Abstract
Plasma membrane clathrin-coated vesicles form after the directed assembly of clathrin and the adaptor complex, AP2, from the cytosol onto the membrane. In addition to these structural components, several other proteins have been implicated in clathrin-coated vesicle formation. These include the large molecular weight GTPase, dynamin, and several Src homology 3 (SH3) domain–containing proteins which bind to dynamin via interactions with its COOH-terminal proline/arginine-rich domain (PRD). To understand the mechanism of coated vesicle formation, it is essential to determine the hierarchy by which individual components are targeted to and act in coated pit assembly, invagination, and scission.
To address the role of dynamin and its binding partners in the early stages of endocytosis, we have used well-established in vitro assays for the late stages of coated pit invagination and coated vesicle scission. Dynamin has previously been shown to have a role in scission of coated vesicles. We show that dynamin is also required for the late stages of invagination of clathrin-coated pits. Furthermore, dynamin must bind and hydrolyze GTP for its role in sequestering ligand into deeply invaginated coated pits.
We also demonstrate that the SH3 domain of endophilin, which binds both synaptojanin and dynamin, inhibits both late stages of invagination and also scission in vitro. This inhibition results from a reduction in phosphoinositide 4,5-bisphosphate levels which causes dissociation of AP2, clathrin, and dynamin from the plasma membrane. The dramatic effects of the SH3 domain of endophilin led us to propose a model for the temporal order of addition of endophilin and its binding partner synaptojanin in the coated vesicle cycle.
Keywords: dynamin, amphiphysin, endophilin, synaptojanin, coated pits
Introduction
The directed assembly of clathrin and the AP2 adaptor complex from the cytosol onto the membrane leads to the formation of clathrin-coated pits (Smythe 1996; Schmid 1997). In addition to these structural components, many other proteins have been implicated in clathrin-coated vesicle formation. In particular, the large molecular weight GTPase, dynamin, has been implicated in the late stages of coated vesicle budding. Several Src homology (SH) 3 domain–containing proteins which bind to dynamin via interactions with its COOH-terminal proline/arginine-rich domain (PRD) (Schmid et al. 1998) have also been shown to have a role in endocytosis. These include amphiphysin (Shupliakov et al. 1997; Wigge et al. 1997) and endophilin (Ringstad et al. 1997; Schmidt et al. 1999).
Dynamin was first identified as having a role in endocytosis when it was shown to be the mammalian homologue of the shibire protein in Drosophila (van der Bliek and Meyerowitz 1991). Temperature-sensitive mutations in shibire give rise to a paralytic phenotype at the nonpermissive temperature. Morphological analysis revealed an accumulation of membrane invaginations and coated pits at the neuromuscular junction suggestive of a defect in the scission of coated pits to form coated vesicles (Kosaka and Ikeda 1983). Electron-dense collars believed to be composed of dynamin were visible around the necks of these pits. Dynamin was later shown to self-assemble into rings and spirals when incubated in buffers of low ionic strength (Hinshaw and Schmid 1995). These rings and spirals had the same dimensions as the collars seen in shibire mutant flies and spirals that formed around invaginations on permeabilized rat brain synaptosomes in the presence of GTPγS (Takei et al. 1995). The self-assembly of dynamin stimulated its GTPase activity (Warnock et al. 1996) and a model was proposed in which dynamin would assemble around the neck of an invaginated pit. The energy released by GTP hydrolysis would allow it to act as a mechanochemical enzyme in the final stage of scission (Warnock and Schmid 1996; McNiven 1998).
The role of dynamin as a “pinchase” has been somewhat controversial, however (Kelly 1999; Yang and Cerione 1999; Sever et al. 2000b), and recent studies have suggested that rather than requiring GTP hydrolysis to perform its function, dynamin functions as a regulator in its GTP conformation, recruiting other binding partners which are then required for scission (Sever et al. 1999, Sever et al. 2000a).
Several proteins have been demonstrated to bind to the COOH-terminal PRD of dynamin via their SH3 domains (Schmid et al. 1998). These include amphiphysin and endophilin. Amphiphysin has been implicated in the endocytic process by studies where an inhibition of endocytosis was observed when its SH3 domain was microinjected into the synapse of the giant lamprey (Shupliakov et al. 1997) or transfected into fibroblasts (Wigge et al. 1997). In addition to binding dynamin, amphiphysin can also bind to the appendage domain of α-adaptin, suggesting that it acts to recruit dynamin to coated pits (Wang et al. 1995; Wigge and McMahon 1998). This model has been supported by observations showing that amphiphysin can enhance the association of clathrin buds with dynamin on purified liposomes. Amphiphysin can also coassemble with dynamin into rings and spirals (Takei et al. 1999).
Endophilin is another SH3 domain–containing protein whose major binding partner is the inositol 5-phosphatase, synaptojanin, although it also binds dynamin (Micheva et al. 1997; Ringstad et al. 1997). Recent studies have shown that endophilin is required for the formation of synaptic-like microvesicles at the plasma membrane (Schmidt et al. 1999). Endophilin has lysophosphatidic acid acyl transferase activity which is essential for its function in synaptic-like microvesicle formation. Synaptojanin knockout mice have elevated levels of phosphatidylinositol 4,5-bisphosphate (PtdInsP2) and show an accumulation of clathrin-coated vesicles indicative of a role for synaptojanin in uncoating (Cremona et al. 1999).
To understand the mechanism of coated vesicle formation, it is essential to determine the hierarchy by which individual components are targeted to and act in coated pit assembly. To address the role of dynamin and its binding partners in the early stages of endocytosis, we have used well-established in vitro assays for the late stages of coated pit invagination and coated vesicle scission (Smythe et al. 1989; Schmid and Smythe 1991; Smythe et al. 1992). Using these functional assays, we demonstrate that dynamin, in addition to its role in scission, is also required for the late stages of coated pit invagination. Furthermore, both GTP binding and hydrolysis are required for this step. We also demonstrate that the SH3 domain of endophilin causes a reduction in PtdInsP2 levels, a loss of AP2 from the permeabilized cell membranes, and a concomitant inhibition of clathrin-coated pit invagination and scission. This leads us to suggest a model for the sequential action of endophilin and synaptojanin in the coated vesicle cycle.
Materials and Methods
Cell Lines and Tissues
Human adenocarcinoma A431 cells were grown in DME supplemented with 10% FCS and 100 U/ml each of penicillin and streptomycin. Cells were passaged every 2 d by trypsinization. Cells required for an in vitro experiment were seeded overnight as described previously (Schmid and Smythe 1991; Smythe et al. 1992).
Antibodies
AP.6 and X22 expressing cells were obtained from the American Tissue Culture Collection. Anti–glutathione S-transferase (GST) antibodies were a generous gift from Nick Helps (Medical Research Council, Protein Phosphorylation Unit, University of Dundee). Antidynamin and antisynaptojanin antibodies were generated by the Scottish Antibody Production Unit using peptides derived from dynamin (ARDVLENKLLPLRRGYIGVVNRSQKD) and synaptojanin (SLRVDSSDEDRISEVRK). The antibodies were affinity purified using peptide columns as described (Harlow and Lane 1988). Antitransferrin receptor monoclonal antibody, HTR 68.4, was a generous gift from Prof. Colin Hopkins (Imperial College, London, UK). Monoclonal antibodies against clathrin (CHC5.9) and α-adaptin (100/2) used for Western blotting were obtained from ICN Biomedicals and Sigma-Aldrich.
Cloning and Purification of Proteins
cDNAs encoding the fusion proteins of GST and the SH3 domains of endophilin, amphiphysin, and amphiphysin with a D to R conversion at residues 530 (GST–endoSH3, GST–amph2 SH3, and GST–amph2 SH3D36R) were a generous gift from Harvey McMahon (Laboratory of Molecular Biology, Cambridge, UK). D36R corresponds to residue 530 of full-length amphiphysin 2 and was mutated to arginine by PCR mutagenesis. GST constructs were expressed in Escherichia coli and purified as described (Smith and Johnson 1988).
Dynamin was purified from frozen rat brains (obtained from Harlan Sera-Lab) as described (Stowell et al. 1999). Purified dynamin was dialyzed overnight with three further changes of buffer into KSHM buffer (100 mM potassium acetate, 85 mM sucrose, 20 mM Hepes, 1 mM magnesium chloride, pH 7.4). Dynamin was determined to be >95% pure by Coomassie gel analysis (Fig. 3 a). For the preparation of wild-type and mutant dynamin, human embryonic kidney (HEK)293 cells were transfected with pCMV encoding untagged wild-type or mutant bovine dynamin1 (a generous gift from Harvey McMahon), using calcium phosphate (Sambrook et al. 1989). Cells were harvested 48 h after transfection and dynamin purified as described (Stowell et al. 1999). Dynamin T65A was generated using the Stratagene QuikChange site-directed mutagenesis kit according to the manufacturer's instructions. The sequence of the mutated protein was verified using an ABI automated DNA sequencer (PE Biosystems). Dynamin GTPase assays were performed according to the method of Gout et al. 1993 and Warnock et al. 1996. Synaptojanin was prepared by expressing a cDNA encoding synaptojanin in pEF Bos (a generous gift from Harvey McMahon) in HEK293 cells. The protein was purified using GST–amph2 SH3D36R as an affinity ligand as described for dynamin (Stowell et al. 1999).
Figure 3.
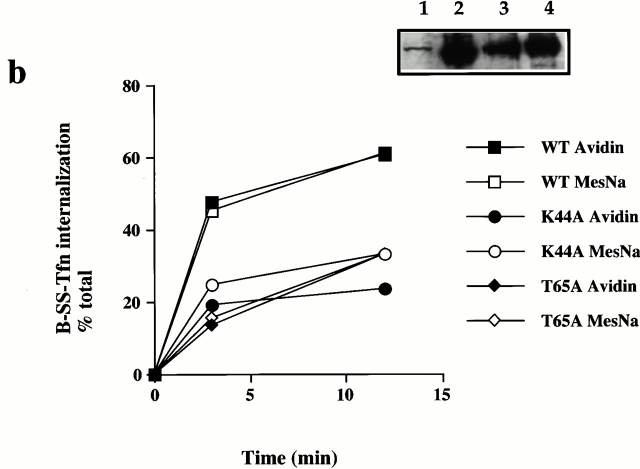
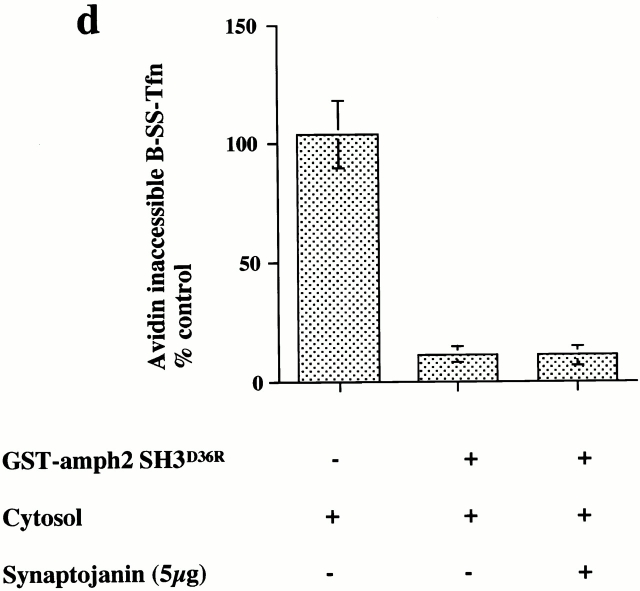
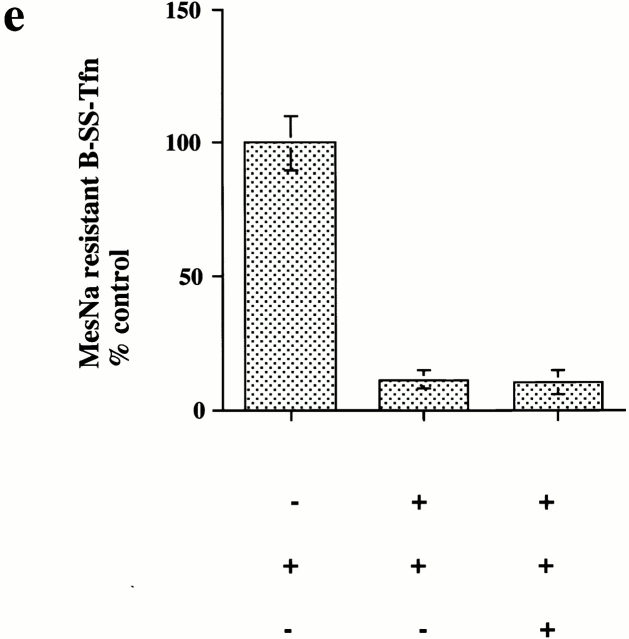
GTP binding and hydrolysis are required for the late stages of coated pit invagination. (a) Permeabilized cells were preincubated with GST–amph2 SH3D36R for 5 min at 30°C. The membranes were collected by centrifugation and assayed for the ability to support B-SS-Tfn sequestration using the avidin inaccessibility assay in the presence of cytosol (2.5 mg/ml) and 5 μg of wild-type (WT), S45N, K44A, T65A, or rat brain (RB) dynamin as indicated. Each point represents the mean ± SD of two experiments each performed in duplicate. (b) Internalization of B-SS-Tfn in HEK293 cells transfected with wild-type, K44A, or T65A dynamin was measured using the avidin inaccessibility or MesNa resistance assays as described in Materials and Methods. Results are from a representative experiment where each point is the mean of duplicates where the range is <10%. The insert shows a Western blot probed with antidynamin antibody showing equivalent protein loadings of HEK293 cells which were mock-transfected (lane 1) or transfected with wild-type, dynamin (lane 2), T65A dynamin (lane 3), or K44A dynamin (lane 4).
Cytosol was prepared from frozen bovine brain (Pel-Freez Ltd.) as described (McLauchlan et al. 1998). Dynamin/synaptojanin-depleted cytosol was prepared by incubation of 200 μg GST–amph2 SH3D36R or GST–endoSH3 with glutathione-agarose beads for 30 min at 4°C. The beads were washed and incubated with 500 μl cytosol (10 mg/ml) for 2 h at 4°C. The beads were pelleted and the cytosol analyzed by Western blot to determine the extent of dynamin depletion. Cytosol was mock treated by incubating with glutathione-agarose alone.
Permeabilized Cell Assays for Clathrin-coated Vesicle Formation
Assays in permeabilized A431 cells after the uptake of biotinylated transferrin (B-SS-Tfn) were performed as described previously (Schmid and Smythe 1991; Smythe et al. 1994).
For SH3 domain preincubation experiments, permeabilized cell membranes were incubated at 30°C for 5 min with the SH3 domain in a volume of 10 μl per assay. The membranes were pelleted at 14,000 rpm for 5 s and resuspended in KSHM buffer. Cytosol, dynamin, synaptojanin, B-SS-Tfn, and the ATP regenerating system were added as indicated in the figure legends, and the membranes were incubated at 37°C for 30 min to allow internalization. Assays containing dynamin were supplemented with 100 μM GTP.
For immunofluorescence experiments, endocytic assay mixtures were centrifuged at 14,000 rpm for 20 s, resuspended in 10% (wt/vol) BSA and centrifuged onto coverslips using a cytospin at 250 rpm for 1 min. Cells were then fixed in methanol (−20°C) for 5 min. The cells were then processed for immunofluorescence. AP2 was detected using AP.6 (Chin et al. 1989). Clathrin was detected using X22 (Brodsky 1985), and synaptojanin was detected using sheep antisynaptojanin antibodies described above.
Transfection Assays
To measure the uptake of B-SS-Tfn in HEK 293 cells expressing wild-type or mutant dynamin, cells were grown in DMEM supplemented with FCS (10%) and plated on 10-cm dishes (1.4 × 106 cells per dish) 1–2 h before transfection. DNA (10 μg per dish) was introduced into the cells using calcium phosphate as described (Sambrook et al. 1989), and the cells were grown for a further 12 h. They were then incubated in serum-free medium for 30 min, washed once in PBS+ (PBS containing 1 mM MgCl2, 1 mM CaCl2, 0.5 mM glucose, and 0.2% [wt/vol] BSA) and incubated for 1–2 min in PBS+ before being lifted gently from the dish. The cells were then pelleted at 850 rpm for 5′ and washed one more time with PBS+. The cells were then incubated with 5 μg/ml B-SS-Tfn in PBS+ for 40 min at 4°C, then washed two times in PBS+, and aliquoted (50 μl containing ∼100,000 cells per tube) before being warmed at 30°C to allow internalization. The cells were then chilled and treated with avidin or 2-Mercaptoethanesulfonic acid and the amount of internalization quantitated as described (Smythe et al. 1994). In general >90% of the cells were transfected using this protocol as assessed by transfection of a cDNA encoding green fluorescence protein (data not shown).
Determination of PtdIns(4,5)P2 and PtdIns(3,4,5)P3 Levels
TCA pellets were extracted for 20 min on ice with 0.5 ml CHCl3/MeOH/conc. HCl (40/80/1 by vol). The organic phase was removed after separation of the phases, induced by adding 0.175 ml CHCl3 and 0.3 ml 0.1 M HCl, vigorous shaking, and centrifugation for 3 min at 12,000 rpm. The upper and interphase were reextracted once with 0.35 ml CHCl3 and the pooled organic phases were vacuum dried.
The dried lipids were alkaline hydrolyzed for 30 min at 100°C in 0.1 ml 0.5 M KOH. After cooling on ice, 0.05 ml of 1 M acetic acid was added, and the samples were extracted twice with 0.8 ml of butan-1-ol/petroleum ether (boiling point 40–60°C)/ethyl acetate (20/4/1 by vol). The lower phases were vacuum dried and reconstituted in 0.125 ml of 0.1 M acetic acid to give a final pH of 5.0.
The samples were assayed for Ins(1,4,5)P3 (derived from PtdIns[4,5]P2) and Ins(1,3,4,5)P4 (derived from PtdIns[3,4,5]P3) using radioligand displacement of [3H]Ins(1,4,5)P3 from a crude membrane preparation of bovine adrenal cortex (Chilvers et al. 1991) and [33P]Ins(1,3,4,5)P4 from recombinant GST–GTPase activity protein 1IP4BP using authentic Ins(1,4,5)P3 and Ins(1,3,4,5)P4 as standards (van der Kaay et al. 1998). For the determination of PtdIns(4,5)P2 and PtdIns(3,4,5)P3, 0.001 and 0.1 ml of each sample was used, respectively.
Results
Many different proteins have been implicated in endocytosis (Damke et al. 1994; Wigge et al. 1997; Benmerah et al. 1998; Chen et al. 1998) by experiments involving overexpression of mutants in living cells, but it is now important to decipher at which stage in the process they act. In vitro assays in permeabilized cells can distinguish between formation of deeply invaginated coated pits and events leading to coated vesicle scission (Schmid and Smythe 1991; Smythe et al. 1992). Transferrin, biotinylated via a cleavable disulphide linkage (B-SS-Tfn), binds to its receptor in permeabilized cells and is further sequestered into deeply invaginated coated pits and also internalized into coated vesicles in a cytosol- and ATP-dependent manner (Schmid and Smythe 1991; Smythe et al. 1992). This assay system distinguishes between deep invagination and scission by testing the accessibility of B-SS-Tfn to exogenously added reagents. B-SS-Tfn which is sequestered into deeply invaginated coated pits is inaccessible to exogenously added avidin but is still sensitive to small membrane impermeant reducing agents such as MesNa which can access B-SS-Tfn through the narrow neck of a deeply invaginated pit. Ligand which has been internalized into coated vesicles, however, is resistant to MesNa reduction. Thus the avidin inaccessibility assay measures the sequestration of ligand into deeply invaginated coated pits and its internalization into coated vesicles, whereas the MesNa resistance assay measures the internalization of ligand into coated vesicles only (Schmid and Smythe 1991; Smythe et al. 1992). We have used these functional assays to investigate the role of dynamin at different stages of clathrin-coated pit invagination and coated vesicle formation.
GTP Hydrolysis by Dynamin Is Required to Form a Deeply Invaginated Coated Pit
Dynamin binds via its PRD to a variety of different SH3 domain–containing proteins. Amphiphysin is one such SH3 domain–containing protein which is proposed to recruit dynamin to sites of endocytosis where it participates either directly or indirectly in vesicle scission (Sever et al. 1999; Stowell et al. 1999). We have tested the effect of a GST fusion protein of a mutant form of the SH3 domain of amphiphysin, GST–amph2 SH3D36R, on B-SS-Tfn sequestration and internalization in permeabilized A431 cells. GST–amph2 SH3D36R binds with a higher affinity to dynamin and thus more strongly inhibits endocytosis in vivo than the wild-type SH3 domain (Owen et al. 1998). Addition of this fusion protein to our in vitro assays revealed a potent inhibition of both ligand sequestration into deeply invaginated coated pits and internalization into coated vesicles as measured by the avidin inaccessibility assay (Fig. 1 a). Half-maximal inhibition occurred at a concentration of ∼0.4 μM GST–amph2 SH3D36R. In a separate assay which allows us to measure the invagination of newly formed coated pits only, the adaptor-dependent stimulation of sequestration (Smythe et al. 1992; McLauchlan et al. 1998), B-SS-Tfn sequestration into new pits was also inhibited by GST–amph2 SH3D36R and similar results were obtained with a GST fusion protein encoding the wild-type amphiphysin SH3 domain (data not shown). Similarly, GST alone is not inhibitory and the SH3 domain of Grb2 is inhibitory but only at a 10-fold higher concentration (data not shown). Grb2 has also been demonstrated to bind to dynamin (Gout et al. 1993) but overexpression of the SH3 domain does not inhibit endocytosis (Wang and Moran 1996; Wigge et al. 1997).
Figure 1.
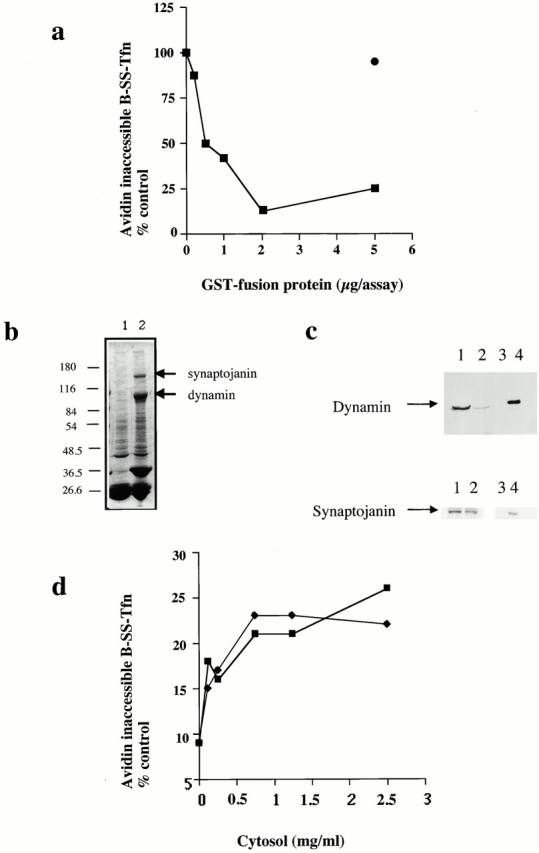
The SH3 domain of amphiphysin inhibits ligand sequestration and internalization in permeabilized A431 cells. (a) The sequestration of B-SS-Tfn into deeply invaginated coated pits and its internalization into coated vesicles was measured using the avidin internalization assay in the presence of the indicated concentrations of GST fusion proteins containing GST alone (filled circles), or the mutant SH3 domain of amphiphysin GST–amph2 SH3D36R (filled squares). Results are from a typical experiment where each assay point is the mean of duplicates which differed by <10%. (b) Bovine brain cytosol (500 μl of 10 mg/ml) was treated with glutathione-agarose alone or coupled to GST–amph2 SH3D36R for 2 h at 4°C. The beads were pelleted and washed three times in PBS before being electrophoresed on a 10% SDS-PAGE and Coomassie stained. Lane 1, GSH beads alone; lane 2, GST–amph2 SH3D36R beads. (c) Western blot of cytosol after treatment with GST–amph2 SH3D36R. Cytosol was treated as described in b and equivalent volumes of supernatant (lanes 1 and 2) or beads (lanes 3 and 4) were probed on Western blots using either antidynamin or antisynaptojanin antibodies. (d) The avidin inaccessibility assay was carried out in the presence of increasing concentrations of bovine brain cytosol which had been treated with GSH beads alone (filled squares) or with GSH beads coupled to GST–amph2 SH3D36R (filled diamonds). (e and f) Permeabilized cells were preincubated with GST (filled circles) or GST–amph2 SH3D36R (filled squares) at the indicated concentrations for 5 min at 30°C. The membranes were then pelleted by centrifugation and assayed either for (e) avidin inaccessibility or (f) MesNa resistance of B-SS-Tfn in the presence of cytosol (2.5 mg/ml) and ATP.
To investigate whether the target(s) of GST–amph2 SH3D36R was located on the membranes or in the cytosol, we first incubated cytosol with GST–amph2 SH3D36R. Treatment of cytosol with GST–amph2 SH3D36R led to specific depletion of both dynamin and also synaptojanin (Fig. 1b and Fig. c). Western blotting showed that with GST–amph2 SH3D36R, >90% of the dynamin and ∼50% of synaptojanin were depleted from cytosol (Fig. 1 c). Treatment with GST–amph2 SH3D36R had no significant effect on the ability of cytosol to support avidin inaccessibility of B-SS-Tfn compared with mock-treated cytosol (Fig. 1 d). Mock-treated cytosol supported both sequestration and internalization to the same extent as untreated cytosol (data not shown). We then preincubated the permeabilized cells with GST–amph2 SH3D36R, collected the membranes by centrifugation, and assayed them in both the avidin inaccessibility and the MesNa resistance assays. After preincubation with GST–amph2 SH3D36R, the ability of permeabilized cells to support ATP- and cytosol-dependent B-SS-Tfn sequestration and internalization in the avidin and MesNa assays was essentially abolished (Fig. 1e and Fig. f). Thus GST–amph2 SH3D36R blocks endocytic vesicle formation in vitro by interfering with one or more membrane-associated proteins.
The major binding partners for the SH3 domain of amphiphysin are dynamin and the inositol 5-phosphatase, synaptojanin (David et al. 1996; Micheva et al. 1997; Wigge et al. 1997) (see Fig. 1b and Fig. c). We wanted to investigate whether the inhibitory effects of the SH3 domain were specific either for dynamin or synaptojanin. Therefore, we investigated whether purified dynamin and synaptojanin could rescue the membranes which had been preincubated with GST–amph2 SH3D36R. Dynamin was purified from rat brain and synaptojanin was purified after overexpression in HEK293 cells using the wild-type GST–amph2 SH3 fusion protein as an affinity ligand (Stowell et al. 1999). Purified dynamin (Fig. 2 a) could restore the ability of the preincubated membranes to support ligand sequestration into deeply invaginated coated pits (Fig. 2 b). Strikingly, however, dynamin was unable to rescue the inhibitory effects of GST–amph2 SH3D36R in the MesNa resistance assay (Fig. 2 c). By contrast, purified synaptojanin was unable to rescue the inhibitory effects of GST–amph2 SH3D36R in either the avidin inaccessibility or MesNa resistance assays (Fig. 2d and Fig. e). To assess whether dynamin effected rescue by competing the SH3 domain of amphiphysin from the membrane, membranes which had been treated with GST–amph2 SH3D36R were incubated in the presence of cytosol alone or cytosol plus dynamin or synaptojanin. They were then Western blotted and probed with an antibody raised against GST. In the presence of dynamin or synaptojanin there was no reduction in the amount of GST–amph2 SH3D36R associated with the membrane (Fig. 2 f). This shows that when dynamin rescues pretreated membranes, it is not acting simply to displace GST–amph2 SH3D36R from the membranes. The inability of dynamin to restore MesNa resistance may be due to depletion of another protein by the SH3 domain of amphiphysin, but it could equally well be due to disruption of the sequential order and timing of events during endocytic vesicle formation.
Figure 2.
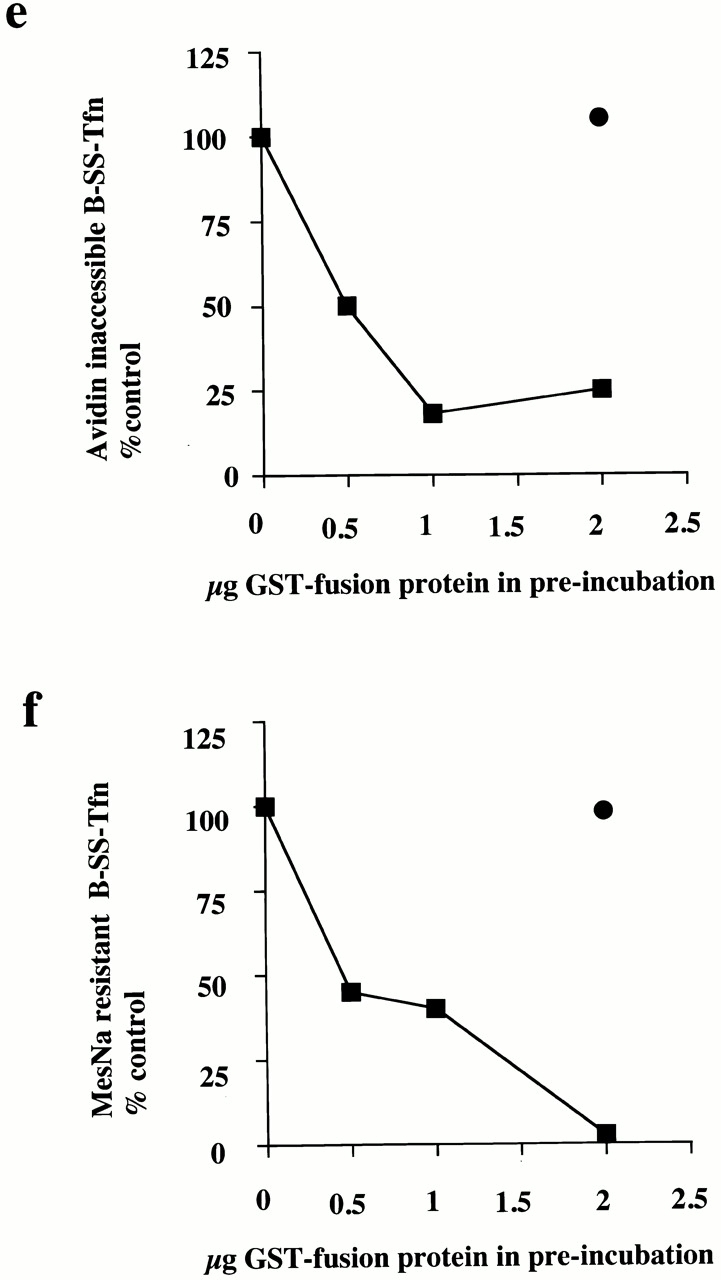
Dynamin can rescue the inhibitory effects of GST–amph2 SH3D36R in the avidin inaccessibility but not the MesNa resistance assay. (a) Dynamin, purified from rat brain was electrophoresed on a 10% SDS-PAGE gel and visualized by Coomassie staining. Permeabilized cell membranes were preincubated in the presence or absence of GST–amph2 SH3D36R (0.1 mg/ml) for 5 min. They were then collected by centrifugation and assayed in the presence of ATP, cytosol (2.5 mg/ml), and dynamin (5 μg) as indicated for the ability to support avidin inaccessibility (b) or MesNa resistance (c) of B-SS-Tfn. Results are expresssed as the mean ± SEM of three experiments each performed in duplicate. Permeabilized cell membranes were preincubated in the presence or absence of GST–amph2 SH3D36R (0.1 mg/ml) for 5 min. They were then collected by centrifugation and assayed in the presence of ATP, cytosol (2.5 mg/ml), and synaptojanin (5 μg) as indicated for the ability to support avidin inaccessibility (d) or MesNa resistance (e) of B-SS-Tfn. Results are expresssed as the mean ± SD of two experiments each performed in duplicate. (f) Permeabilized membranes which had been preincubated in the presence of GST–amph2 SH3D36R and then subsequently incubated for 30 min in the presence of ATP and cytosol (lanes 1–3), plus dynamin (5 μg; lane 2), plus synaptojanin (5 μg; lane 3) were solubilized and electrophoresed on a 10% SDS gel, transferred to nitrocellulose, and probed for the presence of GST–amph2 SH3D36R using anti-GST antibodies.
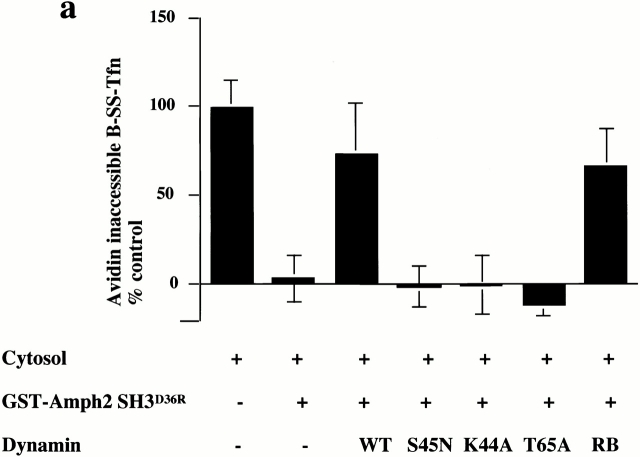
To characterize further the role of dynamin in invagination, we tested a range of dynamin mutants for their ability to rescue the inhibitory effects of GST–amph2 SH3D36R. While both rat brain dynamin and wild-type dynamin purified from HEK293 cells were capable of rescuing the inhibitory effects of GST–amph2 SH3D36R, dynamin which is incapable of binding GTP (dyn S45N) was unable to rescue (Fig. 3 a). Similarly, dyn K44A, which is deficient in both binding and hydrolysis (van der Bliek et al. 1993), was also unable to rescue the inhibitory effects of the SH3 domain of amphiphysin. We also examined the effect of mutant dynamin deficient in GTP hydrolysis. By analogy with known mutations in the structure of Ras GTPase (Pai et al. 1990), a point mutation in dynamin (T65A) generated a mutant form of dynamin which although capable of binding GTP as well as wild-type (data not shown) showed a significant impairment of dynamin GTPase activity (Table ). This mutant protein was also unable to rescue the inhibitory effects of the SH3 domain. These results show that dynamin needs to be able both to bind and hydrolyze GTP to reconstitute the formation of deeply invaginated coated pits in vitro.
Table 1.
GTPase Activity of Wild-Type and Mutant Dynamin
| Dynamin | Rat brain | Wild-type | Dynamin T65A |
|---|---|---|---|
| μM | |||
| 0.5 | 3.5 ± 0.6 | 3.8 ± 0.3 | 0.25 ± 0.01 |
| 1.5 | 5.5 ± 0.7 | 8.0 ± 1.0 | 0.05 ± 0.01 |
GTPase assays were carried out as described by Warnock et al. 1996. Results are expressed as the mean ± SEM of four different determinations of the specific activity (pmol GTP hydrolyzed/min/pmol dynamin) of dynamin GTPase.
The requirement for GTP binding and hydrolysis for invagination in vitro has significant implications for the mechanism of action of dynamin (for a review of current models see Sever et al. 2000b). Our in vitro data suggested that overexpression of dynamin T65A should have a dominant negative effect on transferrin internalization. It has already been demonstrated that measurement of the internalization of B-SS-Tfn in intact cells using either the avidin inaccessibility or MesNa resistance assay can distinguish between inhibition of invagination and scission (Schmid and Carter 1990; van der Bliek et al. 1993). For example, overexpression of K44A results in a significant inhibition of internalization as measured by both the avidin inaccessibility and MesNa resistance assays, leading to the conclusion that mutations in human dynamin block an intermediate stage in coated vesicle formation. Therefore, we measured B-SS-Tfn internalization after overexpression of dynamin T65A in HEK293 cells and compared it with the effect of overexpressing K44A and wild-type dynamin. Strikingly, overexpression of dynamin T65A resulted in a significantly reduced uptake of transferrin compared with wild-type and the amount internalized was the same whether uptake was measured using the avidin inaccessibility or MesNa resistance assays (Fig. 3 b). Overexpression of dynamin K44A also showed reduced uptake of transferrin in both the avidin inaccessibility and MesNa resistance assays, consistent with previously published data (van der Bliek et al. 1993). These data further support the requirement for GTP hydrolysis in the formation of deeply invaginated coated pits.
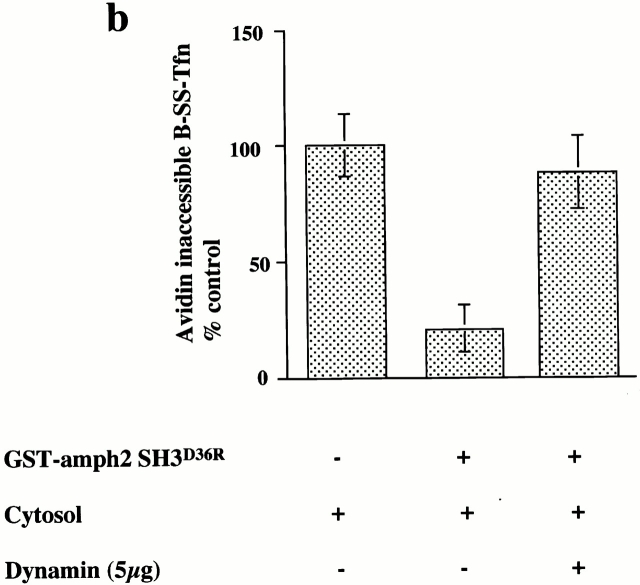
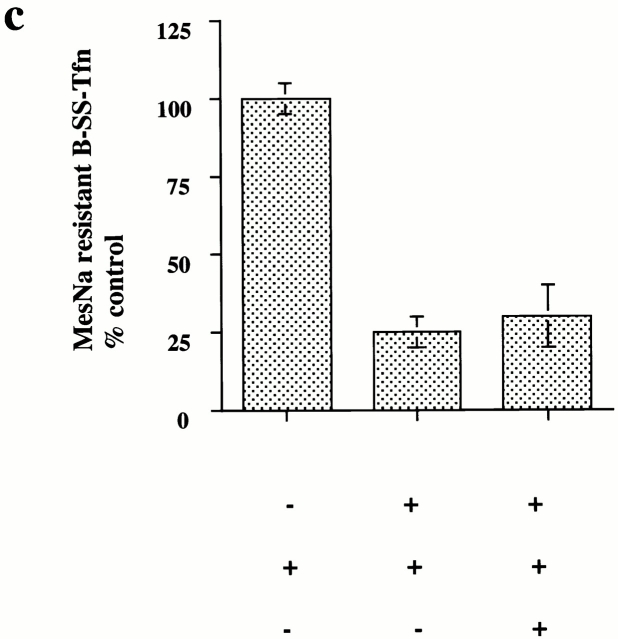
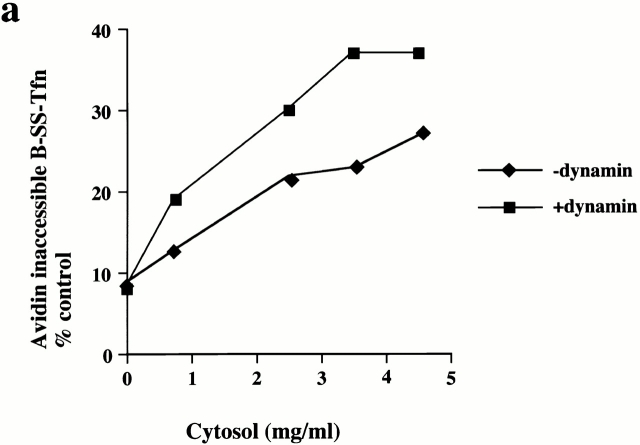
Coated pits on membranes which have been preincubated with GST–amph2 SH3D36R were able to become deeply invaginated and sequester ligand in the presence of added dynamin. This indicated that it is possible to reconstitute the recruitment of dynamin from the cytosol onto the membranes of permeabilized cells. Given our result that the SH3 domain of amphiphysin can inhibit both the late stages of coated pit invagination (measured by the avidin inaccessibility assay) and clathrin-coated vesicle scission (measured by MesNa resistance), we were interested to know whether adding additional dynamin directly into assay mixes could stimulate B-SS-Tfn sequestration. Therefore, we measured the effects of purified dynamin in the endocytic assays. Fig. 4 a shows a cytosol titration in the presence of purified dynamin in the avidin inaccessibility assay. There is a significant stimulation of ligand sequestration. The maximum stimulation with dynamin in the avidin inaccessibility assay was observed at high cytosol concentrations and half-maximal stimulation was seen at ∼0.3 μM dynamin (Fig. 4 b). In contrast, addition of purified dynamin to the MesNa resistance assay had no significant effect on transferrin internalization into sealed coated vesicles (Fig. 4 b). Exogenously added dynamin can therefore stimulate the formation of deeply invaginated coated pits in a cytosol-dependent manner but has no effect on coated vesicle scission in permeabilized A431 cells. Thus, dynamin cannot rescue coated vesicle scission in GST–amph2 SH3D36R treated membranes nor can it stimulate this stage in untreated membranes.
Figure 4.
Exogenously added dynamin stimulates the late stages of invagination but not scission in permeabilized A431 cells. (a) B-SS-Tfn sequestration and internalization were measured in the avidin inaccessibility assay in the presence of increasing amounts of cytosol as indicated, in the presence or absence of 5 μg of purified dynamin. Results are from a typical experiment where each assay point was carried out in duplicate which differed from each other <10%. (b) B-SS-Tfn sequestration and internalization measured by the avidin inaccessibility assay (filled squares) and internalization measured by the MesNa resistance assay (filled diamonds) were analyzed in the presence of cytosol (2.5 mg/ml) in the presence of increasing amounts of dynamin. Results are from a typical experiment where each assay point is the mean of duplicates which differed by <10%.
The SH3 Domain of Endophilin Inhibits Clathrin-coated Vesicle Formation
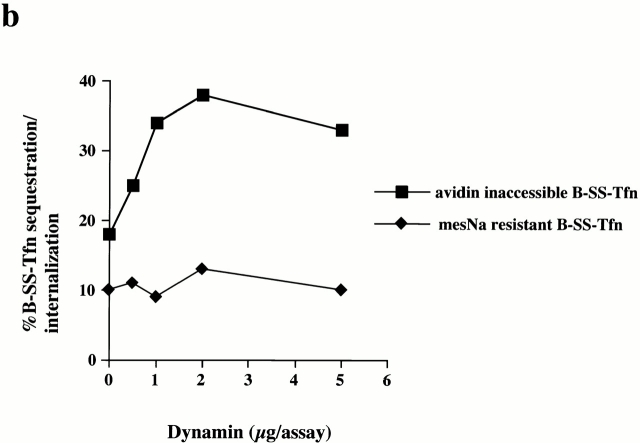
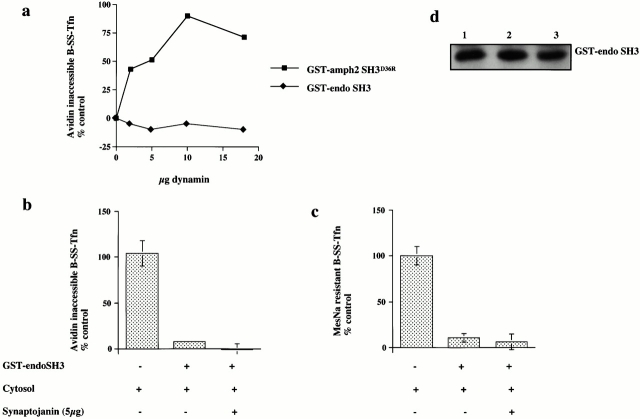
The SH3 domain of endophilin (GST–endoSH3) also binds dynamin, although synaptojanin is its major binding partner (Micheva et al. 1997; Ringstad et al. 1997). Given the specificity of the inhibition of GST–amph2 SH3D36R on invagination, we were interested to see if the SH3 domain of endophilin might also reveal some of the molecular and temporal requirements for invagination and scission. Addition of the SH3 domain of endophilin resulted in a dose-dependent inhibition of both ligand sequestration and internalization (Fig. 5 a). As with the SH3 domain of amphiphysin, we were interested to see if the target of the SH3 domain of endophilin was at the membrane or in the cytosol. The SH3 domain of endophilin can effectively deplete both synaptojanin and, to a lesser extent, dynamin, from cytosol (Fig. 5b and Fig. c). Mock-treated and GST–endoSH3-depleted cytosols were equally efficient at supporting invagination and scission as measured by the avidin inaccessibility and MesNa resistance assays (Fig. 5 c) as was untreated cytosol (data not shown). Similar to the effect of the SH3 domain of amphiphysin, preincubation of the membranes with the SH3 domain of endophilin resulted in a complete inhibition of both transferrin sequestration and internalization (Fig. 5d and Fig. e). However, in contrast to the inhibitory effect of the SH3 domain of amphiphysin, it was not possible to rescue the inhibitory effects of the SH3 domain of endophilin with purified dynamin even at high concentrations (Fig. 6 a; compare with Fig. 2b and Fig. c). Purified synaptojanin was also unable to rescue the inhibitory effects of GST–endoSH3 in either the avidin inaccessibility or MesNa resistance assay (Fig. 6b and Fig. c). Western blot analysis of membranes pretreated with GST–endoSH3 showed that subsequent incubation in the presence of cytosol and either dynamin or synaptojanin had no effect on the amount of SH3 domain associated with the membranes compared with the amount associated with cytosol alone (Fig. 6 d).
Figure 5.
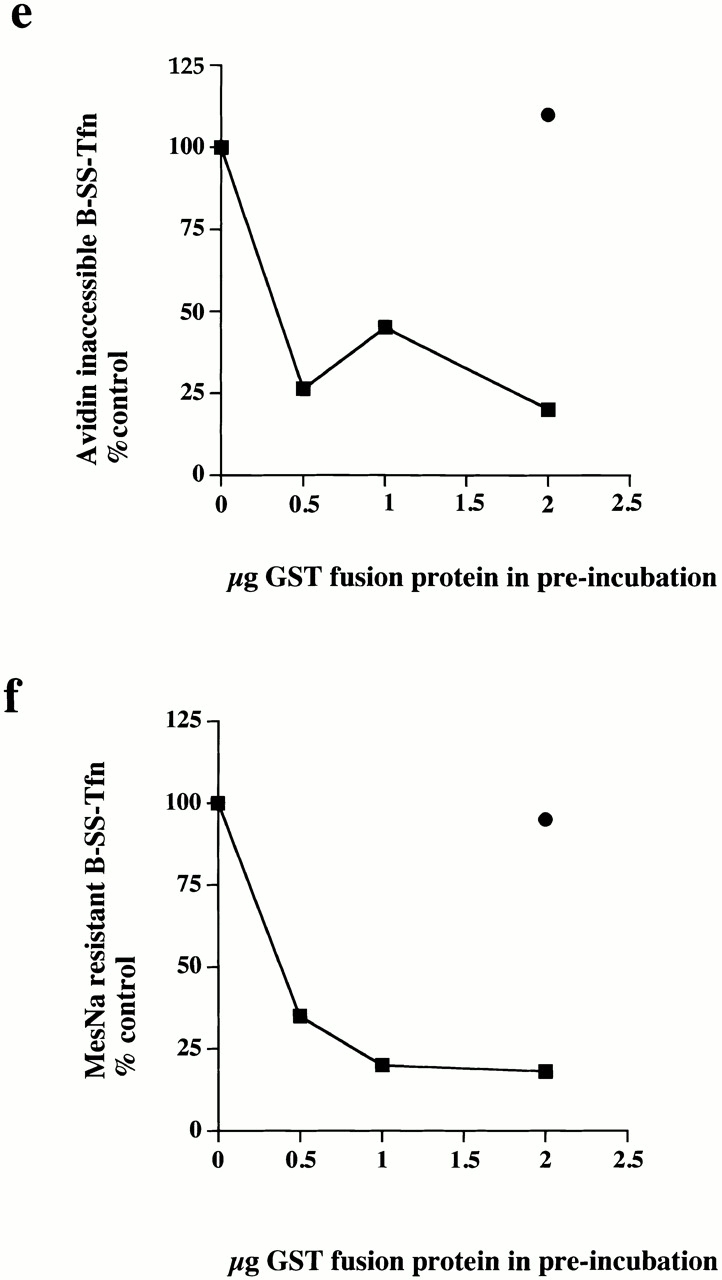
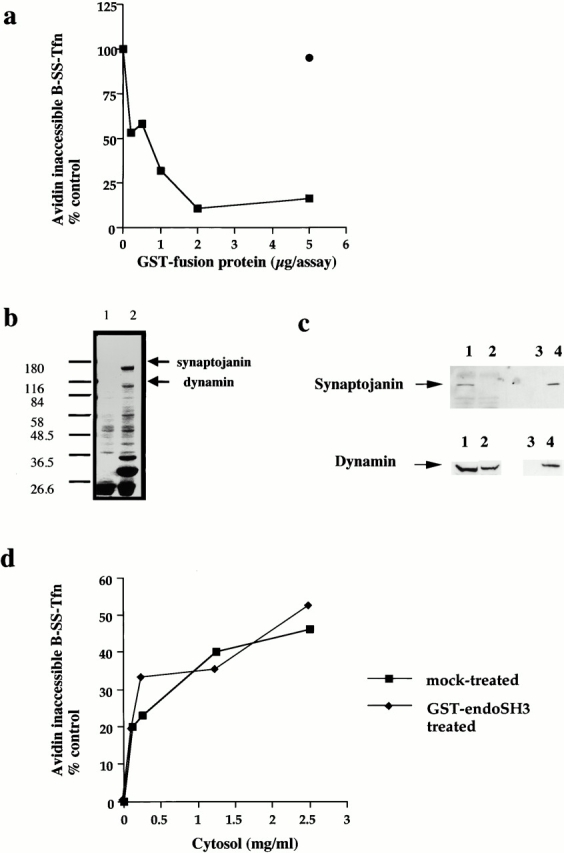
The SH3 domain of endophilin inhibits ligand sequestration and internalization in permeabilized A431 cells. (a) The sequestration of B-SS-Tfn into deeply invaginated coated pits and its internalization into coated vesicles were measured using the avidin internalization assay in the presence of the indicated concentrations of GST fusion proteins containing GST alone (filled circles) or GST–endoSH3 (filled squares). Results are from a typical experiment where each assay point is the mean of duplicates which differed by <10%. (b) Bovine brain cytosol (500 μl of 10 mg/ml) was treated with glutathione-agarose alone or coupled to the GST–endoSH3 fusion proteins for 2 h at 4°C. The beads were pelleted and washed three times in PBS before being electrophoresed on a 10% SDS-PAGE and Coomassie stained. Lane 1, GSH beads alone; lane 2, GST–endoSH3 beads. (c) Western blot of cytosol after treatment with GST–endoSH3. Cytosol was treated as described in b and equivalent volumes of supernatant (lanes 1 and 2) or beads (lanes 3 and 4) were probed on Western blots using either antidynamin or antisynaptojanin antibodies. (c) The avidin inaccessibility assay was carried out in the presence of increasing concentrations of bovine brain cytosol which had been treated with GSH beads alone (filled squares) or with GSH beads coupled to GST–endoSH3 (filled diamonds). (e and f) Permeabilized cells were preincubated with GST (filled circles) or GST–endoSH3 (filled squares) at the indicated concentrations for 5 min at 30°C. The membranes were then pelleted by centrifugation and assayed either for avidin inaccessibility (e) or MesNa resistance (f) of B-SS-Tfn in the presence of cytosol (2.5 mg/ml) and ATP.
Figure 6.
Both synaptojanin and dynamin are unable to rescue the inhibitory effects of GST–endoSH3. (a) Permeabilized cells which had been preincubated with either GST–amph2 SH3D36R (filled squares) or GST–endoSH3 (filled diamonds) for 5 min at 30°C were collected by centrifugation and assayed in the presence of ATP, cytosol (2.5 mg/ml), and increasing concentrations of dynamin for the ability to support B-SS-Tfn sequestration using the avidin inaccessibility assay. (b and c) Permeabilized cell membranes were preincubated in the presence or absence of GST–endoSH3 (0.1 mg/ml) for 5 min. They were then collected by centrifugation and assayed in the presence of ATP, cytosol (2.5 mg/ml), and synaptojanin (5 μg) as indicated for the ability to support avidin inaccessibility (b) or MesNa resistance (c) of B-SS-Tfn. Results are expresssed as the mean ± SD of two experiments each performed in duplicate. (d) Permeabilized membranes which had been preincubated in the presence of GST–endoSH3 and then subsequently incubated for 30 min in the presence of ATP and cytosol (lanes 1–3), plus dynamin (5 μg) (lane 2), plus synaptojanin (5 μg) (lane 3) were solubilized and electrophoresed on a 10% SDS gel, transferred to nitrocellulose, and probed for the presence of GST–endoSH3 using anti-GST antibodies.
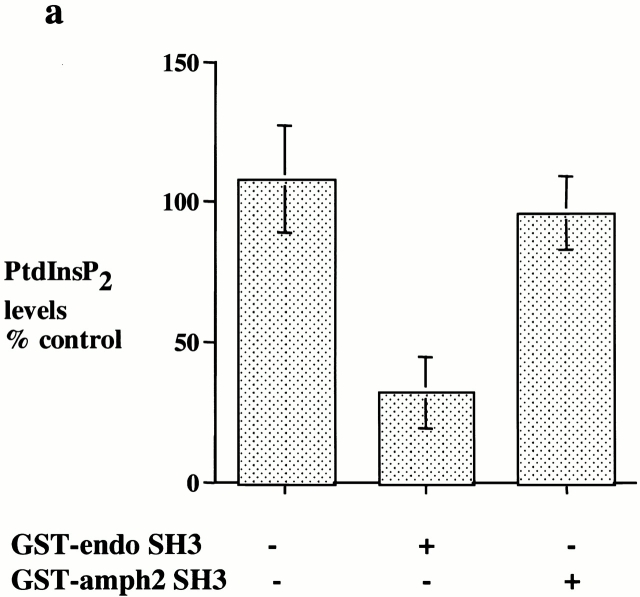
Synaptojanin is an inositol 5-phosphatase whose major in vivo substrates are likely to be PtdInsP2 and PtdInsP3 (Woscholski et al. 1997). Recently, a synaptojanin knockout mouse has been generated by de Camilli and colleagues (Cremona et al. 1999). Neurons isolated from these knockout mice exhibit elevated levels of PtdInsP2 and an accumulation of clathrin-coated vesicles, which implies a role for synaptojanin in uncoating clathrin-coated vesicles. To address whether the SH3 domain of endophilin might act by interfering with the biological function of synaptojanin, we investigated if the SH3 domain of endophilin affected PtdInsP2 or PtdInsP3 levels in the permeabilized cells. Treatment of the permeabilized cells with the SH3 domain of endophilin had no effect on the levels of PtdInsP3 (data not shown), but the levels of PtdInsP2 were reduced by >60% by the addition of the SH3 domain of endophilin while the SH3 domain of amphiphysin had no effect (Fig. 7 a). These results strongly suggest that the SH3 domain of endophilin may inhibit coated pit invagination and scission by mislocalizing synaptojanin or by activating synaptojanin at an inappropriate site resulting in a reduction of PtdInsP2 levels.
Figure 7.
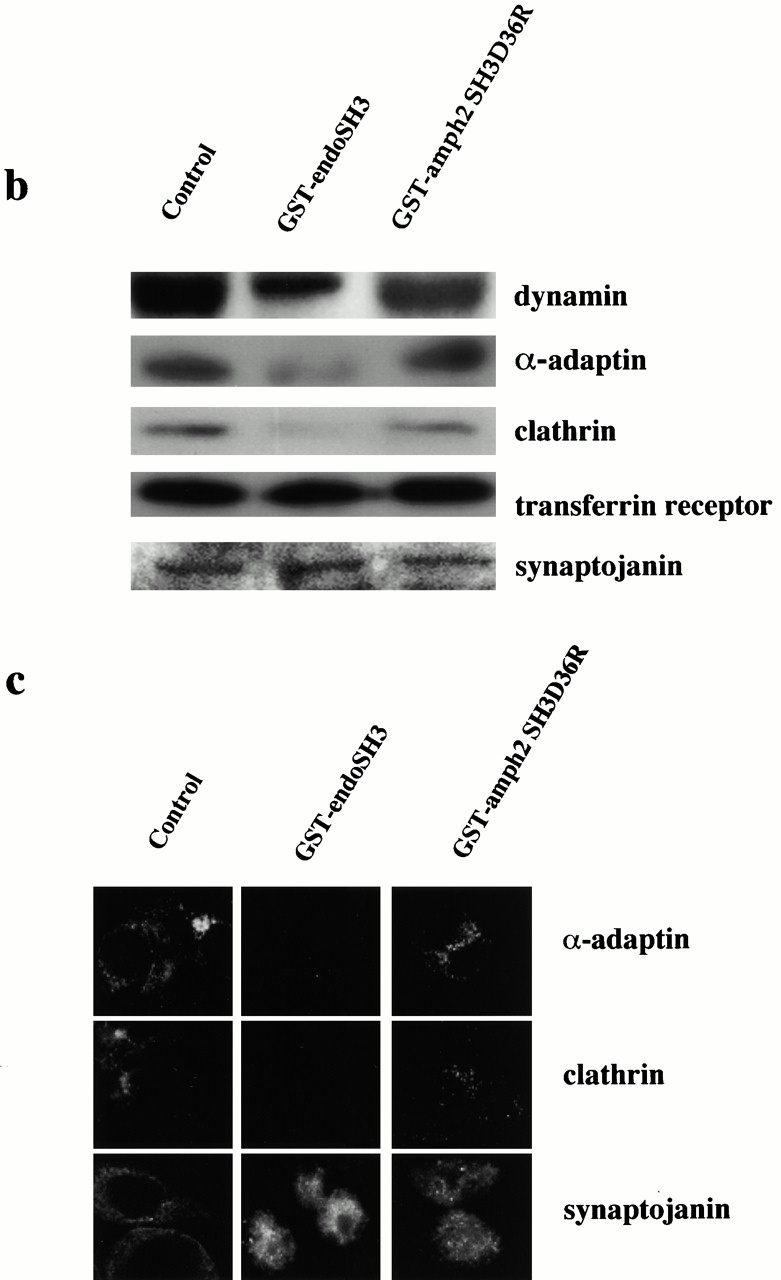
The SH3 domain of endophilin inhibits invagination and scission by reducing PtdInsP2 levels with a concomitant loss of coated pit components from the plasma membrane. (a) Permeabilized cell membranes which had been incubated with cytosol (2.5 mg/ml) and ATP for 30 min at 37°C in the presence or absence of GST–endoSH3 or GST–amph2 SH3D36R (25 μg/ml) were collected by centrifugation and assayed for PtdInsP2 levels. Results are expressed as the mean ± SD of two different experiments each performed in triplicate. (b) Western blots of the permeabilized cell membranes obtained from assay mixtures which had been incubated in the absence or presence of GST–endoSH3 or GST–amph2 SH3 D36R. The membranes were probed for α-adaptin, clathrin, dynamin, synaptojanin, and transferrin receptor as indicated. (c) Permeabilized cells which had been incubated with cytosol (2.5 mg/ml), ATP, and in the absence or presence of GST–endoSH3 or GST–amph2 SH3D36R were centrifuged onto coverslips and analyzed by indirect immunofluorescence for the presence of α-adaptin, clathrin, and dynamin.
Since PtdInsP2 has been implicated in the membrane association of both AP2 and dynamin (Achiriloaie et al. 1999; Lee et al. 1999; Vallis et al. 1999), we examined whether the reduction in PtdInsP2 altered the association of these and other components of the coated pit with the plasma membrane. Control permeabilized cell membranes and those which had been incubated in the presence of GST–endoSH3 or GST–amph2 SH3D36R were analyzed by Western blotting with antibodies specific for AP2, dynamin, clathrin, synaptojanin, and transferrin receptor. Fig. 7 b shows that treatment with GST–endoSH3 compared with control cells results in a significant loss of immunoreactivity to dynamin, AP2, and clathrin while there is no significant loss of immunoreactivity to synaptojanin or transferrin receptor. Treatment with GST–amph2 SH3D36R causes a slight reduction in both dynamin and clathrin immunoreactivity. Quantitative Western blotting revealed that this was no more than 5–10% (data not shown). We confirmed the Western blot results by immunofluorescence using antibodies against α-adaptin, clathrin, and synaptojanin (Fig. 7 c). Consistent with the results of the Western blots, treatment of permeabilized cells with GST–endoSH3 resulted in a loss of the characteristic punctate staining of clathrin and AP2 which was evident in both the control samples and those treated with GST–amph2 SH3D36R. Again consistent with the results of the Western blots, membrane association of synaptojanin was unaltered by either of the SH3 domains. These results indicate a direct link between PtdInsP2 levels and AP2, dynamin, and indirectly, clathrin membrane association.
Discussion
The formation of clathrin-coated pits requires an array of structural and regulatory proteins. Key issues concern the precise mechanism of action of these components and how they work together to effect coated pit assembly and coated vesicle formation. In this paper we have used the SH3 domains of two proteins implicated in endocytosis, amphiphysin and endophilin, as tools to investigate the nature of these interactions. Using the SH3 domain of amphiphysin, we have analyzed the requirement for dynamin during the late stages of clathrin-coated pit invagination and coated vesicle scission in permeabilized A431 cells. We demonstrate that dynamin is required not only for scission of coated vesicles but also for the late stages of clathrin-coated pit invagination. Using the SH3 domain of endophilin, we demonstrate that this domain is sufficient to cause a dramatic reduction in PtdInsP2 levels and a concomitant inhibition of ligand sequestration and internalization.
The Role of Dynamin in Invagination and Scission
The SH3 domain of amphiphysin inhibits both ligand sequestration and internalization. We define ligand sequestration as the loss of accessibility of B-SS-Tfn to exogenously added avidin. The deeply invaginated structures which sequester B-SS-Tfn have not yet pinched off because the biotinylated ligand is still sensitive to the small membrane-impermeant reducing agent MesNa. By contrast, B-SS-Tfn which is internalized into coated vesicles is inaccessible to avidin and also resistant to MesNa reduction. The SH3 domain of amphiphysin interacts strongly with dynamin and our results show that the inhibitory effects result from interactions with membrane-associated dynamin. The inhibitory effects of the SH3 domain of amphiphysin are consistent with results in intact cells where transfection of the SH3 domain into fibroblasts (Wigge et al. 1997) or injection into lamprey synapses (Shupliakov et al. 1997) resulted in an inhibition of receptor-mediated endocytosis via clathrin-coated pits. Injection of the SH3 domain of amphiphysin into lamprey synapses resulted in an accumulation of invaginated coated pits many of which still have a clearly discernible “neck.” Given that this SH3 domain in the permeabilized A431 cell system causes an increase in the accessibility of avidin to B-SS-Tfn, it would be interesting to know if these coated pits might represent a stage of invagination in which receptor-bound ligand is still accessible to avidin. In permeabilized A431 cells which have been preincubated with GST–amph2 SH3D36R, Western blotting reveals that the bulk of dynamin remains associated with the membranes (Fig. 7 b), indicating that GST–amph2 SH3D36R acts by binding to dynamin on the membrane and preventing it from performing its biological function.
The major binding partners of the SH3 domain of amphiphysin are dynamin and synaptojanin, but the inhibitory effects of GST–amph2 SH3D36R on the avidin inaccessibility assay were only rescued by purified dynamin and not by synaptojanin. Rescue is not apparently due to displacement of the inhibitory fusion protein. A more likely possibility is that dynamin is being recruited directly to deeply invaginating coated pits and so compensates for nonfunctional dynamin already on the membrane. It has been proposed (Shupliakov et al. 1997) that the effect of the SH3 domain is to block the recruitment of dynamin since after injection of this domain there was no electron-dense collar as is observed in the shibire mutant of Drosophila at the nonpermissive temperature. This electron-dense collar is thought to be composed of dynamin although this has not yet been formally demonstrated. Therefore, it is likely that recruitment of dynamin to the neck of pits is blocked when the permeabilized cells are preincubated with the SH3 domain of amphiphysin. Another possibility is that it interferes with the assembly/disassembly of dynamin as was demonstrated for the SH3 domain in vitro, thus preventing the formation of a dynamin collar (Owen et al. 1998). The ability of dynamin rather than synaptojanin to rescue coated pit invagination indicates that the inhibitory effect of the SH3 domain of amphiphysin is specific for dynamin.
Purified dynamin, however, is unable to rescue the inhibitory effects of GST–amph2 SH3D36R in the MesNa resistance assay. This suggests that for coated vesicle scission, the SH3 domain of amphiphysin has a dominant negative effect. This is consistent with our previous studies which indicated that the population of coated pits that are capable of pinching off in A431 cells is “primed” for scission and has many of the components required for the late stages of coated vesicle budding already assembled (Schmid and Smythe 1991; Smythe et al. 1992). The dominant negative effect of the SH3 domain on the MesNa resistance assay may operate in one of two ways. It is possible that GST–amph2 SH3D36R acts directly to interfere with dynamin function. Alternatively, it may exert its inhibitory effects by blocking access of other binding partners of dynamin, e.g., full length endophilin, required for the final scission step. However, because we were unable to rescue the inhibitory effects of GST–amph2 SH3D36R with concentrated cytosol, dynamin, or synaptojanin, we cannot rule out the possibility that there is another target of this SH3 domain in addition to dynamin that is essential for the final scission step.
The requirement for dynamin to execute both the late stages of invagination and scission is consistent with experiments in cells transfected with GTP binding domain mutants of dynamin where endocytosis was blocked after coat assembly and preceding the sequestration of ligand into deeply invaginated coated pits (van der Bliek et al. 1993). Similarly the GED (GTPase effector domain) of dynamin inhibits B-SS-Tfn as measured by the avidin inaccessibility assay in permeabilized A431 cells (Sever et al. 1999). Our results are also consistent with studies on the assembly of dynamin onto liposomes which indicate that dynamin can tubulate liposomes and cause them to evaginate (Sweitzer and Hinshaw 1998; Takei et al. 1998).
Consistent with the rescue experiments described above, the addition of purified dynamin to untreated permeabilized cells resulted in a stimulation of the avidin inaccessibility assay by 10–15%. This increase in the avidin inaccessibility signal likely corresponds to the increased invagination of a population of coated pits where dynamin is limiting and which, in the presence of exogenously added dynamin, can become sufficiently invaginated to confer avidin inaccessibility. Although the addition of exogenous dynamin can increase the extent of invagination of these pits, it is insufficient to cause them to pinch off and therefore become MesNa resistant. This is again consistent with the idea that those pits which are capable of pinching off have already assembled all of the essential components. However, the inhibitory effect of the SH3 domain of amphiphysin indicates that, as expected, dynamin function appears still to be required for the late stages of coated scission. Binding of the SH3 domain interferes with this function.
GTP Binding and Hydrolysis Are Required for Coated Pit Invagination
The rescue by dynamin is dependent on the ability of dynamin to bind GTP. In contrast to the ability of wild-type dynamin to rescue the inhibitory effects of the SH3 domain of amphiphysin, neither S45N nor K44A dynamin was capable of rescue. The S45N mutant is deficient in the ability to bind GTP. The K44A mutant, although considered as an hydrolysis mutant, also has reduced binding of GTP (van der Bliek et al. 1993). However, even in the presence of high concentrations of GTP (500 μM), the K44A mutant failed to rescue the inhibitory effects of the amphiphysin SH3 domain (data not shown). Surprisingly, mutant dynamin deficient in the ability to hydrolyze GTP (T65A) was also unable to rescue the inhibitory effects of the SH3 domain of amphiphysin. This implies that dynamin must bind and hydrolyze GTP in order to promote the formation of deeply invaginated coated pits in vitro which are inaccessible to avidin. Our in vitro results were confirmed by studies in HEK293 cells overexpressing wild-type and mutant forms of dynamin. Overexpression of both dynamin K44A and dynamin T65A resulted in an inhibition of B-SS-Tfn uptake as measured by both the avidin inaccessibility and MesNa resistance assays. It has previously been demonstrated that these assays can distinguish between the formation of deeply invaginated coated pits and pinched off coated vesicles in intact cells (Schmid and Carter 1990). These results are in contrast to recent results reporting an increase in the uptake of transferrin on overexpression of mutant dynamin deficient in GTP hydrolysis and assembly (Sever et al. 1999, Sever et al. 2000a). Using dynamin which has point mutations in the GED (GTPase effector domain), these workers concluded that dynamin functions as a classical GTPase, recruiting effectors when in the GTP conformation. Our results indicate, however, that GTP hydrolysis by dynamin is required for the late stages of invagination. Our data are therefore most consistent with a ratchet model of dynamin action (Smirnova et al. 1999) whereby dynamin molecules would interact with those molecules on an adjacent rung of the dynamin collar. The energy of GTP hydrolysis would then be harnessed to the movement of the dynamin molecules resulting in an increase in constriction which would result in an increase in avidin inaccessibility in our system (Fig. 8). The action of dynamin would thus be akin to that of kinesin and myosin (Vale 1996; Smirnova et al. 1999).
Figure 8.
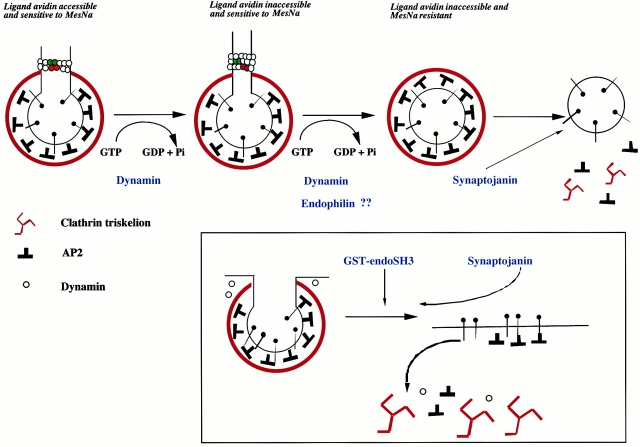
Model of the role of dynamin and some of its binding partners in clathrin-coated vesicle formation. In this model, dynamin is recruited to participate in both the late stages of invagination and scission. GTP binding and hydrolysis by dynamin are required to form a deeply invaginated coated pit. Dynamin may increase the invagination of a coated pit such that avidin is excluded in a manner analogous to a ratchet where the energy of GTP hydrolysis is used to promote movement. This is indicated by the red dynamin molecules ratchetting over the green ones and causing a constriction of the neck. During invagination and scission, dynamin may recruit/interact with other binding partners, e.g., endophilin. After scission, endophilin may undergo a conformational change which would activate synaptojanin. The insert shows that addition of the SH3 domain of endophilin causes an inhibition of endocytosis by reducing PtdInsP2 levels, which results in the dissociation of AP2 from the membrane. The drop in PtdInsP2 levels may result from a mistiming in the activation of synaptojanin.
Despite the stimulatory effect of dynamin in the avidin inaccessibility assay, depletion of dynamin from bovine brain cytosol had no significant effect on the extent of avidin inaccessibility. This suggests either that the concentration of dynamin (0.1 μM) present at the highest concentration of cytosol used was too little to have a significant effect or, alternatively, that in the preparation of bovine brain cytosol used the dynamin is somehow inactivated. It has recently been reported that several SH3 domains inhibit clathrin-mediated endocytosis in permeabilized 3T3L1 cells (Simpson et al. 1999). However, in contrast to our results, the SH3 domains of amphiphysin and endophilin only inhibited scission in this cell line. This study also showed that depletion of dynamin from cytosol inhibited scission as measured by the MesNa resistance assay in permeabilized 3T3L1 cells. The reasons for these differences are unknown, but one possible explanation for the discrepancies may be that the cytosol preparation used by Simpson and colleagues contains an active pool of dynamin. If this were the case then this cytosol might be capable of overcoming the inhibitory effects of the amphiphysin and endophilin SH3 domains on invagination. This would also explain why the inhibitory effects of these SH3 domains in 3T3L1 cells were only observed after preincubation of the permeabilized cell membranes and with concentrations of SH3 domains that are 10-fold higher than those used in this study.
The current study does not allow us to draw any conclusions about the mechanism of action of dynamin in scission. As discussed above, the specificity of the inhibitory effects of GST–amph2 SH3D36R on invagination and its similar concentration-dependent inhibition of scission lead us to conclude that it is required for scission, as has been shown by many other laboratories (Takei et al. 1995; Sweitzer and Hinshaw 1998; Stowell et al. 1999). Although the A431 permeabilized cell system has several limitations for the study of scission, these limitations actually provide an advantage for the study of the formation of deeply invaginated coated pits since they allow us to dissociate the two events.
The SH3 Domain of Endophilin Modulates PtdInsP2 Levels at the Plasma Membrane of Permeabilized Cells
The SH3 domain of endophilin also inhibited both deep invagination and scission in permeabilized A431 cells. The major physiological binding partner for endophilin is synaptojanin although it also binds dynamin to a lesser extent (Micheva et al. 1997; Ringstad et al. 1997). Synaptojanin was unable to rescue the inhibitory effects of GST–endoSH3 in either the avidin inaccessibiltiy or MesNa resistance assays. In contrast to the inhibitory effect of the SH3 domain of amphiphysin, dynamin (at concentrations up to twofold higher than that required for full rescue of GST–amph2 SH3D36R) was also unable to rescue the inhibitory effects of the SH3 domain of endophilin.
Synaptojanin is an inositol 5-phosphatase whose principal substrates are PtdInsP2 and PtdInsP3 (Woscholski et al. 1997). There is no significant effect on the levels of PtdInsP3 in permeabilized cells treated with GST–endoSH3 (data not shown). However, the presence of GST–endoSH3 resulted in a dramatic reduction in PtdInsP2 levels. The reduction in PtdInsP2 levels was specific for GST–endoSH3 since GST–amph2 SH3D36R had no effect. Phosphoinositides have been implicated in the coated vesicle cycle in the binding of both AP2 and dynamin to the plasma membrane. AP2 can bind a variety of phospholipids and its biological activity is modulated by these interactions. For example, PtdInsP2 and PtdInsP3 enhance the binding of AP2 to internalization motifs, and the enhancement is comparable to the binding of AP2 to receptor tails within assembled coats although the latter is independent of phosphoinositides (Gaidarov et al. 1996; Rapoport et al. 1997). A phosphoinositide binding site on the α-subunit of AP2 has been identified which is essential for correct targeting of AP2 to clathrin-coated pits at the plasma membrane (Gaidarov and Keen 1999). The PH domain of PLCδ1, which specifically binds PtdInsP2, has been shown to interfere with ligand sequestration and internalization in permeabilized A431 cells (Jost et al. 1998). In this study, we demonstrate that GST–endoSH3 reduced PtdInsP2 levels by >60%, leading to the loss of AP2 from the permeabilized cell membranes and a concomitant inhibition of ligand sequestration and internalization. Similarly, the pleckstrin homology domain of dynamin which interacts with membrane phospholipids has been shown to be essential for endocytosis (Achiriloaie et al. 1999; Lee et al. 1999; Vallis et al. 1999), and interestingly this interaction is dependent on its oligomeric state (Lee et al. 1999). GST–endoSH3-induced reduction of PtdInsP2 levels also leads to a loss of dynamin from the permeabilized cell membranes, providing further evidence of the requirement for PtdInsP2 in the association of dynamin with membranes. Treatment of the permeabilized cells with GST–endoSH3 also caused a loss in membrane-associated clathrin, measured both by Western blotting and by immunofluorescence. This presumably is an indirect effect of the loss of AP2 from the plasma membrane and further confirms the requirement for PtdInsP2 in coated pit assembly. By contrast, there is no significant loss of membrane-associated synaptojanin or transferrin receptor after treatment with either of the SH3 domains, indicating that the reduction in PtdInsP2 levels has a specific effect on clathrin-coated pit components and is not due to gross disruption of membranes. GST–amph2 SH3D36R caused a small reduction in the amount of clathrin and dynamin associated with the membranes, but the reduction was much less than that observed in the presence of GST–endoSH3. We have estimated that the amount lost is no more than 5–10% of the total.
Synaptojanin was unable to rescue the inhibitory effects of either of the SH3 domains. The inability of synaptojanin to rescue GST–amph2 SH3D36R treated membranes further demonstrates the specificity of the rescue of these membranes by dynamin, indicating that dynamin is the true target for this SH3 domain. The lack of rescue of GST–endoSH3 treated membranes is consistent with our results, showing that these membranes have reduced PtdInsP2 levels. Since synaptojanin, if recruited to the membrane, would be predicted to itself cause a reduction in PtdInsP2 levels, the most likely mechanism by which it could rescue the inhibitory effects of the SH3 domain would be by competition of the SH3 domain from the membrane. Western blots using anti-GST antibodies revealed that there was no significant reduction in the amount of GST–endoSH3 associated with the membrane in the presence of either dynamin or synaptojanin. It is worth noting that we have estimated that we are adding a molar excess of at least 100-fold of dynamin and synaptojanin over the amount of SH3 domain associated with the membrane. This suggests that the fusion proteins may either form a tighter association with membrane rather than cytosolic components or, alternatively, be incorporated into a relatively stable complex.
Recent data using a mouse knockout for synaptojanin have strongly implicated synaptojanin as having a role in uncoating of clathrin-coated vesicles (Cremona et al. 1999). Cortical neurons from the knockout mouse exhibited increased levels of clathrin-coated vesicles and also of PtdInsP2, providing a link between the requirement to hydrolyze PtdInsP2 and uncoating. In Caenorhabditis elegans, mutations in synaptojanin lead to pleiotropic defects in vesicle trafficking including synaptic vesicle budding and uncoating (Harris et al. 2000). Our data are most consistent with a model where it appears that the SH3 domain of endophilin causes an untimely reduction in PtdInsP2 levels, most likely by mislocalization and/or activation of synaptojanin (Fig. 8). Western blotting of membranes separated from assay mixtures which had been incubated in the presence or absence of GST–endoSH3 showed no significant recruitment of recombinant synaptojanin by GST–endoSH3 (data not shown). Similarly, addition of recombinant synaptojanin had only a very modest inhibitory effect on coated pit invagination and scission and caused a similar modest reduction in PtdInsP2 levels (data not shown). This strongly suggests that the functional pool of synaptojanin in permeabilized cells is associated with the membrane, and its untimely activation prevents coat assembly and invagination and probably scission (see below). However, we would predict that normally synaptojanin works later in the endocytic cycle, in the uncoating of coated vesicles.
This hypothesis is supported by recent studies carried out in parallel with ours (Gad et al. 2000). These workers showed that microinjection of antisynaptojanin antibodies and also of a peptide which specifically interferes with the interaction of endophilin with synaptojanin and dynamin caused an accumulation of coated vesicles in the lamprey synapse. Microinjection of the peptide and also GST–endoSH3 caused an accumulation of coated pits, but interestingly, synapses microinjected with GST–endoSH3 showed no uncoating defect. These results suggest that the interaction between endophilin and synaptojanin is important for uncoating while SH3-mediated effects of endophilin (perhaps by interaction with dynamin) are necessary for scission (Schmidt et al. 1999). In our permeabilized cell system, GST–endoSH3 has inhibitory effects on both invagination and scission. The inhibition appears to result principally from a loss of coat components from the membrane as a result of the reduction in PtdInsP2 levels. However, we cannot rule out the possibility that the inhibition of scission is a direct effect on this process as is observed in the lamprey synapse. The differential effects of GST–endoSH3 seen in the lamprey synapse versus permeabilized A431 cells are most likely due to compensatory mechanisms within the synapse which are lost in permeabilized cells and perhaps allow a replenishment of PtdInsP2 levels. Such mechanisms appear to exist in vivo to maintain the levels of essential phosphoinositides because, for example, the increase in PtdInsP2 in the synaptojanin knockout mouse, although significant, is only 1.6-fold (Cremona et al. 1999).
In summary, we have shown that dynamin, in addition to its well-established role in scission, is also necessary for the late stages of coated pit invagination. Its function in invagination is dependent on GTP binding and hydrolysis, pointing, at the very least, to a mechanochemical role. However, our findings do not exclude the possibility of additional regulatory components in this process. Our results with the SH3 domain of endophilin indicate the importance of PtdInsP2 in the recruitment of AP2 and dynamin to the membrane to initiate coated vesicle formation.
Acknowledgments
We are indebted to Harvey McMahon (Laboratory of Molecular Biology, Cambridge, UK) for his generous provision of constructs without which much of this work would not have been possible. We would especially like to acknowledge him for his first identification of T65A as a GTPase-defective mutant and also for numerous lively and stimulating discussions throughout these studies. We thank Prof. Colin Hopkins (Imperial College, London, UK) for providing antitransferrin receptor monoclonal antibody, HTR 68.4. We thank Oyinkan Olusanya for excellent technical assistance, Agnes Saint-Pol, Andrew Osborne, and Alex Flett for useful discussions, and Colin Watts for critical reading of the manuscript.
This work was supported by the Medical Research Council (E. Smythe and C.P. Downes). E. Smythe is a Medical Research Council Senior Research Fellow. E. Hill is the recipient of a Medical Research Council PhD studentship. E. Smythe and C.P. Downes are members of the Dundee Eukaryotic Membrane Dynamics Co-operative Group.
Footnotes
Abbreviations used in this paper: B-SS-Tfn, biotinylated transferrin; GST; glutathione S-transferase; HEK, human embryonic kidney; PRD, proline/argine-rich domain; PtdInsP2, phosphoinositide 4,5-bisphosphate; SH, Src homology.
References
- Achiriloaie M., Barylko B., Albanesi J.P. Essential role of the dynamin pleckstrin homology domain in receptor-mediated endocytosis. Mol. Cell. Biol. 1999;19:1410–1415. doi: 10.1128/mcb.19.2.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmerah A., Lamaze C., Begue B., Schmid S.L., Dautry V.A., Cerf B.N. AP-2/Eps15 interaction is required for receptor-mediated endocytosis. J. Cell Biol. 1998;140:1055–1062. doi: 10.1083/jcb.140.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky F.M. Clathrin structure characterized by monoclonal antibodies. I. Analysis of multiple antigenic sites. J. Cell Biol. 1985;101:2047–2054. doi: 10.1083/jcb.101.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Fre S., Slepnev V.I., Capua M.R., Takei K., Butler M.H., Di F.P., De Camilli P. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature. 1998;394:793–797. doi: 10.1038/29555. [DOI] [PubMed] [Google Scholar]
- Chilvers E.R., Batty I.H., Challiss R.A., Barnes P.J., Nahorski S.R. Determination of mass changes in phosphatidylinositol 4,5-bisphosphate and evidence for agonist-stimulated metabolism of inositol 1,4,5-trisphosphate in airway smooth muscle. Biochem. J. 1991;275:373–379. doi: 10.1042/bj2750373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin D.J., Straubinger R.M., Acton S., Nathke I., Brodsky F.M. 100-kda polypeptides in peripheral clathrin-coated vesicles are required for receptor-mediated endocytosis. Proc. Natl. Acad. Sci. USA. 1989;86:9289–9293. doi: 10.1073/pnas.86.23.9289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremona O., Di P.G., Wenk M.R., Luthi A., Kim W.T., Takei K., Daniell L., Nemoto Y., Shears S.B., Flavell R.A., McCormick D.A., De Camilli P. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell. 1999;99:179–188. doi: 10.1016/s0092-8674(00)81649-9. [DOI] [PubMed] [Google Scholar]
- Damke H., Baba T., Warnock D.E., Schmid S.L. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David C., McPherson P.S., Mundigl O., de Camilli P. A role of amphiphysin in synaptic vesicle recycling suggested by its binding to dynamin in nerve terminals. Proc. Natl. Acad. Sci. USA. 1996;93:331–335. doi: 10.1073/pnas.93.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad H., Ringstad N., Low P., Kjaerulff O., Gustafsson J., Wenk M., Di Paolo G., Nemoto Y., Crum J., Ellisman M.H. Fission and uncoating of synaptic clathrin-coated vesicles are perturbed by disruption of interactions with the SH3 domain of endophilin. Neuron. 2000;27:301–312. doi: 10.1016/s0896-6273(00)00038-6. [DOI] [PubMed] [Google Scholar]
- Gaidarov I., Keen J.H. Phosphoinositide–AP-2 interactions required for targeting to plasma membrane clathrin-coated pits. J. Cell Biol. 1999;146:755–764. doi: 10.1083/jcb.146.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidarov I., Chen Q., Falck J.R., Reddy J.K.K., Keen J.H. A functional phosphatidylinositol 3,4,5-trisphosphate/phosphoinositide binding domain in the clathrin adaptor AP-2 alpha subunit. Implications for the endocytic pathway. J. Biol. Chem. 1996;271:20922–20929. doi: 10.1074/jbc.271.34.20922. [DOI] [PubMed] [Google Scholar]
- Gout I., Dhand R., Hiles I.D., Fry M.J., Panayotou G., Das P., Truong O., Totty N.F., Hsuan J., Booker G.W. The GTPase dynamin binds to and is activated by a subset of SH3 domains. Cell. 1993;75:25–36. [PubMed] [Google Scholar]
- Harlow E., Lane D. AntibodiesA Laboratory Manual 1st ed 1988. Cold Spring Harbor Laboratory, ; Cold Spring Harbor, NY: pp. 721 pp [Google Scholar]
- Harris T.W., Hartweig E., Horvitz H.R., Jorgensen E.M. Mutations in synaptojanin disrupt synaptic vesicle recycling. J. Cell Biol. 2000;150:589–599. doi: 10.1083/jcb.150.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw J.E., Schmid S.L. Dynamin self-assembles into rings suggesting a mechanism for coated vesicle budding. Nature. 1995;374:190–192. doi: 10.1038/374190a0. [DOI] [PubMed] [Google Scholar]
- Jost M., Simpson F., Kavran J.M., Lemmon M.A., Schmid S.L. Phosphatidylinositol-4,5-bisphosphate is required for endocytic coated vesicle formation. Curr. Biol. 1998;8:1399–1402. doi: 10.1016/s0960-9822(98)00022-0. [DOI] [PubMed] [Google Scholar]
- Kelly R.B. New twists for dynamin. Nat. Cell Biol. 1999;1:E8–E9. doi: 10.1038/8957. [DOI] [PubMed] [Google Scholar]
- Kosaka T., Ikeda K. Reversible blockage of membrane retrieval and endocytosis in the garland cell of the temperature-sensitive mutant of Drosophila melanogaster, shibirets1 . J. Cell Biol. 1983;97:499–507. doi: 10.1083/jcb.97.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., Frank D.W., Marks M.S., Lemmon M.A. Dominant-negative inhibition of receptor-mediated endocytosis by a dynamin-1 mutant with a defective pleckstrin homology domain. Curr. Biol. 1999;9:261–264. doi: 10.1016/s0960-9822(99)80115-8. [DOI] [PubMed] [Google Scholar]
- McLauchlan H., Newell J., Morrice N., Osborne A., West M., Smythe E. A novel role for rab5-GDI in ligand sequestration into clathrin-coated pits. Curr. Biol. 1998;8:34–45. doi: 10.1016/s0960-9822(98)70018-1. [DOI] [PubMed] [Google Scholar]
- McNiven M.A. Dynamina molecular motor with pinchase action. Cell. 1998;94:151–154. doi: 10.1016/s0092-8674(00)81414-2. [DOI] [PubMed] [Google Scholar]
- Micheva K.D., Kay B.K., McPherson P.S. Synaptojanin forms two separate complexes in the nerve terminal. Interactions with endophilin and amphiphysin. J. Biol. Chem. 1997;272:27239–27245. doi: 10.1074/jbc.272.43.27239. [DOI] [PubMed] [Google Scholar]
- Owen D.J., Wigge P., Vallis Y., Moore J.D., Evans P.R., McMahon H.T. Crystal structure of the amphiphysin-2 SH3 domain and its role in the prevention of dynamin ring formation. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:5273–5285. doi: 10.1093/emboj/17.18.5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai E.F., Krengal U., Petsko G.A., Goody R.S., Kabsch W., Wittinghofer A. Refined crystal structure of the triphosphate conformation of H-ras p21 at 1.35A resolutionimplications for the mechanism of GTP hydrolysis. EMBO (Eur. Mol. Biol. Organ.) J. 1990;9:2351–2359. doi: 10.1002/j.1460-2075.1990.tb07409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport I., Miyazaki M., Boll W., Duckworth B., Cantley L.C., Shoelson S., Kirchhausen T. Regulatory interactions in the recognition of endocytic sorting signals by AP-2 complexes. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:2240–2250. doi: 10.1093/emboj/16.9.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringstad N., Nemoto Y., De Camilli P. The SH3p4/Sh3p8/SH3p13 protein familybinding partners for synaptojanin and dynamin via a Grb2-like Src homology 3 domain. Proc. Natl. Acad. Sci. USA. 1997;94:8569–8574. doi: 10.1073/pnas.94.16.8569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T. Molecular CloningA Laboratory Manual 2nd ed 1989. Cold Spring Harbor Laboratory, ; Cold Spring Harbor, NY: p. 18.88. [Google Scholar]
- Schmid S.L. Clathrin-coated vesicle formation and protein sortingan integrated process. Annu. Rev. Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- Schmid S.L., Carter L.L. ATP is required for receptor-mediated endocytosis in intact cells. J. Cell Biol. 1990;111:2307–2318. doi: 10.1083/jcb.111.6.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S.L., Smythe E. Stage-specific assays for coated pit formation and coated vesicle budding in vitro. J. Cell Biol. 1991;114:869–880. doi: 10.1083/jcb.114.5.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S.L., McNiven M.A., de Camilli P. Dynamin and its binding partnersa progress report. Curr. Opin. Cell Biol. 1998;10:504–512. doi: 10.1016/s0955-0674(98)80066-5. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Wolde M., Thiele C., Fest W., Kratzin H., Podtelejnikov A.V., Witke W., Huttner W.B., Soling H.D. Endophilin I mediates synaptic vesicle formation by transfer of arachidonate to lysophosphatidic acid. Nature. 1999;401:133–141. doi: 10.1038/43613. [DOI] [PubMed] [Google Scholar]
- Sever S., Muhlberg A.B., Schmid S.L. Impairment of dynamin's GAP domain stimulates receptor-mediated endocytosis. Nature. 1999;398:481–486. doi: 10.1038/19024. [DOI] [PubMed] [Google Scholar]
- Sever S., Muhlberg A.B., Schmid S.L. Dynamin:GTP controls the formation of constricted coated pits, the rate limiting step in clathrin-mediated endocytosis J. Cell Biol. 150 2000. 1137 1148a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sever S., Damke H., Schmid S.L. Garrotes, Springs, Ratchets, and Whipsputting dynamin models to the test Traffic. 1 2000. 385 392b [DOI] [PubMed] [Google Scholar]
- Shupliakov O., Low P., Grabs D., Gad H., Chen H., David C., Takei K., De Camilli P., Brodin L. Synaptic vesicle endocytosis impaired by disruption of dynamin-SH3 domain interactions. Science. 1997;276:259–263. doi: 10.1126/science.276.5310.259. [DOI] [PubMed] [Google Scholar]
- Simpson F., Hussain N.K., Qualmann B., Kelly R.B., Kay B.K., McPherson P.S., Schmid S.L. SH3-domain-containing proteins function at distinct steps in clathrin-coated vesicle formation. Nat. Cell Biol. 1999;1:119–124. doi: 10.1038/10091. [DOI] [PubMed] [Google Scholar]
- Smirnova E., Shurland D.L., Newman-Smith E.D., Pishvaee B., van der Bliek A.M. A model for dynamin self-assembly based on binding between three different protein domains. J. Biol. Chem. 1999;274:14942–14947. doi: 10.1074/jbc.274.21.14942. [DOI] [PubMed] [Google Scholar]
- Smith D.B., Johnson K.S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Smythe E. Endocytosis. Subcell. Biochem. 1996;27:51–92. doi: 10.1007/978-1-4615-5833-0_3. [DOI] [PubMed] [Google Scholar]
- Smythe E., Pypaert M., Lucocq J., Warren G. Formation of coated vesicles from coated pits in broken A431 cells. J. Cell Biol. 1989;108:843–853. doi: 10.1083/jcb.108.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythe E., Carter L.L., Schmid S.L. Cytosol-dependent and clathrin-dependent stimulation of endocytosis in vitro by purified adapters. J. Cell Biol. 1992;119:1163–1171. doi: 10.1083/jcb.119.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythe E., Smith P.D., Jacob S.M., Theobald J., Moss S.E. Endocytosis occurs independently of annexin-VI in human A431 cells. J. Cell Biol. 1994;124:301–306. doi: 10.1083/jcb.124.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell M.H., Marks B., Wigge P., McMahon H.T. Nucleotide-dependent conformational changes in dynaminevidence for a mechanochemical molecular spring. Nat. Cell Biol. 1999;1:27–32. doi: 10.1038/8997. [DOI] [PubMed] [Google Scholar]
- Sweitzer S.M., Hinshaw J.E. Dynamin undergoes a GTP-dependent conformational change causing vesiculation. Cell. 1998;93:1021–1029. doi: 10.1016/s0092-8674(00)81207-6. [DOI] [PubMed] [Google Scholar]
- Takei K., McPherson P.S., Schmid S.L., De Camilli P. Tubular membrane invaginations coated by dynamin rings are induced by GTP-gammaS in nerve terminals. Nature. 1995;374:186–190. doi: 10.1038/374186a0. [DOI] [PubMed] [Google Scholar]
- Takei K., Haucke V., Slepnev V., Farsad K., Salazar M., Chen H., De Camilli P. Generation of coated intermediates of clathrin-mediated endocytosis on protein-free liposomes. Cell. 1998;94:131–141. doi: 10.1016/s0092-8674(00)81228-3. [DOI] [PubMed] [Google Scholar]
- Takei K., Slepnev V.I., Haucke V., De Camilli P. Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat. Cell Biol. 1999;1:33–39. doi: 10.1038/9004. [DOI] [PubMed] [Google Scholar]
- Vale R.D. Switches, latches and amplifierscommon themes of G proteins and molecular motors. J. Cell Biol. 1996;135:291–302. doi: 10.1083/jcb.135.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallis Y., Wigge P., Marks B., Evans P.R., McMahon H.T. Importance of the pleckstrin homology domain of dynamin in clathrin-mediated endocytosis. Curr. Biol. 1999;9:257–260. doi: 10.1016/s0960-9822(99)80114-6. [DOI] [PubMed] [Google Scholar]
- van der Bliek A.M., Meyerowitz E.M. Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature. 1991;351:411–414. doi: 10.1038/351411a0. [DOI] [PubMed] [Google Scholar]
- van der Bliek A.M., Redelmeier T.E., Damke H., Tisdale E.J., Meyerowitz E.M., Schmid S.L. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J. Cell Biol. 1993;122:553–563. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kaay J., Cullen P.J., Downes C.P. Phosphatidylinositol(3,4,5)trisphosphate (Ptdins(3,4,5)P3) mass measurement using a radioligand displacement assay. Methods Mol. Biol. 1998;105:109–125. doi: 10.1385/0-89603-491-7:109. [DOI] [PubMed] [Google Scholar]
- Wang L.H., Sudhof T.C., Anderson R.G.W. The appendage domain of alpha-adaptin is a high affinity binding site for dynamin. J. Biol. Chem. 1995;270:10079–10083. doi: 10.1074/jbc.270.17.10079. [DOI] [PubMed] [Google Scholar]
- Wang Z., Moran M.F. Requirement for the adapter protein GRB2 in EGF receptor endocytosis. Science. 1996;272:1935–1939. doi: 10.1126/science.272.5270.1935. [DOI] [PubMed] [Google Scholar]
- Warnock D.E., Schmid S.L. Dynamin GTPase, a force-generating molecular switch. Bioessays. 1996;18:885–893. doi: 10.1002/bies.950181107. [DOI] [PubMed] [Google Scholar]
- Warnock D.E., Hinshaw J.E., Schmid S.L. Dynamin self-assembly stimulates its GTPase activity. J. Biol. Chem. 1996;271:22310–22314. doi: 10.1074/jbc.271.37.22310. [DOI] [PubMed] [Google Scholar]
- Wigge P., McMahon H.T. The amphiphysin family of proteins and their role in endocytosis at the synapse. Trends Neurosci. 1998;21:339–344. doi: 10.1016/s0166-2236(98)01264-8. [DOI] [PubMed] [Google Scholar]
- Wigge P., Vallis Y., McMahon H.T. Inhibition of receptor-mediated endocytosis by the amphiphysin SH3 domain. Curr. Biol. 1997;7:554–560. doi: 10.1016/s0960-9822(06)00254-5. [DOI] [PubMed] [Google Scholar]
- Woscholski R., Finan P.M., Radley E., Totty N.F., Sterling A.E., Hsuan J.J., Waterfield M.D., Parker P.J. Synaptojanin is the major constitutively active phosphatidylinositol-3,4,5-trisphosphate 5-phosphatase in rodent brain. J. Biol. Chem. 1997;272:9625–9628. doi: 10.1074/jbc.272.15.9625. [DOI] [PubMed] [Google Scholar]
- Yang W., Cerione R.A. Is dynamin a ‘blue collar’ or' ‘white collar’ worker? Curr. Biol. 1999;9:R511–R514. doi: 10.1016/s0960-9822(99)80323-6. [DOI] [PubMed] [Google Scholar]