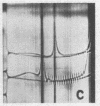
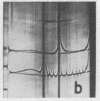
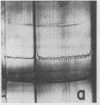
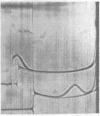
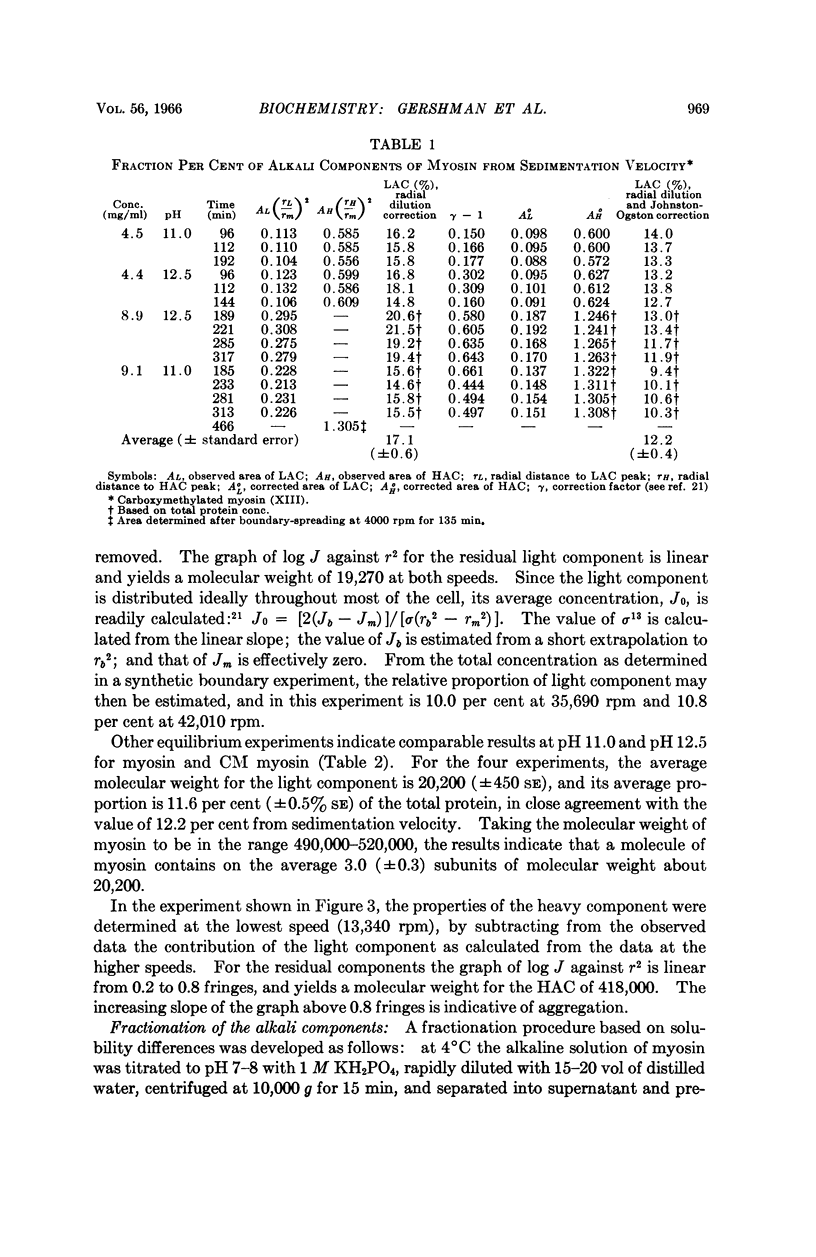
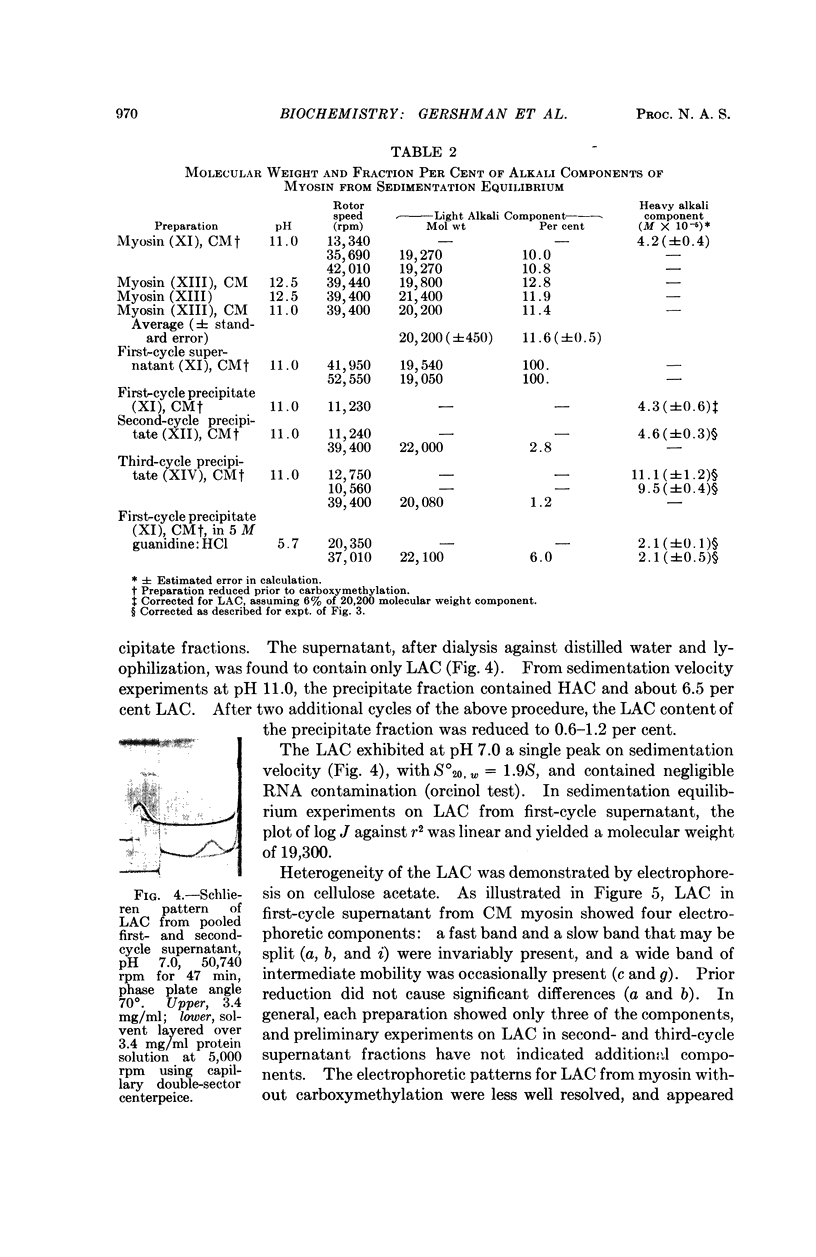
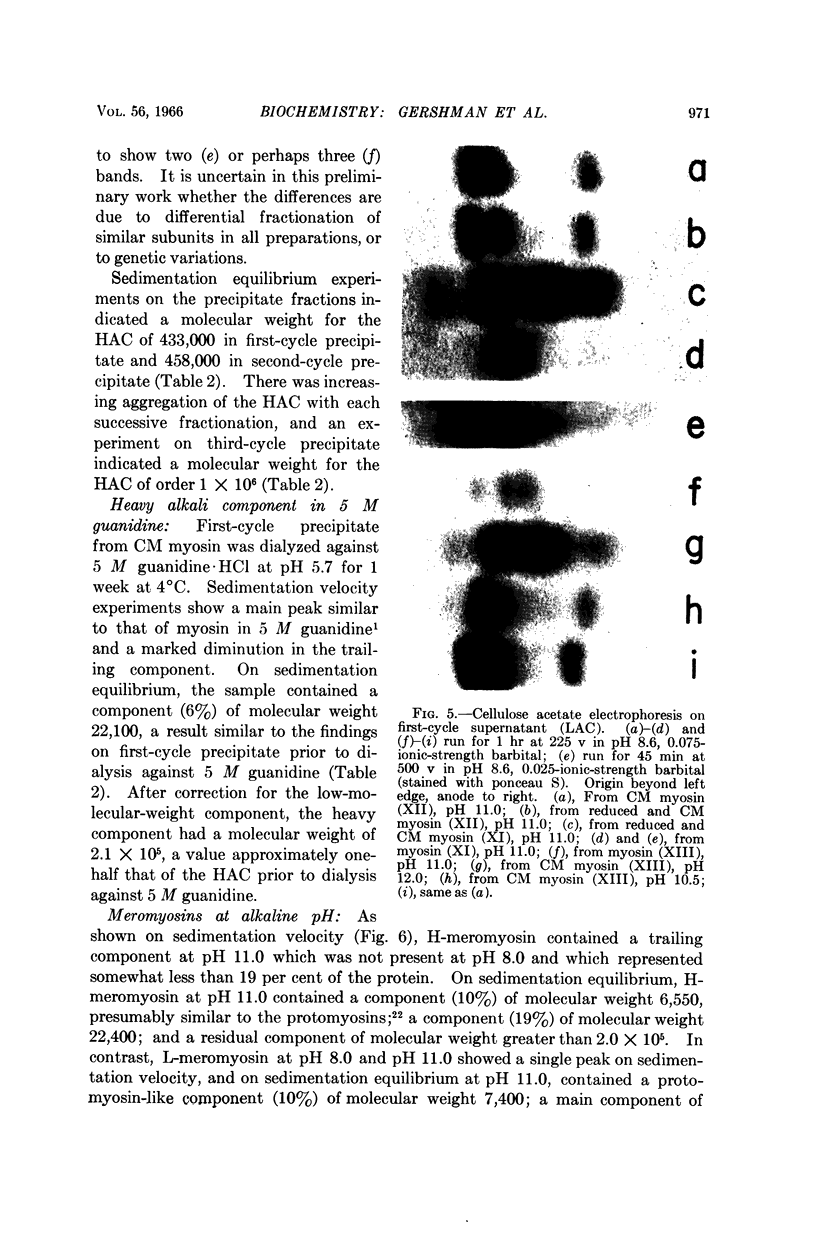
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dreizen P., Hartshorne D. J., Stracher A. The subunit structure of myosin. I. Polydispersity in 5 M guanidine. J Biol Chem. 1966 Jan 25;241(2):443–448. [PubMed] [Google Scholar]
- KAY C. M. The partial specific volume of muscle proteins. Biochim Biophys Acta. 1960 Mar 11;38:420–427. doi: 10.1016/0006-3002(60)91277-4. [DOI] [PubMed] [Google Scholar]
- KIELLEY W. W., HARRINGTON W. F. A model for the myosin molecule. Biochim Biophys Acta. 1960 Jul 15;41:401–421. doi: 10.1016/0006-3002(60)90037-8. [DOI] [PubMed] [Google Scholar]
- KOMINZ D. R., HOUGH A., SYMONDS P., LAKI K. The amino acid composition of action, myosin, tropomyosin and the meromyosins. Arch Biochem Biophys. 1954 May;50(1):148–159. doi: 10.1016/0003-9861(54)90017-x. [DOI] [PubMed] [Google Scholar]
- LOWEY S., COHEN C. Studies on the structure of myosin. J Mol Biol. 1962 Apr;4:293–308. doi: 10.1016/s0022-2836(62)80007-2. [DOI] [PubMed] [Google Scholar]
- LOWEY S., HOLTZER A. The homogeneity and molecular weights of the meromyosins and their relative proportions in myosin. Biochim Biophys Acta. 1959 Aug;34:470–484. doi: 10.1016/0006-3002(59)90300-2. [DOI] [PubMed] [Google Scholar]
- MUELLER H. MOLECULAR WEIGHT OF MYOSIN AND MEROMYOSINS BY ARCHIBALD EXPERIMENTS PERFORMED WITH INCREASING SPEED OF ROTATIONS. J Biol Chem. 1964 Mar;239:797–804. [PubMed] [Google Scholar]
- Mueller H. Characterization of the molecular region containing the active sites of myosin. J Biol Chem. 1965 Oct;240(10):3816–3828. [PubMed] [Google Scholar]
- RICE R. V. Conformation of individual macromolecular particles from myosin solution. Biochim Biophys Acta. 1961 Sep 30;52:602–604. doi: 10.1016/0006-3002(61)90427-9. [DOI] [PubMed] [Google Scholar]
- STRACHER A., CHAN P. C. Effect of p-chloromercuribenzoate on myosin A-adenosinetriphosphatase activity. Arch Biochem Biophys. 1961 Dec;95:435–440. doi: 10.1016/0003-9861(61)90173-4. [DOI] [PubMed] [Google Scholar]
- SZENT-GYORGYI A. G., BORBIRO M. Depolymerization of light meromyosin by urea. Arch Biochem Biophys. 1956 Jan;60(1):180–197. doi: 10.1016/0003-9861(56)90410-6. [DOI] [PubMed] [Google Scholar]
- TSAO T. C. Fragmentation of the myosin molecule. Biochim Biophys Acta. 1953 Jul;11(3):368–382. doi: 10.1016/0006-3002(53)90056-0. [DOI] [PubMed] [Google Scholar]
- Tonomura Y., Appel P., Morales M. On the molecular weight of myosin. II. Biochemistry. 1966 Feb;5(2):515–521. doi: 10.1021/bi00866a017. [DOI] [PubMed] [Google Scholar]
- WETLAUFER D. B., EDSALL J. T. Sedimentation of myosin in urea solutions. Biochim Biophys Acta. 1960 Sep 9;43:132–134. doi: 10.1016/0006-3002(60)90417-0. [DOI] [PubMed] [Google Scholar]
- WOODS E. F., HIMMELFARB S., HARRINGTON W. F. Studies on the structure of myosin in solution. J Biol Chem. 1963 Jul;238:2374–2385. [PubMed] [Google Scholar]
- YOUNG D. M., HIMMELFARB S., HARRINGTON W. F. ON THE STRUCTURAL ASSEMBLY OF THE POLYPEPTIDE CHAINS OF HEAVY MEROMYOSIN. J Biol Chem. 1965 Jun;240:2428–2436. [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]