transfer and the equal loading were verified using Ponceau Red and β-actin respectively. The densitometrical value of cleaved caspase 3 (activated caspase) was expressed as a per cent of the total caspase 3 present in the sample (the sum of cleaved and non-cleaved caspase 3).
Statistical analysis
Factorial one- and two-way analysis of variance (ANOVA) using STAT view 4.5. (Abacus Concepts Inc, Berkeley, CA, USA) was used for comparisons between more than two groups. Fisher's protected least-significance difference post hoc test was applied for between-group comparisons where a significant F-value had been obtained. Results were considered significant when P⩽0.05. All results were displayed as group means±standard error (s.e.).
Materials
Human recombinant erythropoietin (Roche, WERK Penzberg, Germany) was added to Tyrode buffer in concentrations ranging from 25, 50 to 150 ng ml−1. The PI3 kinase inhibitor, LY294002 (2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one hydrochloride, Sigma-Aldrich, Poole, UK) was dissolved in dimethyl sulphoxide (DMSO), Sigma-Aldrich) and added to the buffer to a final concentration of 15 μM LY294002. The p42/44 MAP kinase inhibitor, U0126 (Sigma-Aldrich) was dissolved in DMSO and subsequently added to Tyrode buffer to reach a final concentration of 10 μM. The final concentration of DMSO was <0.01%
Results
A maximum of three trabeculae from each patient could be obtained and these were randomly assigned to various experimental protocols. Fifteen trabeculae were excluded from the study as a consequence of prolonged dissections times or because they were too small, as previously discussed.
EPO at reoxygenation confers protection against hypoxia/reoxygenation in both non-diabetic and diabetic human atrial trabeculae
The recovery of the developed force of contraction was used as the end point in these experiments. The values were expressed as a percentage of the force of contraction of the trabeculae prior to the hypoxia/reoxygenation injury (baseline).
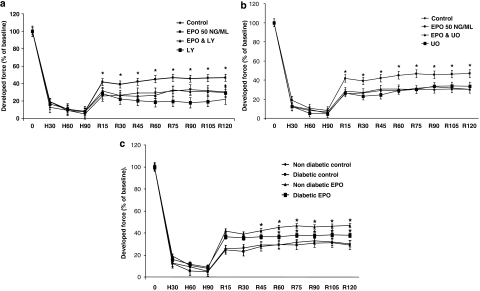
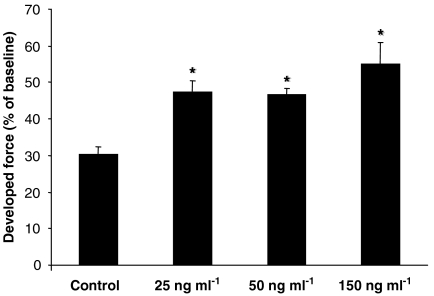
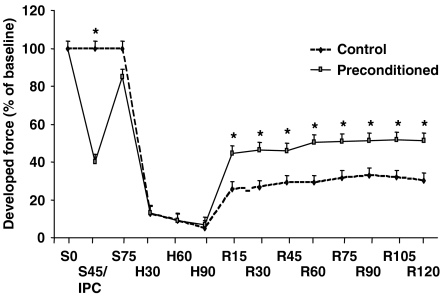
The trabeculae from non-diabetic patients, treated with 50 ng ml−1 EPO throughout reoxygenation demonstrated a significantly improved recovery of contractility over the control tissues (Figure 2a,b). Other EPO concentrations were also used and similar degrees of protection was observed at 25 and 150 ng ml−1 (Figure 3). The protection observed following EPO administration was comparable with that of a preconditioning stimulus which was used as a positive control (Figure 4).
Figure 2.
Developed force in human trabeculae under a variety of conditions. The developed force was expressed as a percentage of baseline developed force in all the different test groups. (a) Illustrates a direct comparison between control, EPO, EPO+LY294002 (LY) and LY alone at each assessed time interval (shown as min). (b) Effects of the ERK 1/2 inhibitor U0126 (U0) are shown on controls and EPO-treated tissues. (c) Responses of non-diabetic control, diabetic control, non-diabetic+EPO and diabetic+EPO are shown, at each assessed time interval. 0 –H90=time of hypoxia; H90–R120=time in reoxygenation. *P<0.05 vs corresponding control values.
Figure 3.
Erythropoietin (EPO) protects the human atrial myocardium from hypoxia/reoxygenation injury at various concentrations. EPO was superfused at 25, 50 or 150 ng ml−1 throughout the 120 min reoxygenation period. *P<0.05 vs control.
Figure 4.
Direct comparison between control and preconditioning groups at each assessed time interval. The preconditioning stimulus consisted of 5 min of hypoxia (at S45/IPC) followed by 20 min of reoxygenation before the standard period of hypoxia (between S75 and H90; see Figure 1). Reoxygenation was for the usual 120 min (to R120) S=stabilization; IPC=preconditioning hypoxia;, R=reoxygenation; all times in min. *P<0.05 vs control.
Interestingly, in the diabetic group, EPO also significantly improved the developed force of contraction post-hypoxia, but the degree of cardioprotection was less than that observed in the non-diabetic group (P<0.005) (Figure 2c).
EPO-mediated cardioprotection in the human myocardium is via activation of the reperfusion injury salvage kinases, PI3K and ERK 1/2
In order to ascertain the degree of involvement of the pro-survival kinases in the protective effects of EPO, the phosphatidylinositol-3 kinase (PI3K) inhibitor LY294002 and/or ERK 1/2 inhibitor, U0126 were co-administered with EPO. These experiments were performed on tissue samples from non-diabetic patients only. The cardioprotective effect of EPO was abolished by the PI3K inhibitor, LY294002 (Figure 2a) and by the ERK 1/2 inhibitor, U0126 (Figure 2b). Administration of these inhibitors alone at reoxygenation did not have any effect upon the recovery of the contractile function, with results similar to that seen for the control values (Figure 2a,b).
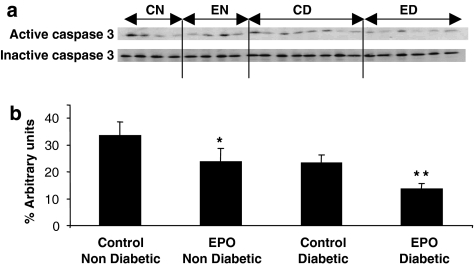
EPO reduces caspase 3 activation
Figure 5 depicts the level of caspase 3 activation at the end of reoxygenation in both the non-diabetic and diabetic tissue. The blots were quantified by densitometry and the values presented in Figure 5b were calculated as the proportion of active caspase 3 relative to total caspase 3 (sum of active and inactive bands). There was significantly less activation of caspase 3 in the EPO-treated groups, relative to the corresponding untreated groups, for both non-diabetic and diabetic tissues.
Figure 5.
Active caspase 3 in atrial trabeculae from non-diabetic and diabetic patients after hypoxia/reoxygenation injury. (a) Western blots for active (cleaved) and inactive caspase 3 in the experimental groups (CN, control non-diabetic, n=4, EN, EPO-treated non-diabetic, n=4, CD, control diabetic, n=8; ED, EPO-treated diabetic, n=6); (b) densitometry values of active caspase 3, as per cent of total caspase 3 (the sum of the active and inactive bands). *P=0.05 vs control non-diabetic, **=P<0.01 vs control diabetic.
Discussion and conclusions
The data presented in this study demonstrate, for the first time, that EPO administered at the point of reoxygenation provides a direct and immediate protective effect on human myocardial tissue. Needless to say, human tissue samples are difficult to obtain and this imposed certain limitations in our study. These limitations refer mainly to using atrial instead of ventricular tissue and having a sample population of large variability in terms of age, gender and previous treatments. The improved recovery of contractility of the atrial trabeculae was observed in tissue samples, derived from both non-diabetic and diabetic patients. This cardioprotective action of EPO against hypoxia/reoxygenation injury in human muscle appears to be mediated by the reperfusion-injury salvage kinase pathway incorporating both PI3K/Akt and ERK1/2 and is also correlated with reduced caspase-3 activation. Inhibition of these pro-survival kinases using the PI3K inhibitor LY294002 or the ERK1/2 inhibitor, U0126 resulted in the loss of the beneficial effect of EPO. This study therefore, demonstrates the pivotal role that these pro-survival kinases play in protecting the human muscle from hypoxia/reoxygenation injury. These observations are in accordance with other studies that have also implicated the same pro-survival kinases in EPO-driven cardiac protection in other mammalian models, in vitro and or in vivo (Bullard et al., 2005; Hirata et al., 2005; Rafiee et al., 2005).
Activation of PI3K and its downstream target Akt, play an important role in modulating cardiomyocyte death in ischaemia–reperfusion injury. Studies by Tramontano et al. (2003) have demonstrated that activation of PI3K/Akt by EPO inhibits apoptosis in neonatal rat ventricular myocytes exposed to hypoxia. Activation of Akt results in the activation of both antiapoptotic factors (eNOS, p70S6K) and the inhibition of pro-apoptotic factors (BAD, BAX, BIM and caspases (Rafiee et al., 2005). EPO is known to inhibit apoptosis of erythroid progenitor stem cells in the bone marrow and it is also known to inhibit apoptosis in various tissue types such as cardiac (Tada et al., 2006) and neuronal tissue (Junk et al., 2002). Similarly, EPO can activate the mitogen-activated protein kinases such as ERK 1/2, which are involved in cell differentiation and survival leading to cellular protection in the setting of ischaemia–reperfusion injury (Rafiee et al., 2005). In addition to activating Akt and ERK, previously demonstrated in animal models and now in this human atrial muscle model, EPO is also capable of reducing cell death by inhibiting caspase activation (Tramontano et al., 2003; Tada et al., 2006). It could be argued that one limitation of our study related to the fact that we did not demonstrate a direct relation between these two beneficial effects of EPO treatment (that is Akt activation and less cleaved caspase 3). However, we propose that an increase in the cell survival reflected by these data will have a beneficial impact on the recovery of the atrial muscle contractility. Harnessing the antiapoptotic effects of EPO in cardiac tissue opens up the potential to salvage more cardiomyocytes following acute coronary syndromes resulting in an increased left ventricular mass and thus improving functional recovery.
One could consider that EPO is protective due to its primary function of increasing the haematocrit and therefore improving the oxygen delivery to the heart. This could also be considered the case in chronic administration, although this was not the aim of our investigation. Importantly however, studies using EPO given acutely (Junk et al., 2002; Cai and Semenza, 2004; Bullard et al., 2005) or using EPO analogues (Leist et al., 2004), which do not have erythropoietic properties, have eliminated improved oxygen delivery as a possible cause for the cardioprotection witnessed in ischaemia–reperfusion injury, as they did not alter the overall haematocrit level.
We also report in this study that human type II (non-insulin-dependent diabetes mellitus (NIDDM)) diabetic myocardium can also be protected from hypoxia/reoxygenation injury when EPO was administered at the point of reoxygenation, following hypoxia. However, although significant, the cardiac protection observed was less than that observed in the non-diabetic muscle. An explanation for this difference could be associated with the known cellular defects that occur in various cellular pathways such as PI3K/Akt (Steiler et al., 2003) in diabetes. Studies from our group (Tsang et al., 2005) have already shown that while the total amount of Akt is similar in both diabetic and non-diabetic animal myocardium, there is kinase downregulation in the diabetic setting. Moreover, the type II diabetic phenotype is characterized by increased levels of the protein SOCS (suppressor of cytokine signalling) (Ueki et al., 2004), which may negatively regulate the cells response to EPO (Jegalian and Wu, 2002). These differences could explain the blunted EPO response in the human diabetic myocardium. However, the exact mechanism underlying the attenuated response to EPO in diabetic myocardium has yet to be fully elucidated.
In conclusion, we report for the first time that EPO administered acutely, at the point of reoxygenation (after a prolonged hypoxic event) can protect human myocardial tissues in vitro. This protection is mediated by activation of the pro-survival kinases that constitute the RISK pathway, namely, PI3K/Akt and ERK1/2. Protection was also observed in the diabetic myocardium although the degree of protection observed was attenuated. There was a significant decrease in caspase-3 activation as a result of EPO treatment in atrial trabeculae from both non-diabetic as well as diabetic patients. These data therefore support the potential use of EPO as a novel cardioprotective strategy when used alone or as an adjunct in the clinical setting alongside existing reperfusion therapies such as percutaneous coronary intervention, thrombolysis and coronary artery bypass grafting.
Acknowledgments
We thank the British Heart Foundation for continuing support.
Abbreviations
- Akt
protein kinase B
- AMI
acute myocardial infarction
- BAD
Bcl-2-associated death protein
- BAX
Bcl-2-associated X protein
- BIM
Bcl-2-interacting mediator of cell death
- CABG
coronary artery bypass grafting
- DMSO
dimethyl sulphoxide
- EPO
erythropoietin
- ERK1/2
extracellular signal-regulated kinases
- LY294002
(2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one hydrochloride)
- NIDDM
non-insulin- dependent diabetes mellitus
- PCI
percutaneous coronary intervention
- PI3K
phosphatidylinositol-3 kinase
- U0126
1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene
Conflict of interest
The authors state no conflict of interest.
References
- Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword. J Clin Invest. 1985;76:1713–1719. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullard AJ, Govewalla P, Yellon DM. Erythropoietin protects the myocardium against reperfusion injury in vitro and in vivo. Basic Res Cardiol. 2005;100:397–403. doi: 10.1007/s00395-005-0537-4. [DOI] [PubMed] [Google Scholar]
- Cai Z, Manalo DJ, Wei G, Rodriguez ER, Fox-Talbot K, Lu H, et al. Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia–reperfusion injury. Circulation. 2003;108:79–85. doi: 10.1161/01.CIR.0000078635.89229.8A. [DOI] [PubMed] [Google Scholar]
- Cai Z, Semenza GL. Phosphatidylinositol-3-kinase signaling is required for erythropoietin-mediated acute protection against myocardial ischemia/reperfusion injury. Circulation. 2004;109:2050–2053. doi: 10.1161/01.CIR.0000127954.98131.23. [DOI] [PubMed] [Google Scholar]
- Calvillo L, Latini R, Kajstura J, Leri A, Anversa P, Ghezzi P, et al. Recombinant human erythropoietin protects the myocardium from ischemia–reperfusion injury and promotes beneficial remodeling. Proc Natl Acad Sci USA. 2003;100:4802–4806. doi: 10.1073/pnas.0630444100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeping R, Kawakami K, Ocker H, Wagner JM, Heringlade M, Noetzold A, et al. Expression of the erythropoietin receptor in human heart. J Thorac Cardiovasc Surg. 2005;130:877. doi: 10.1016/j.jtcvs.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Efthymiou CA, Mocanu MM, Yellon DM. Atorvastatin and myocardial reperfusion injury: new pleiotropic effect implicating multiple prosurvival signaling. J Cardiovasc Pharmacol. 2005;45:247–252. doi: 10.1097/01.fjc.0000154376.82445.06. [DOI] [PubMed] [Google Scholar]
- Hirata A, Minamino T, Asanuma H, Sanada S, Fujita M, Tsukamoto O, et al. Erythropoietin just before reperfusion reduces both lethal arrhythmias and infarct size via the phosphatidylinositol-3 kinase-dependent pathway in canine hearts. Cardiovasc Drugs Ther. 2005;19:33–40. doi: 10.1007/s10557-005-6895-1. [DOI] [PubMed] [Google Scholar]
- Jegalian AG, Wu H. Differential roles of SOCS family members in EpoR signal transduction. J Interferon Cytokine Res. 2002;22:853–860. doi: 10.1089/107999002760274863. [DOI] [PubMed] [Google Scholar]
- Junk AK, Mammis A, Savitz SI, Singh M, Roth S, Malhotra S, et al. Erythropoietin administration protects retinal neurons from acute ischemia–reperfusion injury. Proc Natl Acad Sci USA. 2002;99:10659–10664. doi: 10.1073/pnas.152321399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- Moon C, Krawczyk M, Ahn D, Ahmet I, Paik D, Lakatta EG, et al. Erythropoietin reduces myocardial infarction and left ventricular functional decline after coronary artery ligation in rats. Proc Natl Acad Sci USA. 2003;100:11612–11617. doi: 10.1073/pnas.1930406100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa CJ, Matsumoto A, Kim J, Riel RU, Pascal LS, Walton GB, et al. A novel protective effect of erythropoietin in the infarcted heart. J Clin Invest. 2003;112:999–1007. doi: 10.1172/JCI18200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiee P, Shi Y, Su J, Pritchard KA, Jr, Tweddell JS, et al. Erythropoietin protects the infant heart against ischemia–reperfusion injury by triggering multiple signaling pathways. Basic Res Cardiol. 2005;100:187–197. doi: 10.1007/s00395-004-0508-1. [DOI] [PubMed] [Google Scholar]
- Sadamoto Y, Igase K, Sakanaka M, Sato K, Otsuka H, Sakaki S, et al. Erythropoietin prevents place navigation disability and cortical infarction in rats with permanent occlusion of the middle cerebral artery. Biochem Biophys Res Commun. 1998;253:26–32. doi: 10.1006/bbrc.1998.9748. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Wen TC, Matsuda S, Masuda S, Morishita E, Nagao M, et al. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci USA. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiler T, Galuska D, Leng Y, Chilbalin A, Gilbert M, Zierath J. Effect of hyperglycemia on signal transduction in skeletal muscle from Goto-Kakizaki rats. Endocrinology. 2003;144:5259–5267. doi: 10.1210/en.2003-0447. [DOI] [PubMed] [Google Scholar]
- Tada H, Kagaya Y, Takeda M, Ohta J, Asaumi Y, Satoh K, et al. Endogenous erythropoietin system in non-hematopoietic lineage cells plays a protective role in myocardial ischemia/reperfusion. Cardiovasc Res. 2006;71:466–477. doi: 10.1016/j.cardiores.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Tramontano AF, Muniyappa R, Black AD, Blendea MC, Cohen I, Deng L, et al. Erythropoietin protects cardiac myocytes from hypoxia-induced apoptosis through an AKT-dependent pathway. Biochem Biophys Res Commun. 2003;308:990–994. doi: 10.1016/s0006-291x(03)01503-1. [DOI] [PubMed] [Google Scholar]
- Tsang A, Hausenloy DJ, Mocanu MM, Carr RD, Yellon DM. Preconditioning the diabetic heart: the importance of AKT phosphorylation. Diabetes. 2005;54:2360–2364. doi: 10.2337/diabetes.54.8.2360. [DOI] [PubMed] [Google Scholar]
- Ueki K, Kondo T, Kahn CR. Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol Cell Biol. 2004;24:5434–5446. doi: 10.1128/MCB.24.12.5434-5446.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Walker JM, Pugsley WB, Pattison CW, Yellon DM. Preconditioning in isolated superfused human muscle. J Mol Cell Cardiol. 1995;27:1349–1357. doi: 10.1016/s0022-2828(05)82397-1. [DOI] [PubMed] [Google Scholar]
- Wright GL, Hanlon P, Amin K, Steenbergen C, Murphy E, Arcasoy MO. Erythropoietin receptor expression in adult rat cardiomyocytes is associated with an acute cardioprotective effect for recombinant erythropoietin during ischemia–reperfusion injury. FASEB J. 2004;18:1031–1033. doi: 10.1096/fj.03-1289fje. [DOI] [PubMed] [Google Scholar]
- World Medical Association Declaration of Helsinki. Cardiovasc Res. 1997;35:2–3. [PubMed] [Google Scholar]