Abstract
Previous work has shown that Xenopus blastula cells sense activin concentration by assessing the absolute number of occupied receptors per cell (100 and 300 molecules of bound activin activate Xbra and Xgsc transcription, respectively; a difference of only 3-fold). We now ask how quantitative differences in the absolute number of occupied receptors lead to the qualitatively distinct gene responses in the nucleus through SMAD2, a transducer of concentration-dependent gene responses to activin. We show that the injection of 0.2 or 0.6 ng of Smad2 mRNA activates Xbra or Xgsc transcription, respectively, involving, again, only a 3-fold difference. Furthermore, Xbra transcription is down-regulated by overexpression of SMAD2 as it is after activin signaling. We have developed a method to isolate nuclei from animal cap cells and subsequently have quantified the amount of nuclear SMAD2 protein. We find that the injection of 0.2 or 0.6 ng of Smad2 mRNA into an egg leads to only a 3-fold difference in the amount of SMAD2 protein in the nuclei of the blastula cells that express Xbra or Xgsc. We conclude that a 3-fold difference in the absolute number of occupied activin receptors can be maintained only as a 3-fold difference in the level of nuclear SMAD2 protein. Therefore, in this example of morphogen action, there appears to be no amplification of a key cytoplasmic transduction response, and a small but developmentally important change in extracellular signal concentration is relayed directly to the nucleus.
Cell differentiation during vertebrate development is determined, to a large extent, by a series of sequential cell interactions. One of the fundamental mechanisms in this process involves the action of morphogens, where an individual cell can make at least three different responses to a single signaling molecule, the morphogen, in a concentration-dependent manner (1). This mechanism is particularly important in development, because a single signaling molecule can specify the formation of different cell types spatially related to the source of the signal and, hence, can generate positional information (2). There is an increasing number of examples of concentration-dependent responses to morphogens in development (3, 4). Within the vertebrates, the effect of activin on Xenopus blastula cells is believed to reflect the mesoderm-forming induction. Blastula cells show a morphogen-like response to activin by expressing different genes according to the concentration of activin (5–7). Furthermore, our previous work has demonstrated that the increasing occupancy of a single activin receptor type at the cell surface can cause cells to switch gene expression and has shown that cells sense 3-fold changes in ligand concentration by the absolute number of occupied receptors per cell [100 and 300 molecules of bound activin activate Xbrachyury (Xbra) and Xenopus goosecoid (Xgsc) transcription, respectively] (8).
Activin signals through serine-threonine kinase receptors I and II at the cell surface (9–12). Binding of activin induces the formation of heteromeric complexes of these receptors, and signaling is initiated when receptor I is phosphorylated and activated by receptor II. The activated receptor I phosphorylates SMAD2. Activated SMAD2 associates with SMAD4 and mediates nuclear translocation of the heteromeric complex. In the nucleus, the SMAD2/SMAD4 complex is thought to activate specific gene transcription through cooperative interactions with DNA and other DNA-binding proteins such as FAST-1 and FAST-2 (13, 14).
Overexpression of SMAD2 in Xenopus blastula cells results in the activation of Xbra and Xgsc transcription in a way similar to the concentration-dependent gene responses to activin, although Xbra transcription has been reported not to be down-regulated as it is after activin signaling (15). It is not at all understood, however, how a small increase in morphogen concentration outside a cell is transduced rapidly into qualitatively distinct gene responses inside a cell, a basic characteristic of morphogen action. In the example analyzed here, Xbra transcription is activated at a low receptor occupancy (low gene response) and Xgsc transcription is activated at a 3-fold-higher occupancy (high gene response) through the SMAD2-signaling pathway. There are two possible mechanisms by which extracellular signal concentration can be quantitatively transduced to the nucleus: (i) the 3-fold difference in receptor occupancy is maintained at each step inside the cell so that there still is only a 3-fold difference in the level of nuclear SMAD2, or (ii) the 3-fold difference at the receptor level is increased substantially during intracellular transduction as the signal is transmitted to the nucleus. We show here that the former mechanism operates in this system. This helps to clarify further the mechanism by which a cell responds to its position in a concentration gradient of morphogen.
MATERIALS AND METHODS
Materials.
The capped mRNAs were synthesized in vitro (16) by using Ambion Megascript (Ambion, Austin, TX). pSP64TNE-Smad2 was a gift of D. A. Melton (Howard Hughes Medical Institute, Harvard University) that was linearized by XbaI and transcribed to untagged Smad2 mRNA by SP6. pT7TSHA-HA was constructed by inserting a DNA fragment encoding the HA (influenza hemagglutinin) epitope into the cloning site of pT7TSHA (17). pT7TSHA-HA-Smad2 was constructed by inserting Smad2 cDNA from pSP64TNE-Smad2 into the cloning site of pT7TSHA-HA, linearized by XbaI, and transcribed to HA-HA Smad2 mRNA by T7. pCS2+MT-Smad2 was constructed by inserting Smad2 cDNA into the cloning site of pCS2+MT (18), linearized by NotI, and transcribed to Myc6 Smad2 mRNA by SP6. A maltose-binding protein–HA-HA SMAD2 fusion protein was purified from overexpressing Escherichia coli (19). Recombinant HA-HA SMAD2 was cleaved from maltose-binding protein by factor Xa, following the manufacturer’s recommendations (New England Biolabs). Anti-HA and anti-α-tubulin mAbs were purchased from Boehringer Mannheim and Sigma, respectively.
Microinjection of mRNA.
Embryos were fertilized in vitro, dejellied, and cultured as described (20). Each embryo was injected in the animal pole with an indicated dose of mRNAs at the two-cell stage into both blastomeres by using a Drummond Scientific Nanoject system (Drummond Scientific, Broomall, PA). Animal caps were dissected at stage 8.5 in 1× strength modified Barth-Hepes saline and cultured to stage 10.5.
RNase Protection Assays.
Five animal caps at stage 10.5 were frozen on dry ice. RNase protection assays were carried out as described (21). The fibroblast growth factor receptor, Xbra, and Xgsc probes were the same as those used previously (22). Quantitation of RNase protection assay gels was performed by using a Bio-imaging analyzer, BAS-2500, and the macbas 2.3 software package (Fuji).
Preparation of Animal Cap Homogenate.
Ten animal caps at stage 10.5 were pipetted in 0.1 ml buffer A [20 mM Hepes⋅KOH, pH 7.4/10 mM MgCl2/0.1 mM NaF/1 mM sodium orthovanadate/0.1 mM spermidine/1 mM CaCl2/0.5 mM DTT/0.1 mM PMSF/1× protease inhibitor mixture (Boehringer Mannheim)] and sonicated on ice. Twelve micrograms of total protein was subjected to SDS/PAGE, followed by Western blotting with the anti-HA antibody.
Preparation of Nuclear Fraction.
The nuclear fraction was prepared as described with modifications (23). Each embryo was injected with an indicated dose of HA-HA Smad2 mRNA and with [3H]thymidine (8,500 cpm/0.8 pmol per embryo). All manipulations were performed at 0–4°C. Fifty animal caps at stage 10.5 were rinsed with buffer B (buffer A containing 0.25 M sucrose) and homogenized with 0.5 ml buffer A containing 2 M sucrose in a Wheaton glass–glass homogenizer (1 ml, loose type) gently with 40 strokes. The homogenate was layered onto a 2.05 M sucrose cushion, followed by centrifugation at 200,000 × g for 1 h. The pellet was suspended in 40 μl buffer B and transferred to a new tube. After addition of 0.4 ml buffer B, the sample was centrifuged at 900 × g for 10 min. The pellet was suspended in 40 μl buffer B and used as the nuclear fraction. To determine the recovery of nuclear DNA, 10-μl aliquots of the homogenate and the nuclear fraction were frozen in liquid nitrogen and thawed at 25°C. They were precipitated with 1 ml of 10% trichloroacetic acid (TCA). After centrifugation, the pellets were suspended with 1 ml of 10% TCA, followed by centrifugation. The pellets were resuspended in 25 μl of 5% TCA, heated at 90°C for 15 min, and cooled at 25°C (24). The acid-insoluble radioactivity of [3H]thymidine in each sample was measured by a liquid scintillation system.
Protein Analysis.
HA-HA SMAD2 was transferred to nitrocellulose after SDS/PAGE and detected by Western blotting using the enhanced chemiluminescence plus immunoblotting-detection system (Amersham) with the anti-HA antibody. The density of the bands of HA-HA SMAD2 was measured by densitometric tracing at 420 nm. The amount of HA-HA SMAD2 was determined by using a standard curve based on recombinant HA-HA SMAD2 in a linear range as described (23, 25). Total protein concentration of the homogenate was determined with BSA as a standard protein (26). The amount of recombinant HA-HA SMAD2 in Figs. 3A and 5A was determined with BSA as a standard protein by densitometric tracing of protein bands stained with Coomassie brilliant blue on an SDS-polyacrylamide gel (27).
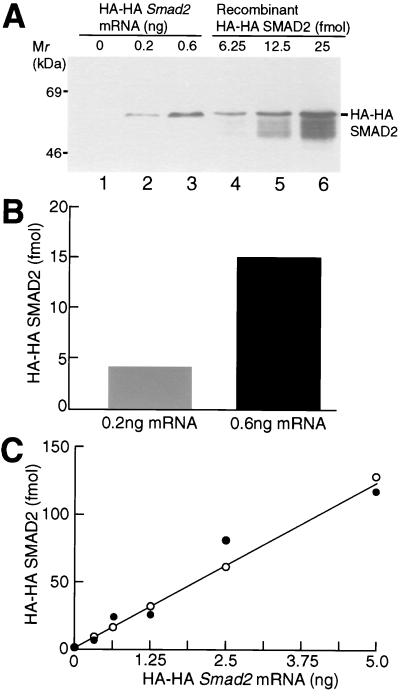
Figure 3.
Quantitation of the amount of HA-HA SMAD2 protein in animal cap cells. (A) Western blotting of HA-HA SMAD2. Each embryo was injected with the indicated dose of HA-HA Smad2 mRNA. Animal caps were homogenized at stage 10.5. Twelve micrograms of total protein from mRNA-injected embryos (lanes 1–3) or the indicated amount of recombinant HA-HA SMAD2 protein (lanes 4–6) was subjected to SDS/PAGE, followed by Western blotting with the anti-HA antibody. The result shown is representative of three independent experiments. (B) Quantitation of the amount of HA-HA SMAD2 protein. The amounts of HA-HA SMAD2 protein in lanes 2 and 3 of A were quantified by using a standard curve based on recombinant HA-HA SMAD2 protein. (C) Quantitation of the amount of HA-HA SMAD2 protein synthesized by embryos injected with various doses of HA-HA Smad2 mRNA. Each embryo was injected with the indicated dose of HA-HA Smad2 mRNA. The amount of HA-HA SMAD2 protein per 12 μg of total protein was quantified as described in B. The results shown are drawn from two independent experiments. In a third experiment, all points were somewhat higher, but fell on a straight line.
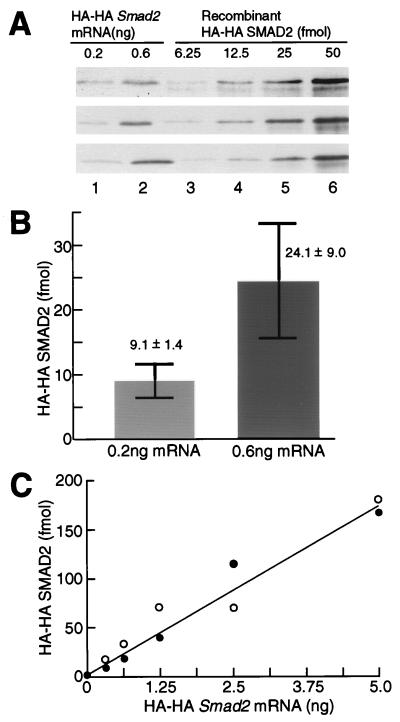
Figure 5.
Quantitation of the amount of HA-HA SMAD2 protein in the nuclei. (A) Western blotting of HA-HA SMAD2 protein. Each embryo was injected with 0.2 or 0.6 ng of HA-HA Smad2 mRNA and with 8,500 cpm of [3H]thymidine. Protein, from the nuclear fractions of mRNA-injected embryos, containing 6,700 cpm of acid-insoluble [3H]thymidine (lanes 1 and 2) or the indicated amount of recombinant HA-HA SMAD2 protein (lanes 3–6) was subjected to SDS/PAGE, followed by Western blotting with the anti-HA antibody. The result shown are from three independent experiments. (B) Quantitation of the amount of HA-HA SMAD2 protein. The amounts of HA-HA SMAD2 in lanes 1 and 2 of A were quantified by using a standard curve based on recombinant HA-HA SMAD2 protein. The values are means ± SEs of three independent experiments. (C) Quantitation of the amount of HA-HA SMAD2 protein synthesized by embryos injected with various doses of HA-HA Smad2 mRNA. Each embryo was injected with the indicated dose of Smad2 mRNA. The amount of HA-HA SMAD2 protein in the nuclear fraction containing 6,700 cpm of acid-insoluble [3H]thymidine was quantified as described in Fig. 5B. The results shown are drawn from two independent experiments. In a third experiment, all points were higher, but fell on a straight line.
RESULTS
HA-HA SMAD2 Is as Effective as Untagged SMAD2 in Activating Xbra and Xgsc Transcription.
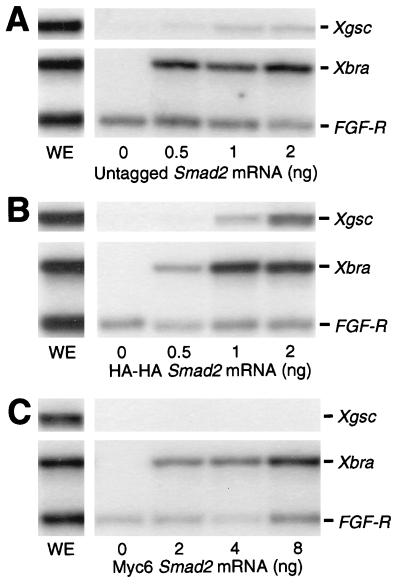
To carry out accurate quantitative experiments, it is necessary to work with epitope-tagged SMAD2. LacZ-tagged mouse SMAD2 is reported to be functional, because it induces secondary axes in injected Xenopus embryos (28). We asked whether epitope-tagged SMAD2 was just as effective as untagged SMAD2 in activating Xbra and Xgsc transcription in animal cap cells. We prepared two kinds of tagged Smad2 mRNAs, HA-HA and Myc6 Smad2 mRNAs. The HA-HA or Myc6 tag was attached to the N terminus of SMAD2. Each embryo was injected with the indicated dose of untagged, HA-HA, or Myc6 Smad2 mRNA. Animal caps were dissected at stage 8.5, cultured to stage 10.5, and analyzed by RNase protection (Fig. 1). Untagged SMAD2 activated Xbra and Xgsc transcription as has been described (15) (Fig. 1A). We found that whereas Myc6 SMAD2 is two to four times less effective in activating Xbra transcription, HA-HA SMAD2 activates Xbra and Xgsc transcription with the same efficiency as untagged SMAD2 (Figs. 1 B and C). We do not know why Myc6 SMAD2 fails to activate Xgsc transcription, though it has been found that the addition of Myc tags can substantially alter the activity of some transcription factors (29). We concluded that HA-HA SMAD2 has the same activity as untagged SMAD2 and could be used to accurately quantify SMAD2 levels in our experiments.
Figure 1.
Xbra and Xgsc transcription is activated by HA-HA SMAD2 as effectively as by untagged SMAD2. Each embryo was injected with the indicated dose of untagged, HA-HA, or Myc6 Smad2 mRNA. Animal caps at stage 10.5 were analyzed by RNase protection. WE, whole embryo. The results shown are representative of three independent experiments.
SMAD2 Activates Xbra and Xgsc Transcription in the Same Dose-Responsive Way as Activin.
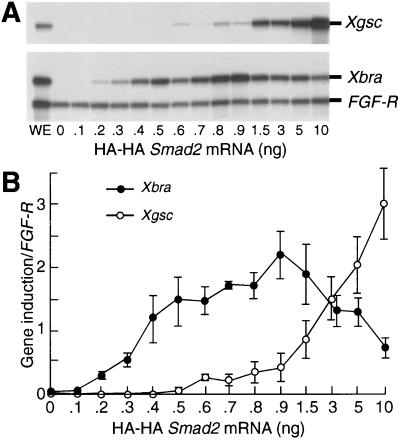
We now asked whether overexpressed SMAD2 activated Xbra and Xgsc transcription in the same dose-responsive way as activin. The injection of increasing doses of untagged Smad2 mRNA from 0.015 to 2 ng has been reported to mimic the concentration-dependent gene responses observed with activin, although Xbra transcription was not seen to be down-regulated as it is after activin signaling (15). We injected embryos with a wider range of doses of HA-HA Smad2 mRNA than reported previously (15), that is, from 0.1 to 10 ng, and observed a repression of Xbra, as seen by activin treatment (8) and by overexpression of Xgsc (30). Animal caps were dissected at stage 8.5, cultured to stage 10.5, and analyzed by RNase protection (Fig. 2A). We saw that Xbra or Xgsc transcription was activated by injection of 0.2 or 0.6 ng of HA-HA Smad2 mRNA, respectively, that was at a 3-fold concentration difference (Fig. 2B). Moreover, Xbra transcription was down-regulated by injection of more than 1.0 ng of mRNA, just as it is after activin signaling (see figure 6D of ref. 8). Some variation was seen in the level of Xgsc transcription at low HA-HA Smad2 mRNA doses up to 1.0 ng, as also was observed in the previous work (15), but a clear and very consistent increase in Xgsc transcription was observed at 1.5 ng of HA-HA Smad2 mRNA and greater. Indeed the amount of Xbra down-regulation by SMAD2 observed here (64% suppression by an 11-fold increase in HA-HA Smad2 mRNA from 0.9 to 10 ng) is very comparable to that observed previously by activin (70% suppression by a 25-fold increase in 35S-activin from 0.3 to 10 μl per 100 μl reaction mixture; see ref. 8). These results indicate that overexpressed SMAD2 can activate Xbra and Xgsc transcription in the same dose-dependent manner as activin. We conclude that overexpression of SMAD2 can be used to analyze the intracellular-signaling mechanism that responds to the concentration-dependent morphogen effects of activin.
Figure 2.
Activation of Xbra and Xgsc transcription by HA-HA SMAD2 in the same dose-responsive way as activin. (A) RNase protection analysis of Xbra and Xgsc expression. Each embryo was injected with the indicated dose of HA-HA Smad2 mRNA. Animal caps at stage 10.5 were analyzed by RNase protection. WE, whole embryo. The result shown is representative of three independent experiments. (B) Quantitation of gel analyses. The levels of Xbra and Xgsc transcription relative to fibroblast growth factor receptor loading control were quantified by a Bio-imaging analyzer, BAS-2500. The values are means ± SEs of three independent experiments.
Smad2 mRNA Is Translated Quantitatively into SMAD2 Protein.
Our finding that overexpressed SMAD2 can activate Xbra and Xgsc transcription in the same way as activin does not necessarily mean that there is the same 3-fold difference in SMAD2 protein in animal cap cells as there was in mRNA injected into eggs. To establish whether there is this quantitative correspondence at the protein level, each embryo was injected with 0.2 or 0.6 ng of HA-HA Smad2 mRNA. Animal caps were dissected at stage 8.5, cultured to stage 10.5, and analyzed by Western blotting (Fig. 3A). HA-HA Smad2 mRNA (0.2 and 0.6 ng) was translated into 4.5 and 15 fmol of HA-HA SMAD2 protein per 12 μg of total protein (about one-third of an animal cap), respectively, a difference of 3-fold (Figs. 3 A and B). These numbers were determined by using a standard curve based on recombinant HA-HA SMAD2 (Fig. 3A; see Materials and Methods). This result indicates that a 3-fold difference in the amount of Smad2 mRNA injected into eggs corresponds to a 3-fold difference in the SMAD2 protein content of animal cap cells. To strengthen the general validity of this correlation, we asked whether it also applies to the higher doses of Smad2 mRNA, at which Xbra transcription declines and Xgsc transcription continues to rise (Fig. 2B). We found that from 0.3 to 5 ng of HA-HA Smad2 mRNA per embryo was translated proportionally to HA-HA SMAD2 protein over the whole of its range (Fig. 3C). We concluded that increasing doses of Smad2 mRNA generate correspondingly increased amounts of SMAD2 protein in a cell.
Preparation of a Nuclear Fraction.
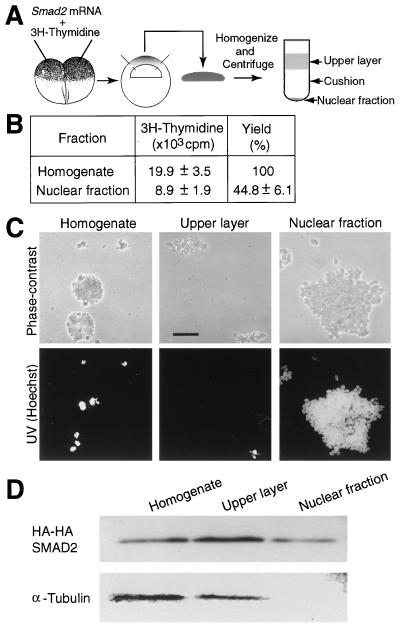
We next asked how much of the ectopic SMAD2 enters nuclei in these experiments. Methods for isolating nuclei from early gastrulation stage Xenopus embryos have been reported in the past (31–33). These published methods, however, are unsatisfactory in one or more of the following ways: they produce nuclei that are heavily contaminated with yolk; the yields are generally poor; and detergents are used that break the nuclear membrane and, therefore, nucleoplasmic proteins are lost. We have developed a method to isolate nuclei from animal cap cells at stage 10.5 that does not use detergents. To determine what proportion of the nuclei in the homogenate are recovered in the nuclear fraction, we coinjected [3H]thymidine with HA-HA Smad2 mRNA and measured the acid-insoluble radioactivity of [3H]thymidine incorporated in DNA (Fig. 4A). In the total homogenate, there were 20 × 103 cpm of [3H]thymidine incorporated in DNA. In the nuclear fraction, 9 × 103 cpm of [3H]thymidine incorporated in DNA were recovered (Fig. 4B). This result indicates that 45% of the nuclei were recovered in the nuclear fraction. We conclude that our method has good yield of nuclei.
Figure 4.
Preparation of a nuclear fraction. (A) Strategy to isolate nuclei. (B) The yield of nuclei. Fifty embryos were injected with [3H]thymidine (8,500 cpm/embryo). The acid-insoluble radioactivity of the homogenate and the nuclear fraction was scintillation counted. The values are means ± SEs of five independent experiments. (C) Photographs of subfractions. The homogenate, the upper layer, and the nuclear fraction were observed and photographed under phase-contrast or UV illumination. (Bar = 0.1 mm.) (D) Western blotting of subfractions. Each embryo was injected with HA-HA Smad2 mRNA (1 ng) and with [3H]thymidine (8,500 cpm). The homogenate (10 μg of total protein), the upper layer (10 μg of total protein), equivalent to about one-third of an animal cap, and the nuclear fraction containing acid-insoluble 1,000 cpm of [3H]thymidine, equivalent to about two animal caps, were subjected to SDS/PAGE, followed by Western blotting with the anti-HA or anti-α-tubulin antibodies. The results shown in C and D are representative of three independent experiments.
We analyzed the purity of the nuclear fraction. The homogenate, the upper layer, and the nuclear fraction were examined under a light microscope (Fig. 4C). The homogenate contained cellular debris, yolk, and nuclei, whereas the upper layer contained cellular debris, yolk, and few nuclei. The nuclear fraction contained many nuclei without cellular debris and yolk. We examined the integrity of the fractions by using an anti-α-tubulin antibody to determine whether cytoplasmic and cytoskeletal components contaminated the nuclear fraction (Fig. 4D) (34). α-Tubulin was detected in the homogenate and the upper layer, but not in the nuclear fraction where HA-HA SMAD2 was observed. These results indicate that the nuclear fraction is free from cytoplasmic and cytoskeletal components. Thus, our method to isolate nuclei results in good recovery and high purity of nuclei.
A 3-Fold Difference in the Level of Smad2 mRNA Leads to a 3-Fold Difference in the Amount of Nuclear SMAD2 Protein.
We have observed that a 3-fold difference in the total cellular SMAD2 protein level can recapitulate the different activin dose responses of Xbra and Xgsc transcription. A critical question is what are the levels of SMAD2 in the nucleus that can elicit these different gene responses. There are three possibilities: i) no difference, ii) more than a 3-fold difference, or iii) only a 3-fold difference, in the level of nuclear SMAD2 protein. We therefore asked how much nuclear SMAD2 protein results from a 3-fold difference in the Smad2 mRNA level? Each embryo was injected with 0.2 or 0.6 ng of HA-HA Smad2 mRNA and with [3H]thymidine. The nuclei were isolated and analyzed by Western blotting (Fig. 5A). The nuclear fractions of embryos injected with 0.2 or 0.6 ng mRNA contained, on average, 9 or 24 fmol of HA-HA SMAD2 protein per acid-insoluble 6,700 cpm of [3H]thymidine, respectively, a difference of 3-fold (Figs. 5 A and B). The numbers were determined by using a standard curve based on recombinant HA-HA SMAD2 (Fig. 5A). Moreover, increasing doses of Smad2 mRNA generated correspondingly increased amounts of nuclear SMAD2 protein (Fig. 5C). We conclude that a 3-fold difference in the amount of Smad2 mRNA leads to a 3-fold difference in the level of nuclear SMAD2 protein in animal cap cells.
DISCUSSION
In this study, we have analyzed a quantitative aspect of the activin-SMAD2 signaling transduction in embryo cells during morphogen action. Although a very large amount of work has been published in recent years identifying molecules that transduce transforming growth factor type β signaling (9–12), the quantitative regulation of this signaling route has been explored relatively little. In one study, the injection of 0.01 ng or 0.1 ng of constitutively active ALK4/activin receptor IB mRNA into an egg has been shown to result in the activation of Xbra or Xgsc transcription, respectively (35). At the level of SMAD2, the injection of 0.12 or 0.25 ng of Smad2 mRNA into an egg has been shown to result in the activation of Xbra or Xgsc, respectively (15). In neither of these cases has the amount of SMAD protein been determined. Having developed an efficient nuclear-isolation procedure for Xenopus blastulae, we have been able to determine the nuclear content of SMAD2 protein.
In this study, we used mRNAs encoding wild-type SMAD2 protein, not mutant or constitutively active forms. Why does injection of wild-type Smad2 mRNA lead to the activation of Xbra and Xgsc transcription? SMAD2 is under negative regulation in the absence of activin (9–12). It seems that overexpression of SMAD2 abolishes the requirement for phosphorylation by the activated type I activin receptor and that overexpressed SMAD2 forms active complexes with endogenous SMAD4, which is presumed not be limiting, and translocates to the nucleus to activate gene transcription. This assumption also has been made in the only other relevant work (15).
We harvested the animal caps at stage 10.5 for the RNase protection assays and Western blotting, and we assume that overexpressed SMAD2-induced transcription turns on just after stage 8, as do many other genes. This means that the amount of overexpressed SMAD2 must be reasonably constant between stages 8 and 10.5. Indeed, the level of SMAD2 protein in an embryo is almost the same between stages 8 and 10.5, when HA-HA Smad2 mRNA-injected embryos are tested by Western analysis (unpublished data).
In Xenopus blastula cells, FAST-1 and SMAD4 have been identified as factors that cooperate with SMAD2 (13, 36). Can they activate Xbra and Xgsc transcription in the same way as SMAD2? Overexpression of SMAD4 in Xenopus blastula cells has been shown to result in the activation of Xbra transcription (36). Overexpression of FAST-1 in Xenopus blastula cells, however, has not been reported. The possibility that amplification of these molecules may help to mediate concentration-dependent responses to activin has not been tested. However, it seems unlikely that such an effect, if it exists, would have a regulatory role because SMAD4 and FAST-1 are not specific to the activin-SMAD2 pathway: thus, SMAD4 cooperates with SMAD1 and SMAD2 in activating both BMP and activin-response genes (36); FAST-1 binds to an activin-response element upstream of the Xenopus homeobox gene, Mix.2; and, in the course of activin signaling, FAST-1 forms a complex with SMAD2 and SMAD4 (37). Thus, SMAD2 is, at present, the only factor known to specifically transduce concentration-dependent gene responses to activin.
Our results enable us to estimate the absolute concentration and number of molecules of nuclear SMAD2 that activate Xbra and Xgsc transcription. A stage 10 blastula has about 3 × 104 cells (38), and our animal caps contain about one-third of this number of cells. An average stage 10 animal cap nucleus has a diameter of 15 μm and volume of 1.8 × 103 μm3. We find that 45% (our recovery) of nuclei from 50 animal caps contain 12 fmol SMAD2. Therefore, we can calculate that Xbra transcription is activated in a nucleus containing 3.3 × 105 (0.31 μM) SMAD2 molecules. These values will be three times greater for the activation of Xgsc. Of course, these figures are only approximate for several reasons, but will be helpful when the time is reached to determine binding affinities. The further analysis of morphogen action will require detailed knowledge of the nuclear molecules that bind to the promoters of early-response genes.
Acknowledgments
We thank Drs. Leslie Dale, Caroline Hill, Fiona Stennard, and Aaron Zorn for advice and comments on the manuscript. This work was supported by the Cancer Research Campaign. K.S. is also supported by the Japan Society for the Promotion of Science.
ABBREVIATIONS
- HA
influenza hemagglutinin
- Xbra
Xbrachyury
- Xgsc
Xenopus gooscoid
References
- 1.Wolpert L. J Theor Biol. 1969;25:1–47. doi: 10.1016/s0022-5193(69)80016-0. [DOI] [PubMed] [Google Scholar]
- 2.Gurdon J B, Dyson S, St. Johnston D. Cell. 1998;95:159–162. doi: 10.1016/s0092-8674(00)81747-x. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence P A, Struhl G. Cell. 1996;85:951–961. doi: 10.1016/s0092-8674(00)81297-0. [DOI] [PubMed] [Google Scholar]
- 4.Neumann C, Cohen S. BioEssays. 1997;19:721–729. doi: 10.1002/bies.950190813. [DOI] [PubMed] [Google Scholar]
- 5.Green J B A, Smith J C. Nature (London) 1990;347:391–394. doi: 10.1038/347391a0. [DOI] [PubMed] [Google Scholar]
- 6.Green J B, New H V, Smith J C. Cell. 1992;71:731–739. doi: 10.1016/0092-8674(92)90550-v. [DOI] [PubMed] [Google Scholar]
- 7.Gurdon J B, Harger P, Mitchell A, Lemaire P. Nature (London) 1994;371:487–492. doi: 10.1038/371487a0. [DOI] [PubMed] [Google Scholar]
- 8.Dyson S, Gurdon J B. Cell. 1998;93:557–568. doi: 10.1016/s0092-8674(00)81185-x. [DOI] [PubMed] [Google Scholar]
- 9.Heldin C H, Miyazono K, ten Dijke P. Nature (London) 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 10.Kretzschmar M, Massagué J. Curr Opin Genet Dev. 1998;8:103–111. doi: 10.1016/s0959-437x(98)80069-5. [DOI] [PubMed] [Google Scholar]
- 11.Attisano L, Wrana J L. Curr Opin Cell Biol. 1998;10:188–194. doi: 10.1016/s0955-0674(98)80141-5. [DOI] [PubMed] [Google Scholar]
- 12.Derynck R, Zhang Y, Feng X H. Cell. 1998;95:737–740. doi: 10.1016/s0092-8674(00)81696-7. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Rubock M J, Whitman M. Nature (London) 1996;383:691–696. doi: 10.1038/383691a0. [DOI] [PubMed] [Google Scholar]
- 14.Labbé E, Silvestri C, Hoodless P A, Wrana J L, Attisano L. Mol Cell. 1998;2:109–120. doi: 10.1016/s1097-2765(00)80119-7. [DOI] [PubMed] [Google Scholar]
- 15.Graff J M, Bansal A, Melton D A. Cell. 1996;85:479–487. doi: 10.1016/s0092-8674(00)81249-0. [DOI] [PubMed] [Google Scholar]
- 16.Krieg P A, Melton D A. Methods Enzymol. 1987;155:397–415. doi: 10.1016/0076-6879(87)55027-3. [DOI] [PubMed] [Google Scholar]
- 17.Zorn A M, Krieg P A. Genes Dev. 1997;11:2176–2190. doi: 10.1101/gad.11.17.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roth M B, Zahler A M, Stolk J A. J Cell Biol. 1991;115:587–596. doi: 10.1083/jcb.115.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.di Guan C, Li P, Riggs P D, Inouye H. Gene. 1988;67:21–30. doi: 10.1016/0378-1119(88)90004-2. [DOI] [PubMed] [Google Scholar]
- 20.Gurdon J B, Tiller E, Roberts J, Kato K. Curr Biol. 1993;3:1–11. doi: 10.1016/0960-9822(93)90139-f. [DOI] [PubMed] [Google Scholar]
- 21.Lemaire P, Gurdon J B. Development (Cambridge, UK) 1994;120:1191–1199. doi: 10.1242/dev.120.5.1191. [DOI] [PubMed] [Google Scholar]
- 22.Ryan K, Garrett N, Mitchell A, Gurdon J B. Cell. 1996;87:989–1000. doi: 10.1016/s0092-8674(00)81794-8. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu K, Shirataki H, Honda T, Minami S, Takai Y. J Biol Chem. 1998;273:6591–6594. doi: 10.1074/jbc.273.12.6591. [DOI] [PubMed] [Google Scholar]
- 24.Schneider W C. J Biol Chem. 1945;161:293–303. [PubMed] [Google Scholar]
- 25.Shirataki H, Yamamoto T, Hagi S, Miura H, Oishi H, Jin-no Y, Senbonmatsu T, Takai Y. J Biol Chem. 1994;269:32717–32720. [PubMed] [Google Scholar]
- 26.Lowry O H, Rosebrough N J, Farr A L, Randall R J. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 27.Weber K, Prinngle J R, Osborn M. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- 28.Baker J C, Harland R M. Genes Dev. 1996;10:1880–1889. doi: 10.1101/gad.10.15.1880. [DOI] [PubMed] [Google Scholar]
- 29.Ferreiro B, Artinger M, Cho K W Y, Niehrs C. Development (Cambridge, UK) 1998;125:1347–1359. doi: 10.1242/dev.125.8.1347. [DOI] [PubMed] [Google Scholar]
- 30.Artinger M, Blitz I, Inoue K, Tran U, Cho K W. Mech Dev. 1997;65:187–196. doi: 10.1016/s0925-4773(97)00073-7. [DOI] [PubMed] [Google Scholar]
- 31.Arms K. Dev Biol. 1971;26:497–502. doi: 10.1016/0012-1606(71)90079-0. [DOI] [PubMed] [Google Scholar]
- 32.Theriault J, Landesman R. Cell Differ. 1974;3:249–257. doi: 10.1016/0045-6039(74)90015-3. [DOI] [PubMed] [Google Scholar]
- 33.Farzaneh F, Pearson C K. J Embryol Exp Morphol. 1978;48:101–108. [PubMed] [Google Scholar]
- 34.Neufeld K, White R L. Proc Natl Acad Sci USA. 1997;94:3034–3039. doi: 10.1073/pnas.94.7.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armes N A, Smith J C. Development (Cambridge, UK) 1997;124:3797–3804. doi: 10.1242/dev.124.19.3797. [DOI] [PubMed] [Google Scholar]
- 36.Lagna G, Hata A, Hemmati-Brivanlou A, Massagué J. Nature (London) 1996;383:832–836. doi: 10.1038/383832a0. [DOI] [PubMed] [Google Scholar]
- 37.Chen X, Weisberg E, Fridmacher V, Watanabe M, Naco G, Whitman M. Nature (London) 1997;389:85–89. doi: 10.1038/38008. [DOI] [PubMed] [Google Scholar]
- 38.Woodland H R, Gurdon J R. J Embryol Exp Morphol. 1968;19:363–385. [PubMed] [Google Scholar]