Abstract
Background and purpose:
Inflammation elevates plasma verapamil concentrations but diminishes pharmacological response. Angiotensin II is a pro-inflammatory mediator. We examined the effect of angiotensin II receptor blockade on the pharmacokinetics and pharmacodynamics of verapamil, as well as the binding properties and amounts of its target protein in calcium channels, in a rat model of inflammation.
Experimental approach:
We used 4 groups of male Sprague–Dawley rats (220–280 g): inflamed-placebo, inflamed-treated, control-placebo and control-treated. Inflammation as pre-adjuvant arthritis was induced by injecting Mycobacterium butyricum on day 0. From day 6 to 12, 30 mg kg−1 oral valsartan or placebo was administered twice daily. On day 12, a single oral dose of 25 mg kg−1 verapamil was administered and prolongation of the PR interval measured and plasma samples collected for verapamil and nor-verapamil analysis. The amounts of the target protein Cav1.2 subunit of L-type calcium channels in heart was measured by Western blotting and ligand binding with 3H-nitrendipine.
Key results:
Inflammation reduced effects of verapamil, although plasma drug concentrations were increased. This was associated with a reduction in ligand binding capacity and amount of the calcium channel target protein in heart extracts. Valsartan significantly reversed the down-regulating effect of inflammation on verapamil's effects on the PR interval, and the lower level of protein binding and the decreased target protein.
Conclusions and implications:
Reduced responses to calcium channel blockers in inflammatory conditions appeared to be due to a reduced amount of target protein that was reversed by the angiotensin II antagonist, valsartan.
Keywords: angiotensin II, inflammation, ion channels, pharmacokinetics
Introduction
Inflammation is a normal response to external and internal stimuli. It is associated with release of inflammatory mediators such as pro-inflammatory cytokines and nitric oxide. These biochemical changes are associated with altered pharmacokinetics and/or pharmacodynamics of some cardiovascular drugs, such as calcium channel blockers (Mayo et al., 2000; Sattari et al., 2003) and β-adrenoceptor antagonists (Kulmatycki et al., 2001; Guirguis and Jamali, 2003; Clements and Jamali, 2007). For example, the potency of verapamil is reduced in patients with active rheumatoid arthritis despite elevated drug plasma concentration (Mayo et al., 2000). Similar changes in verapamil action and disposition have been reported in ageing (Abernethy et al., 1993) and obesity (Abernethy and Schwartz, 1988); two conditions also associated with elevated pro-inflammatory mediators (Visser et al., 1999; Das, 2001; Sarkar and Fisher, 2006). Patients with arthritis, the elderly and obese are more prone to cardiovascular complications than the general population (Oates, 1988; Kannel, 2000). In addition, inflammation is involved in the aetiology of cardiac diseases, such as acute myocardial infarction (Erzen et al., 2006) and heart failure (Mann, 2002) and may contribute to poor therapeutic outcome. For example, elevated interleukin 6 in patients with unstable angina was linked to increased adverse coronary events (Biasucci et al., 1999).
The observed inflammation-induced reduction in response to verapamil, despite increased concentration, has been attributed to inflammation-induced reduction in binding to L-type calcium channels (Sattari et al., 2003). The reduced clearance of highly cleared drugs such as verapamil (Mayo et al., 2000; Sattari et al., 2003; Ling and Jamali, 2005) and propranolol, (Guirguis and Jamali, 2003) observed in response to inflammation is attributed to increased drug plasma protein binding (Mayo et al., 2000; Sattari et al., 2003; Ling and Jamali, 2005) and/or reduced expression of the enzymes responsible for drug metabolism (Ling and Jamali, 2005).
Inflammatory conditions do not reduce response to all cardiovascular drugs. The potency of angiotensin II receptor type I blockers, valsartan (Daneshtalab et al., 2004) and losartan, (Daneshtalab et al., 2006) is not reduced by rheumatoid arthritis. Indeed, for valsartan, a trend towards increased potency has been observed (Daneshtalab et al., 2004). Angiotensin II receptor type I blockers are known to have direct (Seeger et al., 2000) and indirect (Dagenais and Jamali, 2005) anti-inflammatory actions. The direct effect is likely through their free radical scavenging properties, derived from their phenolic moiety (Seeger et al., 2000), while the indirect action is through the inhibition of the pro-inflammatory effects of angiotensin II (Dagenais and Jamali, 2005).
Our hypotheses were (1) the diminished response to verapamil caused by inflammation is due to reduced calcium channel target protein and (2) the diminished potency of verapamil under inflammatory conditions is reversed by angiotensin II inhibition. We used the pre-adjuvant arthritis (pre-AA) model of inflammation (Ling and Jamali, 2005). This newly developed animal model allows studies under systemic inflammatory conditions in the absence of pain and stress associated with the fully developed adjuvant arthritis. Pre-AA is associated with elevated pro-inflammatory mediators' concentration and depressed drug-metabolizing enzymes (Ling and Jamali, 2005).
Methods
Experimental animals
All animal procedures and the study protocol were approved by the Health Sciences Animal Policy and Welfare Committee of the University of Alberta. A newly developed animal model of inflammation ‘pre-AA' was used in this study (Ling and Jamali, 2005). The experiments were carried out on male Sprague–Dawley rats (220–280 g). They were housed in a controlled temperature room with a 12:12 h dark/light cycle.
Pharmacodynamic study
Effect of pre-AA on response to verapamil
The pre-AA model exhibits the systemic signs of adjuvant arthritis without the manifestation of painful physical destruction of the disease (Ling and Jamali, 2005). To test whether the pharmacological effects of verapamil were altered by pre-AA, a brief experiment was conducted on two animal groups (n=5 per group), inflamed (pre-AA) and control (healthy). The inflamed group received 0.2 ml of 50 mg ml−1 Mycobacterium butyricum suspended in squalene into the tail base as intra-lymphatic injections. Control animals received an equal volume of normal saline into the tail base. On day 12, after baseline ECG measurement, each rat was dosed with 25 mg kg−1 verapamil solution p.o., ECG measurements were recorded at 0, 20, 40, 60, 80, 100, 120, 180 and 240 min post-dose and PR intervals were measured.
Effect of valsartan on response to verapamil
Animals (8–9 per group) were randomized to four groups: pre-AA treated with placebo (inflamed-placebo), pre-AA treated with valsartan (inflamed-treated), control healthy rats treated with placebo (control-placebo) and control healthy rats treated with valsartan (control-treated). Pre-AA (Ling and Jamali, 2005) was induced by injecting 0.2 ml of 50 mg ml−1 M. butyricum suspended in squalene into the tail base of the inflamed groups. Control animals received an equal volume of normal saline into the tail base. The day of its injection was marked as day 0. From day 6–12, rats in the treated groups received valsartan (30 mg kg−1) suspended in polyethylene glycol 400, via gastric gavage, every 12 h. The placebo groups received a comparable volume of the vehicle only.
On day 11, modified ECG leads were implanted subcutaneously over the xyphoid process and in the right and left axilla while the animals were under halothane/oxygen anaesthesia. On day 12, after baseline ECG measurement, each rat was dosed with 25 mg kg−1 p.o. of verapamil solution and ECG measurements were taken at 0, 20, 40, 60, 80, 100, 120, 180 and 240 min post-dosing and PR intervals were measured. The ECG device was Honeywell ECG amplifier (Honeywell Electronics for Medicine, Edmonton, Alberta, Canada). Data were recorded using Acknowledge Software (World Precision Instruments, Miami, FL, USA). Verapamil response was calculated as the area under the effect curve (AUEC) using the linear trapezoidal rule by taking the percent change of PR interval from baseline and multiplying by the time interval in minutes. AUECs are expressed as % min.
On day 12, blood samples were taken to analyse for inflammatory mediators.
Pharmacokinetic study
Another four groups of rats (5–6 per group), similar to those described under ‘Effect of valsartan on response to verapamil' section, were treated identically to the latter groups but without the implantation of ECG leads. Instead, on day 11, the right jugular vein was cannulated in all animals for serial blood collections on day 12, while animals were under halothane/oxygen anaesthesia. Briefly, a polyethylene cannula (Dow Corning Corp., Midland, MI, USA) tipped with 2 cm of Silastic tubing (Becton Dickinson, Sparks, MD, USA) was inserted into the right jugular vein and exteriorized by subcutaneous tunnelling to an incision made in the interscapular area. On day 12, 2 h following valsartan dose, a single oral dose of 25 mg kg−1 verapamil was administered orally to all groups. Plasma samples were collected at 0, 20, 40, 60, 120 and 240 min post-dosing for plasma verapamil and nor-verapamil analysis. Valsartan plasma concentrations were measured 2 h post-dose only in the valsartan-treated control and inflamed groups.
Stereospecific assay for verapamil and nor-verapamil
A previously reported assay (Shibukawa and Wainer, 1992) was used. Briefly, 100 μl of plasma sample or standard was added to 75 μl of the internal standard (+)-glaucine (400 ng ml−1), followed by 100 μl of 2 M sodium hydroxide and 0.4 ml of phosphate buffer (pH 7; ionic strength 0.1). Aliquots of 6 ml of heptane/heptafluorobutanol (99:1) were added and vortex mixed for 1 min. The samples were centrifuged at 2000 g for 10 min. The organic layers were transferred to clean tubes and evaporated to dryness. The residues were reconstituted in 200 μl of mobile phase (hexane/ethanol/isopropanol/triethylamine, 92:4:4:0.1 v/v) and injected (100 μl) into the HPLC at a flow rate of 0.7 ml min−1. Peaks were resolved using a Chiralpak AD-H column (Daicel Chemical Inc., Tokyo, Japan) and detected at excitation and emission wavelengths of 272 and 317 nm, respectively. Standard curves were linear over the range of 10–2000 ng ml−1 (coefficient of variation (CV) <10%). Since standard nor-verapamil was not available, plasma nor-verapamil is presented as peak area ratio. The area under the plasma drug or metabolite concentration curve was calculated using the log-linear trapezoidal method.
Valsartan assay
A previously reported assay was used to analyse plasma valsartan concentration (Daneshtalab et al., 2002). Briefly, 100 μl of plasma sample or standard was added to 100 μl of the internal standard losartan (5 μg ml−1), followed by acidification with 125 μl of 1 M phosphoric acid. Aliquots of 10 ml of methyl-tert-butyl ether were added and vortex mixed for 3 min. The samples were centrifuged at 2000 g for 5 min. The organic layers were transferred to clean tubes containing 200 μl of 0.05 M sodium hydroxide and again vortex mixed for 2 min and centrifuged at 2000 g for 5 min. The organic layer was discarded and the aqueous layer is acidified with 75 μl 0.2 M phosphoric acid. Aliquots of 125 μl of the aqueous layer were injected into the HPLC at a flow rate of 1.3 ml min−1. The mobile phase was 70% phosphate buffer pH 2.8 and 30% acetonitrile. Peaks were resolved using a C18 analytical column (Phenomenex, Torrance, Mississauga, Ontario, Canada) attached to a NovaPak C8 Guard-Pak HPLC Pre-column insert (Waters, Millipore, Mississauga, Ontario, Canada) and detected at excitation and emission wavelengths of 265 and 378 nm, respectively. Standard curves were linear over the range of 0.5–5 μg ml−1 (CV <12%).
Serum nitrite analysis
Nitric oxide (NO) concentration was indirectly measured through concentrations of serum nitrite and nitrate, its stable breakdown product, using a previously reported method (Grisham et al., 1996). Briefly, nitrate was reduced to nitrite by incubating 100 μl sample or standard with 10 μl of Aspergillus nitrate reductase (10 U ml−1), 25 μl of 0.1 mM FAD, 50 μl 1 mM NADPH, 25 μl of 1 M HEPES (pH 7.4) and 290 μl of deionized water for 30 min at 37 °C. This was followed by adding 5 μl of lactate dehydrogenase (1500 U ml−1) and 50 μl of 100 mM pyruvic acid and re-incubation for another 10 min at 37 °C. Subsequently, 1 ml of Griess reagent (0.2% naphthalene ethylene diamine and 2% sulphanilamide in 5% phosphoric acid) was added and incubated for 10 min at room temperature. The absorbance of the developed colour was measured at 543 nm using a Vmax Molecular Devices plate reader (Bio-Tek Instruments Inc., Winooski, VT, USA). Standard curves were linear over the range of 6.25–200 μM (CV <20%).
Determination of C-reactive protein
Serum C-reactive protein was analysed using a commercially available rat C-reactive protein ELISA kit (BD Biosciences, Mississauga, Ontario, Canada). Briefly, 100 μl of diluted samples and standard sera was incubated with antibody-coated 96-well microplate for 30 min. After washing, 100 μl of the detection antibody was added to each well and incubated for another 30 min. The plate was again washed and 100 μl of 3,3′,5,5′-tetramethylbenzidine was added and incubated for 10 min. The absorbance was read at 450 nm using the same plate reader used for serum nitrite analysis. Standard curves were linear over the range of 8.75–133μg ml−1 (CV <16%).
Heart membrane preparation
Heart membrane preparations were obtained using a previously used method (Sattari et al., 2003) after minor modifications. Another four groups of rats (four per group), similar to those described under ‘Effect of valsartan on response to verapamil' section, were used but without implanting the ECG leads. Instead, on day 12, rats were anaesthetized with halothane/oxygen, the thoracic cavity of the rats was opened, blood was removed by cardiac puncture and the heart was excised and placed in ice-cold Tris buffer (0.05 M; pH 7.4). Hearts were then weighed and cut into small pieces and put into an ice-cold Tris buffer/protease inhibitor cocktail (19:1) mixture (10 ml g−1 wet tissue), followed by homogenization in a tube immersed in ice using Brinkmann Homogenizer (Kinematica AG, Littau-Lucerne, Switzerland) for 30 s. The crude homogenate was then centrifuged at 5000 g at 4 °C for 10 min to disrupt nuclei and cytoskeleton particles. Aliquots of 100 μl were taken from the supernatant for western blotting and stored at −80 °C. The remainder was centrifuged at 100 000 g at 4 °C for 1 h. The resultant pellet was re-suspended in 10 ml Tris buffer (pH 7.4) and aliquots were stored at −80 °C for the radioligand-binding study.
The amount of protein in samples was determined using the method of Lowry et al. (1951) using a commercially available protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Briefly, a 250 μl of 10% Folin reagent was added to a mixture of 5 μl sample and 25 μl of alkaline copper tartarate solution. The colour developed was measured by photometry at 570 nm using a Vmax Molecular Devices plate reader (Bio-Tek Instruments Inc.). Standard curves were linear over the concentration range between 0.2 and 1.44 mg ml−1 (CV<10%).
Equilibrium radioligand-binding study
A previously reported method (Sattari et al., 2003) was used following minor modifications. 3H-Nitrendipine was used at serial dilutions of 0, 0.025, 0.05, 0.1, 0.2, 0.3, 0.4 and 0.8 nM in a final volume of 500 μl in Tris buffer (pH 7.4; 0.05 M). The reaction was initiated by adding 100 μg protein of the rat heart membrane preparation (four per group) in the presence and absence of an excess cold ligand (4 × 10−5 M nifedipine) for nonspecific and total binding, respectively and then incubated for 90 min. The reaction was terminated by rapid filtration through Whatman Gf/B filters that were soaked previously in Tris buffer. Filters were then immersed in plastic tubes containing 4 ml CytoScint liquid scintillation cocktail (ICN Biochemicals, Costa Mesa, CA, USA), followed by shaking for 30 s. Beckman LS 6500 multi-purpose liquid scintillation counter (Fullerton, CA, USA) was used to measure the radioactivity retained in each filter. The specific binding was determined by subtraction of the nonspecific binding from the total binding. The data analysis, nonlinear curve fitting, determination of the equilibrium dissociation constant (K) and the maximum number of binding sites (Bmax) were carried out using Prism software (GraphPad Software Inc., San Diego, CA, USA).
Western blot analysis
Western immunoblot was used to determine the protein concentration of the Cav1.2 subunit of L-type calcium channels in the heart. The cardiac proteins were denatured by boiling at 100 °C for 5 min, followed by SDS-gel electrophoretic separation of 50 μg aliquots on 7.5% acrylamide for 1 h at 200 V. The resultant separation was transferred to a nitrocellulose membrane. The membrane was incubated overnight in a blocking solution (2% bovine serum albumin, 5% skim milk and 0.05% Tween 20 in Tris-buffered saline) to block the nonspecific binding. Nitrocellulose membranes were incubated with the primary antibody (1:400 dilution of polyclonal rabbit anti-calcium channel (Cav1.2 subunit) (Sigma-Aldrich, St Louis, MO, USA) or 1:1000 dilution of polyclonal rabbit anti-β actin (Abcam Inc., Cambridge, MA, USA) with shaking for 2 h, followed by the secondary antibody (1:7500 dilution of horseradish peroxidase-conjugated goat anti-rabbit IgG antibody; Bio-Rad Laboratories) for 1 h. The resultant interaction was detected by chemiluminescence (ECL Western Blotting Detection reagents; Bio-Rad Laboratories) and the band's density was measured using Image J software (National Institute of Health, Bethesda, MD, USA).
Data analysis
Data are expressed as mean±s.e.m. Statistical significance between the control and inflamed groups in the two-arm study was analysed using a two-tailed t-test. Differences among the four study groups in AUEC, maximum plasma concentration (Cmax), area under the concentration–time curve from 0 to 4 h (AUC0–4 h), Bmax and K were assessed using one-way ANOVA, followed by Bonferroni post-test. The time-course effect data were analysed using two-way ANOVA, followed by Bonferroni post-test. A P-value of less than 0.05 was considered statistically significant.
Materials
Racemic verapamil hydrochloride, the internal standard (+)-glaucine, heptafluorobutanol, Aspergillus nitrate reductase (10 U ml−1), FAD, NADPH, lactic dehydrogenase (1500 U ml−1), Tris base, Tris hydrochloride, protease inhibitor cocktail and pyruvic acid were purchased from Sigma-Aldrich. HPLC-grade hexane and HPLC-grade isopropanol, 98% ethanol, heptane and triethylamine were purchased from Caledon Laboratories (Georgetown, Ontario, Canada). Squalene was purchased from Kodak (Rochester, NY, USA). Polyethylene glycol 400 was purchased from Wiler (London, Ontario, Canada). Heat-killed dried M. butyricum was purchased from Difco (Detroit, MI, USA). Valsartan was supplied by Novartis Pharma (Basel, Switzerland). Losartan, a gift from Merck Research Laboratories (Rahway, NJ, USA), was used as the internal standard.
Results
Verapamil pharmacodynamics
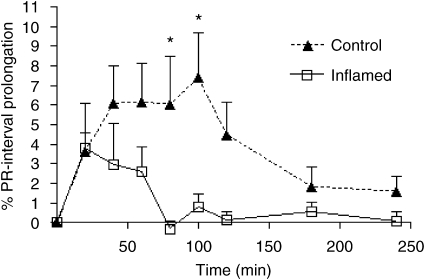
Pre-AA resulted in a significant reduction in the prolongation of the PR interval by verapamil (Figure 1). There was also a significant, 74% reduction in the corresponding AUEC. This observation was made 12 days after the injection of M. butyricum and before emergence of physical signs of adjuvant arthritis. The time-course effect data generated from the four-arm study (Figure 2) were generally lower in the inflamed-placebo group as compared with the control-placebo rats. However, only the 60-min measurements were significantly different between the two groups. The difference in the mean AUEC values was not significant between the latter groups (Figure 2b).
Figure 1.
Verapamil-induced PR interval prolongation in normal and inflamed rats (n=5 per group) following a single oral 25 mg kg−1dose of verapamil. The area under the effect curve (AUEC) in normal animals was significantly higher than in inflamed rats. *P<0.05 vs corresponding values for inflamed rats.
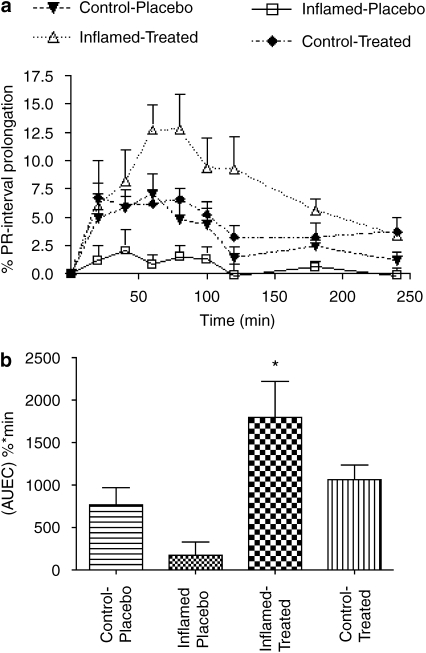
Figure 2.
In (a), the effect of 6 days of valsartan treatment on verapamil-induced PR interval prolongation in normal and inflamed rats (n=8–9 per group) following a single oral 25 mg kg−1 dose of verapamil. Inflamed-placebo vs control-placebo (P<0.05 at 60 min); inflamed-placebo vs inflamed-treated (P<0.05 at 40, 60, 80, 100, 120 min). In (b), the area under the effect curve (AUEC) values derived from the data in panel a are shown. *P<0.05 vs inflamed-placebo.
Six days of treatment with valsartan did not influence the response to verapamil in control rats. However, inflamed rats that were treated with valsartan responded to verapamil to a significantly greater extent than the untreated animals (Figure 2). Significantly greater verapamil potency and greater AUEC values (4- to 10-fold) were observed during 40–120 min post-dose in the inflamed-treated rats as compared with the inflamed-placebo group (Figure 2).
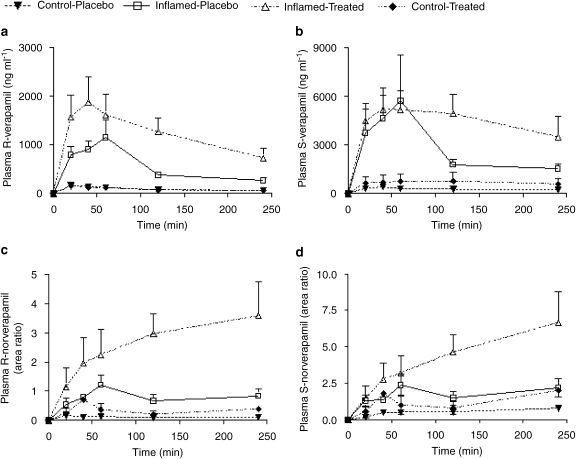
Verapamil and nor-verapamil pharmacokinetics
Table 1 depicts pharmacokinetic indices for both verapamil and nor-verapamil enantiomers in all groups. Plasma verapamil enantiomer concentrations were significantly elevated in inflamed groups as compared to the control groups (Figures 3a and b). Both the area under the curve (AUC0–4 h) and Cmax for the enantiomers in inflamed animals were significantly higher by many magnitudes (Table 1). Valsartan treatment had no significant effect on the pharmacokinetics of S-verapamil in the presence or absence of inflammation. However, it significantly raised the area under the curve of R-verapamil in inflamed animals.
Table 1.
Effect of inflammation and valsartan on the pharmacokinetics of verapamil and on the level of inflammatory markers
| Group | Control-placebo | Inflamed-placebo | Inflamed-treated | Control-treated |
|---|---|---|---|---|
| Cmax (μg ml−1) | ||||
| S-verapamil | 0.45±0.1a | 5.8±2.7b | 5.7±0.9b | 0.74±0.5a |
| R-verapamil | 0.2±0.1a | 1.2±0.3b | 2.0±0.5b | 0.13±0.1a |
| AUC0–4 h (μg min ml−1) | ||||
| S-verapamil | 65±14a | 616±183b,c | 1039±266b | 270±212a,c |
| R-verapamil | 21±7a | 124±19b | 283±73c | 33±21a,b |
| AUC0–4 h (area ratio min−1) | ||||
| S-nor-verapamil | 149±12a | 431±80a | 1029±289b | 243±157a |
| R-nor-verapamil | 20±4.5a | 190±34a | 633±171b | 67±43a |
| Valsartan plasma concentration 2 h post-dosing (μg ml−1) | ND | ND | 2.9±1.3a | 1.5±0.26a |
| Nitrite (μm) | 31.6±2.9a | 138±17b | 145±22b | 29.4±2.1a |
| CRP (μg ml−1) | 307±27a | 406±62a | 344±50a | 238±25a |
Abbreviations: AUC0–4 h, area under the concentration–time curve from 0 to 4 h; Cmax, maximum plasma concentration; ND, not determined.
Control and inflamed animals were given 6 days of treatment with 30 mg kg−1 of valsartan or placebo. Then a single oral dose of 25 mg kg−1 racemic verapamil was given and the variables shown were measured. The inflammatory markers (nitrite and C-reactive protein; CRP) were measured in serum. Values are shown as mean (±s.e.m.). Different superscript letters denote significant differences between means in a row (P<0.05); similar superscript letters indicate lack of significant differences between means.
Figure 3.
Effect of 6 days of valsartan treatment on plasma concentration–time profile of both verapamil and nor-verapamil enantiomers in normal and inflamed rats (n=5–6 per group) following a single oral 25 mg kg−1 dose of verapamil.
Valsartan treatment was associated with a significant rise in the plasma concentration of both nor-verapamil enantiomers in inflamed animals. The plasma concentrations in treated animals were approximately three times higher than those in both inflamed-placebo and control groups (Figures 3c and d).
Valsartan plasma concentrations
As summarized in Table 1, the concentration of valsartan in plasma taken 2 h post-dosing did not significantly differ between the control and the inflamed groups.
Inflammatory markers
Results for the assays of two inflammatory markers are given in Table 1. Serum nitrite concentration, which was significantly elevated by inflammation, was not normalized by valsartan. Neither inflammation nor valsartan significantly affected C-reactive protein concentrations.
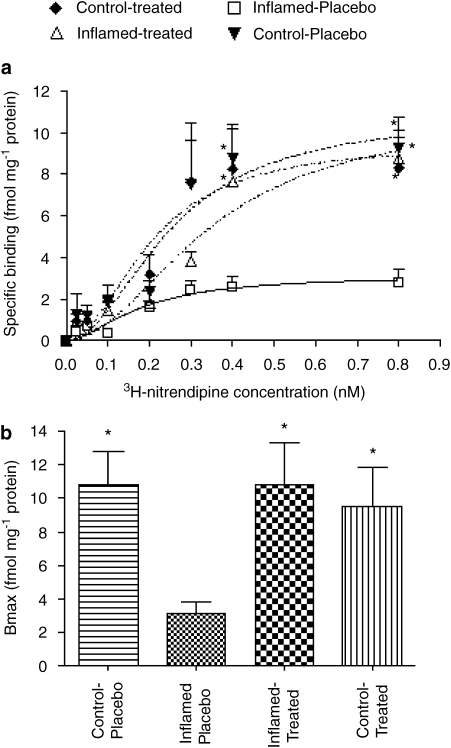
3H-Nitrendipine binding to cardiac L-type calcium channels
As illustrated in Figure 4, inflammation caused a significant reduction in the binding capacity of 3H-nitrendipine to L-type calcium channels in the rat heart (Bmax in inflamed-placebo and control-placebo groups were 3.1±0.7 and 10.8±2.0 fmol mg−1 protein, respectively). On the other hand, there were no significant changes in the equilibrium dissociation constant KD (control-placebo 0.25±0.07 vs inflamed-placebo 0.18±0.06 nM) or Hill coefficient nH (control-placebo 2.0±0.8 vs inflamed placebo 1.7±0.8). Valsartan treatment caused a significant increase in 3H-nitrendipine binding, as shown by the returning of Bmax back to normal (10.8±2.5 fmol mg−1 protein) without affecting KD (0.35±0.1 nM) and nH (2±0.7). Normal rats treated with valsartan did not show any further increase in Bmax (9.5±2.3 fmol mg−1 protein).
Figure 4.
Effect of 6 days of valsartan treatment on 3H-nitrendipine binding to L-type calcium channels in the rat cardiac cell membrane preparations (n=4 per group). (a) Binding obtained at increasing ligand concentrations. (b) Mean Bmax in the four groups. *P<0.001 vs inflamed-placebo.
Western blots of Cav1.2 subunit of cardiac L-type calcium channels
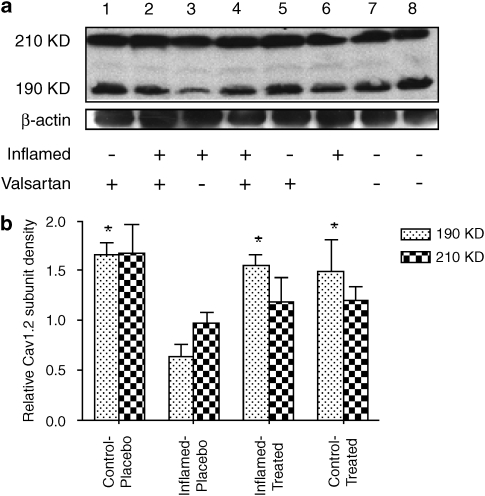
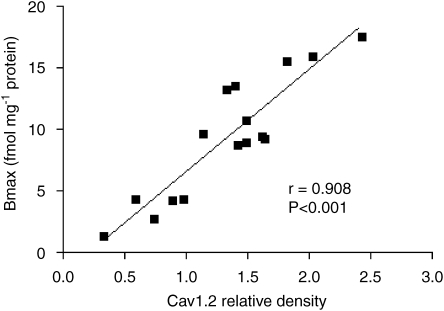
The photometric density of the blots (representing the amount of protein) for the low molecular weight Cav1.2 subunit (190 kDa) was significantly reduced in inflamed animals by 48% as compared with control rats (Figure 5). The high molecular weight subunit (210 kDa) was reduced by 41% but the reduction was not significant. Valsartan treatment caused a significant correction of the blot density of the low molecular weight Cav1.2 subunit back to normal. Moreover, there was a significant direct correlation between the amount of the low molecular weight subunit and the observed Bmax (Figure 6).
Figure 5.
Effect of 6 days of valsartan treatment on cardiac Cav1.2 subunit density in the rat hearts (n=4 per group). In (a), western blots of the Cav1.2 subunit in the rat heart. In (b), mean photometric densities of the low (190 kDa) and high (210 kDa) molecular weight forms in the four groups. *P<0.05 vs inflamed-placebo.
Figure 6.
Correlation between the amount of low molecular weight Cav1.2 subunit (as photometric density of western blot) and the observed maximum binding (Bmax) for the same cardiac cell membrane preparation.
Discussion
Our present data (Figures 1 and 3; Table 1) confirm previous reports that inflammatory conditions alter the pharmacokinetics and pharmacodynamics of verapamil in both humans (Abernethy and Schwartz, 1988; Abernethy et al., 1993; Mayo et al., 2000) and rats (Laethem et al., 1994; Mayo et al., 2000; Sattari et al., 2003; Ling and Jamali, 2005), most likely due to a downregulation of cytochrome P450 and/or increased plasma protein binding (Ling and Jamali, 2005). Despite its elevated plasma concentration, the verapamil-induced prolongation of PR interval was significantly reduced in inflamed rats compared to control (Figure 1). This has been attributed to reduced binding to L-type calcium channels (Sattari et al., 2003). Here for the first time, we present data suggestive of reduced amount of target protein (Figure 5) as an explanation for the reduced binding (Sattari et al., 2003) and hence reduced potency (Mayo et al., 2000; Sattari et al., 2003).
The effect of inflammation on the pharmacokinetics and pharmacodynamics is associated with significant rise in pro-inflammatory mediators (Kulmatycki et al., 2001; Sattari et al., 2003; Ling and Jamali, 2005). Both verapamil clearance and response are reduced in human arthritis (Mayo et al., 2000), ageing (Abernethy et al., 1993) and obesity (Abernethy and Schwartz, 1988); three conditions associated with elevated concentrations of pro-inflammatory mediators. Angiotensin II is a powerful pro-inflammatory mediator (Dagenais and Jamali, 2005). It stimulates the production of reactive oxygen species, such as hydrogen peroxide and superoxides (Rajagopalan et al., 1996), and consequently, activates the intracellular transcription factor, nuclear factor kappa B (Kranzhofer et al., 1999). The latter, in turn, is translocated to the nucleus to act on specific response elements on DNA modulating the expression of some proteins as pro-inflammatory mediators (Christman et al., 1998). L-type calcium channels appear to be regulated by this mechanism as tumour necrosis factor α-induced nuclear factor kappa B activation causes their downregulation (Shi et al., 2005). Accordingly, treatment with valsartan, an angiotensin II receptor antagonist, reversed the downregulating effect of inflammation on verapamil response (Figure 2). This coincided with normalization of the binding capacity (Figure 4) and density (Figure 5) of the low molecular weight Cav1.2 subunit of the target protein. We do not know the exact mechanism of reversal of the effect of inflammation by valsartan. It is known, however, that angiotensin II receptor antagonists have anti-inflammatory effects (Dagenais and Jamali, 2005).
We have previously reported that NO is a reliable marker of inflammation in both humans (Mayo et al., 2000) and rats (Sattari et al., 2003) and we therefore used nitrite, a stable metabolite of NO, as one of the measures of inflammation. Previously, we have reported that the serum nitrite is significantly elevated in inflammation in parallel with serum tumour necrosis factor α concentrations (Ling and Jamali, 2005). Interestingly, the valsartan treatment of inflamed rats did not normalize the observed elevated nitrite concentration (Table 1); that is, the improved pharmacodynamic response to verapamil in inflamed animals was independent of serum NO. This may suggest that the angiotensin II blockade may exert its normalizing effect through increased expression of anti-inflammatory mediators, rather than through inhibition of the pro-inflammatory mediators.
C-reactive protein is also a valuable marker of inflammation in humans (Bassuk et al., 2004). In the rat, however, serum concentrations of C-reactive protein are much higher (Ling and Jamali, 2005; Clements and Jamali, 2007) than in humans (Rifai et al., 1999). Indeed, the concentration observed in healthy rats would be considered toxic in humans. It is, therefore, unclear whether, with regard to this protein, the observations made in the rat can be extrapolated to humans. Nevertheless, the present data (Table 1) indicate an increasing trend, although not statistically significant, in serum concentration of the protein in the inflamed rats that was reduced as a result of valsartan treatment.
The observed altered binding of the calcium channel blocker 3H-nitrendipine to the target protein was only associated with altered Bmax that was normalized by valsartan as the binding affinity (KD) was not significantly altered. Thus, the changes in the binding may be explained by a non-competitive inhibition and/or a change in the amounts of the target protein. The former can be ruled out since both reduced binding caused by inflammation and reversal of the downregulating effect by valsartan were highly correlated with changes in the amounts of the low molecular weight Cav1.2 subunit protein (Figure 6).
Equilibrium radioligand-binding studies were performed using 3H-nitrendipine because it is a specific L-type calcium channel ligand and its electrophysiological effects have been found to be parallel to verapamil (McBride et al., 1984; Green et al., 1985).
Western blots of the functional Cav1.2 subunit of cardiac L-type calcium channels have been reported to exhibit two bands representing a low (190 kDa) and high (210 kDa) molecular weight form (De Jongh et al., 1991) and each band is associated with a different physiological function (De Jongh et al., 1989, 1990, 1991). Our data revealed a positive and significant correlation between the calculated Bmax values and the low but not the high molecular weight band (Figure 6). The parallel changes in the electrophysiological response to verapamil (Figures 1 and 2), the binding to the target protein (Figure 4) and the density of blots for the 190 kDa band (Figures 5 and 6) observed in our study, suggest that the low molecular weight subunit may be more relevant in the context of the present work, that is, the mechanism of involvement of inflammation and valsartan in altering pharmacological response to verapamil.
Six days of treatment with valsartan was sufficient to elevate the reduced amounts of channel protein. Indeed, there is evidence that L-type calcium channel expression takes place very rapidly even within 2 h (Maki et al., 1996). Moreover, other in vitro studies have observed the reduced expression of the functional Cav1.2 subunit in hyperthyroidism (Watanabe et al., 2005), heart failure (Bodi et al., 2005) and atrial fibrillation (Brundel et al., 2001; Bosch et al., 2003), indicating that it is a highly regulated protein.
The downregulating effect of inflammation does not only reduce the amount of L-type target proteins (Figure 5) but it also decreases the enzymes responsible for clearance of verapamil (Ling and Jamali, 2005). Despite reversal of the pharmacodynamic effect of verapamil, valsartan did not reverse the rise in verapamil plasma concentration back to the range noted in normal rats (Figure 3). This may indicate a different mechanism for the decreased enzyme, as compared to either that for the L-type calcium channel target protein, and/or the need for a longer duration and larger doses of valsartan. Another plausible explanation is that valsartan may have its own inhibitory effect on the clearance of verapamil, which may hamper the return to normal caused by the anti-inflammatory effect of the drug. Indeed, in the inflamed rats, treatment with valsartan resulted in elevation of plasma verapamil enantiomer concentrations, which was only significant for the R enantiomer (Table 1; Figure 3a). Nor-verapamil enantiomer concentrations were also significantly increased in inflamed rats (Table 1; Figures 3b, and c). Interestingly, however, no verapamil–valsartan pharmacokinetic interaction was noted in control rats. This may be explained by the abundance of the metabolizing enzyme in the healthy animals as compared to the inflamed rats. Valsartan binds to the cytochrome CYP2C enzyme family that O-demethylate verapamil and nor-verapamil (Busse et al., 1995; Tracy et al., 1999) but only with a moderate affinity (Taavitsainen et al., 2000; Nakashima et al., 2005), In healthy rats, therefore, the inhibitory effect of valsartan on the cytochrome P2C enzymes may be negligible. This effect may become pronounced in inflamed animals as the drug-metabolizing enzymes are relatively decreased.
R-verapamil and nor-verapamil enantiomers have little calcium channel-blocking effect (Echizen et al., 1985) and thus their contribution to the observed enhanced verapamil potency in valsartan treated-inflamed rats is unlikely to be important.
Valsartan plasma concentration 2 h post-dosing was not altered by inflammation, which is in close agreement with that observed in humans (Daneshtalab et al., 2004).
The significance of the downregulating effect of inflammation on verapamil response reported earlier (Sattari et al., 2003) was more evident in our two-arm (Figure 1) than the four-arm study (Figure 2) despite the greater number of animals used in the latter experiment. This can be explained by the inherently lower statistical power ascribed to multi-mean comparison approaches (Steel et al., 1997). Indeed, when the inflamed-placebo rats were compared with the inflamed-treated ones in the absence of the other groups (Figure 2), the observed difference in the AUEC became significant.
The present data demonstrate, for the first time, the effects of the angiotensin II receptor type I antagonism on the pharmacodynamic profile of calcium channel blockers in an inflammatory model. Six days of valsartan treatment reversed the inflammation-induced reduction of verapamil-induced PR interval prolongation in pre-AA rats. This beneficial interaction of valsartan with calcium channel blockers, if demonstrable in humans, may be of value in cardiac patients with existing inflammatory diseases such as rheumatoid arthritis. Moreover, angiotensin II antagonists may increase the L-type calcium channel expression in conditions that are associated with reduced calcium channel expression.
Acknowledgments
SH is a recipient of Government of Egypt Doctoral Scholarship. This work was, in part, supported by a grant from the Canadian Institute of Heath Research.
Abbreviations
- AUEC
area under the effect curve
- pre-AA
pre-adjuvant arthritis
Conflict of interest
The authors state no conflict of interest.
References
- Abernethy DR, Schwartz JB. Verapamil pharmacodynamics and disposition in obese hypertensive patients. J Cardiovasc Pharmacol. 1988;11:209–215. [PubMed] [Google Scholar]
- Abernethy DR, Wainer IW, Longstreth JA, Andrawis NS. Stereoselective verapamil disposition and dynamics in aging during racemic verapamil administration. J Pharmacol Exp Ther. 1993;266:904–911. [PubMed] [Google Scholar]
- Bassuk SS, Rifai N, Ridker PM. High-sensitivity C-reactive protein: clinical importance. Curr Probl Cardiol. 2004;29:439–493. [PubMed] [Google Scholar]
- Biasucci LM, Liuzzo G, Fantuzzi G, Caligiuri G, Rebuzzi AG, Ginnetti F, et al. Increasing levels of interleukin (IL)-1Ra and IL-6 during the first 2 days of hospitalization in unstable angina are associated with increased risk of in-hospital coronary events. Circulation. 1999;99:2079–2084. doi: 10.1161/01.cir.99.16.2079. [DOI] [PubMed] [Google Scholar]
- Bodi I, Mikala G, Koch SE, Akhter SA, Schwartz A. The L-type calcium channel in the heart: the beat goes on. J Clin Invest. 2005;115:3306–3317. doi: 10.1172/JCI27167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch RF, Scherer CR, Rub N, Wohrl S, Steinmeyer K, Haase H, et al. Molecular mechanisms of early electrical remodeling: transcriptional downregulation of ion channel subunits reduces I(Ca,L) and I(to) in rapid atrial pacing in rabbits. J Am Coll Cardiol. 2003;41:858–869. doi: 10.1016/s0735-1097(02)02922-4. [DOI] [PubMed] [Google Scholar]
- Brundel BJ, Van Gelder IC, Henning RH, Tieleman RG, Tuinenburg AE, Wietses M, et al. Ion channel remodeling is related to intraoperative atrial effective refractory periods in patients with paroxysmal and persistent atrial fibrillation. Circulation. 2001;103:684–690. doi: 10.1161/01.cir.103.5.684. [DOI] [PubMed] [Google Scholar]
- Busse D, Cosme J, Beaune P, Kroemer HK, Eichelbaum M. Cytochromes of the P450 2C subfamily are the major enzymes involved in the O-demethylation of verapamil in humans. Naunyn Schmiedebergs Arch Pharmacol. 1995;353:116–121. doi: 10.1007/BF00168924. [DOI] [PubMed] [Google Scholar]
- Christman JW, Lancaster LH, Blackwell TS. Nuclear factor kappa B: a pivotal role in the systemic inflammatory response syndrome and new target for therapy. Intensive Care Med. 1998;24:1131–1138. doi: 10.1007/s001340050735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JD, Jamali F. Pravastatin reverses the down-regulating effect of inflammation on beta-adrenergic receptors: a disease–drug interaction between inflammation, pravastatin, and propranolol. Vascul Pharmacol. 2007;46:52–59. doi: 10.1016/j.vph.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Dagenais NJ, Jamali F. Protective effects of angiotensin II interruption: evidence for antiinflammatory actions. Pharmacotherapy. 2005;25:1213–1229. doi: 10.1592/phco.2005.25.9.1213. [DOI] [PubMed] [Google Scholar]
- Daneshtalab N, Lewanczuk RZ, Jamali F. High-performance liquid chromatographic analysis of angiotensin II receptor antagonist valsartan using a liquid extraction method. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;766:345–349. doi: 10.1016/s0378-4347(01)00507-2. [DOI] [PubMed] [Google Scholar]
- Daneshtalab N, Lewanczuk RZ, Russell A, Jamali F. Rheumatoid arthritis does not reduce the pharmacodynamic response to valsartan. J Clin Pharmacol. 2004;44:245–252. doi: 10.1177/0091270003262951. [DOI] [PubMed] [Google Scholar]
- Daneshtalab N, Lewanczuk RZ, Russell AS, Jamali F. Drug–disease interactions: losartan effect is not downregulated by rheumatoid arthritis. J Clin Pharmacol. 2006;46:1344–1355. doi: 10.1177/0091270006292163. [DOI] [PubMed] [Google Scholar]
- Das UN. Is obesity an inflammatory condition. Nutrition. 2001;17:953–966. doi: 10.1016/s0899-9007(01)00672-4. [DOI] [PubMed] [Google Scholar]
- De Jongh KS, Merrick DK, Catterall WA. Subunits of purified calcium channels: a 212-kDa form of alpha 1 and partial amino acid sequence of a phosphorylation site of an independent beta subunit. Proc Natl Acad Sci USA. 1989;86:8585–8589. doi: 10.1073/pnas.86.21.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jongh KS, Warner C, Catterall WA. Subunits of purified calcium channels. Alpha 2 and delta are encoded by the same gene. J Biol Chem. 1990;265:14738–14741. [PubMed] [Google Scholar]
- De Jongh KS, Warner C, Colvin AA, Catterall WA. Characterization of the two size forms of the alpha 1 subunit of skeletal muscle L-type calcium channels. Proc Natl Acad Sci USA. 1991;88:10778–10782. doi: 10.1073/pnas.88.23.10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echizen H, Vogelgesang B, Eichelbaum M. Effects of d,l-verapamil on atrioventricular conduction in relation to its stereoselective first-pass metabolism. Clin Pharmacol Ther. 1985;38:71–76. doi: 10.1038/clpt.1985.137. [DOI] [PubMed] [Google Scholar]
- Erzen B, Sabovic M, Poredos P, Sebestjen M, Keber I, Simcic S. Inflammation markers in young post-myocardial patients exhibiting various expressions of classic coronary risk factors. Coron Artery Dis. 2006;17:325–330. doi: 10.1097/00019501-200606000-00001. [DOI] [PubMed] [Google Scholar]
- Green FJ, Farmer BB, Wiseman GL, Jose MJ, Watanabe AM. Effect of membrane depolarization on binding of nitrendipine to rat cardiac myocytes. Circ Res. 1985;56:576–585. doi: 10.1161/01.res.56.4.576. [DOI] [PubMed] [Google Scholar]
- Grisham MB, Johnson GG, Lancaster JR., Jr Quantitation of nitrate and nitrite in extracellular fluids. Methods Enzymol. 1996;268:237–246. doi: 10.1016/s0076-6879(96)68026-4. [DOI] [PubMed] [Google Scholar]
- Guirguis MS, Jamali F. Disease–drug interaction: reduced response to propranolol despite increased concentration in the rat with inflammation. J Pharm Sci. 2003;92:1077–1084. doi: 10.1002/jps.10381. [DOI] [PubMed] [Google Scholar]
- Kannel WB. Risk stratification in hypertension: new insights from the Framingham Study. Am J Hypertens. 2000;13:3S–10S. doi: 10.1016/s0895-7061(99)00252-6. [DOI] [PubMed] [Google Scholar]
- Kranzhofer R, Browatzki M, Schmidt J, Kubler W. Angiotensin II activates the proinflammatory transcription factor nuclear factor-kappaB in human monocytes. Biochem Biophys Res Commun. 1999;257:826–828. doi: 10.1006/bbrc.1999.0543. [DOI] [PubMed] [Google Scholar]
- Kulmatycki KM, Abouchehade K, Sattari S, Jamali F. Drug–disease interactions: reduced beta-adrenergic and potassium channel antagonist activities of sotalol in the presence of acute and chronic inflammatory conditions in the rat. Br J Pharmacol. 2001;133:286–294. doi: 10.1038/sj.bjp.0704067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laethem ME, Belpaire FM, Wijnant P, Rosseel MT, Bogaert MG. Influence of endotoxin on the stereoselective pharmacokinetics of oxprenolol, propranolol, and verapamil in the rat. Chirality. 1994;6:405–410. doi: 10.1002/chir.530060508. [DOI] [PubMed] [Google Scholar]
- Ling S, Jamali F. Effect of early phase adjuvant arthritis on hepatic P450 enzymes and pharmacokinetics of verapamil: an alternative approach to the use of an animal model of inflammation for pharmacokinetic studies. Drug Metab Dispos. 2005;33:579–586. doi: 10.1124/dmd.104.002360. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Maki T, Gruver EJ, Davidoff AJ, Izzo N, Toupin D, Colucci W, et al. Regulation of calcium channel expression in neonatal myocytes by catecholamines. J Clin Invest. 1996;97:656–663. doi: 10.1172/JCI118462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res. 2002;91:988–998. doi: 10.1161/01.res.0000043825.01705.1b. [DOI] [PubMed] [Google Scholar]
- Mayo PR, Skeith K, Russell AS, Jamali F. Decreased dromotropic response to verapamil despite pronounced increased drug concentration in rheumatoid arthritis. Br J Clin Pharmacol. 2000;50:605–613. doi: 10.1046/j.1365-2125.2000.00314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride W, Mukherjee A, Haghani Z, Wheeler-Clark E, Brady J, Gandler T, et al. Nitrendipine: effects on vascular responses and myocardial binding. Am J Physiol. 1984;247:H775–H783. doi: 10.1152/ajpheart.1984.247.5.H775. [DOI] [PubMed] [Google Scholar]
- Nakashima A, Kawashita H, Masuda N, Saxer C, Niina M, Nagae Y, et al. Identification of cytochrome P450 forms involved in the 4-hydroxylation of valsartan, a potent and specific angiotensin II receptor antagonist, in human liver microsomes. Xenobiotica. 2005;35:589–602. doi: 10.1080/00498250500158175. [DOI] [PubMed] [Google Scholar]
- Oates JA. Antagonism of antihypertensive drug therapy by nonsteroidal anti-inflammatory drugs. Hypertension. 1988;11:II4–I16. doi: 10.1161/01.hyp.11.3_pt_2.ii4. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifai N, Tracy RP, Ridker PM. Clinical efficacy of an automated high-sensitivity C-reactive protein assay. Clin Chem. 1999;45:2136–2141. [PubMed] [Google Scholar]
- Sarkar D, Fisher PB. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2006;236:13–23. doi: 10.1016/j.canlet.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Sattari S, Dryden WF, Eliot LA, Jamali F. Despite increased plasma concentration, inflammation reduces potency of calcium channel antagonists due to lower binding to the rat heart. Br J Pharmacol. 2003;139:945–954. doi: 10.1038/sj.bjp.0705202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger H, Mueck AO, Lippert TH. Effects of valsartan and 17 beta-estradiol on the oxidation of low-density lipoprotein in vitro. Coron Artery Dis. 2000;11:347–349. doi: 10.1097/00019501-200006000-00008. [DOI] [PubMed] [Google Scholar]
- Shi XZ, Pazdrak K, Saada N, Dai B, Palade P, Sarna SK. Negative transcriptional regulation of human colonic smooth muscle Cav1.2 channels by p50 and p65 subunits of nuclear factor-kappaB. Gastroenterology. 2005;129:1518–1532. doi: 10.1053/j.gastro.2005.07.058. [DOI] [PubMed] [Google Scholar]
- Shibukawa A, Wainer IW. Simultaneous direct determination of the enantiomers of verapamil and norverapamil in plasma using a derivatized amylose high-performance liquid chromatographic chiral stationary phase. J Chromatogr. 1992;574:85–92. doi: 10.1016/0378-4347(92)80101-u. [DOI] [PubMed] [Google Scholar]
- Steel GD, Torrie JH, Dickey DA. Principles and Procedures of Statistics. A Biometrical Approach. McGraw-Hill Companies: New York; 1997. [Google Scholar]
- Taavitsainen P, Kiukaanniemi K, Pelkonen O. In vitro inhibition screening of human hepatic P450 enzymes by five angiotensin-II receptor antagonists. Eur J Clin Pharmacol. 2000;56:135–140. doi: 10.1007/s002280050731. [DOI] [PubMed] [Google Scholar]
- Tracy TS, Korzekwa KR, Gonzalez FJ, Wainer IW. Cytochrome P450 isoforms involved in metabolism of the enantiomers of verapamil and norverapamil. Br J Clin Pharmacol. 1999;47:545–552. doi: 10.1046/j.1365-2125.1999.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Washizuka T, Komura S, Yoshida T, Hosaka Y, Hatada K, et al. Genomic and non-genomic regulation of L-type calcium channels in rat ventricle by thyroid hormone. Endocr Res. 2005;31:59–70. doi: 10.1080/07435800500229227. [DOI] [PubMed] [Google Scholar]