Abstract
During meiosis, two successive divisions occur without any intermediate S phase to produce haploid gametes. The first meiotic division is unique in that homologous chromosomes are segregated while the cohesion between sister chromatids is maintained, resulting in a reductional division. Moreover, the duration of the first meiotic M phase is usually prolonged when compared with mitotic M phases lasting 8 h in mouse oocytes.
We investigated the spindle assembly pathway and its role in the progression of the first meiotic M phase in mouse oocytes. During the first 4 h, a bipolar spindle forms and the chromosomes congress near the equatorial plane of the spindle without stable kinetochore– microtubule end interactions. This late prometaphase spindle is then maintained for 4 h with chromosomes oscillating in the central region of the spindle. The kinetochore–microtubule end interactions are set up at the end of the first meiotic M phase (8 h after entry into M phase). This event allows the final alignment of the chromosomes and exit from metaphase. The continuous presence of the prometaphase spindle is not required for progression of the first meiotic M phase. Finally, the ability of kinetochores to interact with microtubules is acquired at the end of the first meiotic M phase and determines the timing of polar body extrusion.
Keywords: meiosis, microtubule, kinetochore, chromosome, spindle assembly checkpoint
During cell division, a bipolar spindle is assembled to allow the segregation of two sets of chromosomes. The view of spindle assembly and its influence on M phase completion is based mostly on experiments performed on mitotic cells.
In mitosis, spindle assembly proceeds in two major steps. First, the two centrosomes, which have nucleated microtubules, migrate to opposite sides of the nucleus. As a consequence, spindle bipolarity is established before nuclear envelope breakdown. Second, after nuclear envelope breakdown, dynamic microtubules are randomly captured and stabilized by the kinetochores that are located at each chromosome's centromere. Sister kinetochores rarely attach simultaneously, leading to a poleward motion of the chromosomes. The chromosomes move to the equatorial plane of the spindle when both sister kinetochores are connected to opposite poles by kinetochore fibers. This random and continuous process ends with the sharp alignment of all the chromosomes on the metaphase plate (for review see Rieder and Salmon 1998). This allows the transition to anaphase and the segregation of the two sets of chromatids.
During meiosis, two successive M phases occur without an intermediate S phase, so that haploid gametes are produced. The second meiotic division is equational and is effectively a mitotic division. The first meiotic division is unique in that it is reductional: homologous chromosomes that just underwent meiotic recombination are segregated while the cohesion between sister chromatids is maintained.
In the present work, we studied the assembly of the first meiotic spindle and its involvement in the first meiotic M phase completion using the mouse oocyte as a model. In the mouse oocyte, the first meiotic M phase is very long, lasting from 6 to 11 h, depending on the genetic background (Polanski 1986, Polanski 1997). The activity of the major M phase kinase (p34cdc2/cyclin) that directly or indirectly promotes spindle assembly increases slowly and reaches a plateau that is maintained for hours (Choi et al. 1992). One peculiarity of the mouse oocyte is that the oocytes lack centrioles (Szöllösi et al. 1972). The progressive aggregation of numerous microtubule organizing centers (MTOCs)1 form the spindle poles (Maro et al. 1985). Thus, the bipolarity of the spindle is not predefined (reviewed in Vernos and Karsenti 1995) in contrast to mitosis. Finally, a stable bipolar spindle is present in the oocyte during most of the first meiotic M phase (Verlhac et al. 1994).
We show here that the formation of a bipolar spindle and the congression of chromosomes near the equatorial plane of the spindle is performed in the absence of kinetochore fibers. This late prometaphase spindle is then maintained for 4 h. The ability of kinetochores to interact with microtubule ends (to form kinetochore fibers) is acquired at the end of the first meiotic M phase and this event allows the metaphase I to anaphase I transition. Moreover, continuous presence of the prometaphase spindle is not required for progression through the first meiotic M phase.
Materials and Methods
Collection and Culture of Oocytes
To obtain immature oocytes arrested at prophase I of meiosis, the ovaries were removed from 5–6-wk-old Swiss female mice (Animalerie Spécialisée de Villejuif, Center National de la Recherche Scientifique, France) and transferred to prewarmed (37°C) M2 medium supplemented with 4 mg/ml BSA (Whittingham 1971) and 50 μg/ml dibutyryl cyclic AMP (dbcAMP), which prevents immature oocytes from undergoing germinal vesicle breakdown (GVBD). The ovarian follicles were punctured to release the enclosed oocytes, and immature oocytes displaying a germinal vesicle (GV) were collected. Oocytes undergoing meiotic maturation were synchronized by scoring GVBD. Synchronized groups of oocytes were cultured in M2 medium under liquid paraffin oil at 37°C.
Metaphase II-arrested oocytes were recovered from mice superovulated by intraperitoneal injections of pregnant mare gonadotrophin (PMSG; Intervet) and human chorionic gonadotrophin (hCG; Intervet) 48 h apart. Ovulated oocytes were released from the ampullae of oviducts 12.5 or 17 h after hCG. The cumulus cells were dispersed by brief exposure to 0.1 M hyaluronidase (Sigma Chemical Co.) and after careful washing, all oocytes were put in culture.
Immunofluorescence
The fixation and labeling of oocytes were performed as described in Kubiak et al. 1992. As the first layer, we used either the rat mAb YL1/2 specific for tyrosinated α-tubulin (Kilmartin et al. 1982) or a rabbit polyclonal antibody directed against cytoplasmic linker protein 170 (CLIP-170; Dujardin et al. 1998). As the second layer, we used either a fluorescein-conjugated anti-rat antibody or a fluorescein-conjugated anti-rabbit antibody (Miles). The chromatin was visualized using propidium iodide (Molecular Probes; 5 mg/ml in PBS). Samples were observed with a Leica TCS4D confocal microscope.
Video Microscopy
Time-lapse studies on living mouse oocytes were performed with a computer-assisted image analysis system (Oncor Imaging), coupled to a cooled CCD camera (CH 2400; Photometrics) and an epiillumination inverted microscope (Zeiss Axiovert 135). Oocytes were cultured at 37°C in an adaptable cell culture chamber (Life Science Resources) in a 2-ng/ml vital bis benzimide (Hoechst 33258, Sigma Chemical Co.) M2 medium. Chromosome images were acquired with a 20× objective (total optical magnification was 200) and the following filter set: bandpass filter BP 365/12 nm, chromatic beam splitter FT 395 nm, longwave pass filter LP 397 nm. A log 2 neutral filter was placed in the bulb light path to minimize photo damage of oocytes by UV light. Under these conditions, images were acquired every 180 s with a 1 to 2 s exposure time. Images were digitized in 512 × 512 array (binning mode) and fluorescence intensity levels were coded on 16 bits. Images were then converted in 8 bits TIFF format. Image processing was realized with NIH Image 1.62 and Adobe Photoshop 4.0. The printed outputs were done on a sublimation color printer (ColorEase, Kodak).
Transmission EM
For transmission EM, the oocytes were prefixed with 3% paraformaldehyde in PHEM (25 mM Hepes, 60 mM Pipes, 10 mM EGTA, 2 mM MgCl2, pH 7.4) containing 0.5% Triton X-100 for 30 min. Then the oocytes were fixed with 2% glutaraldehyde and 0.2% tannic acid in a phosphate buffer, postfixed on ice with 0.5% OsO4 for 10 min, and stained en bloc with uranyl acetate. Samples were then embedded in epon in Beam's capsule and sectioned on a Reichert ultramicrotome before observations under a Philips EM410 at 80 kV. Thick sections were used to locate the spindles.
Results
Kinetics of the First Meiotic Spindle Formation
1 h after GVBD, short microtubules are radiating around the chromosomes (Fig. 1 A). 2 h after GVBD, the microtubules have formed a bipolar spindle and the chromosomes are dispersed within the structure (Fig. 1 B). 4 h after GVBD, the spindle has acquired a barrel shape morphology, typical of meiosis (Fig. 1 C), and the chromosomes are located near the equatorial plane of the spindle. From 4 to 8 h after GVBD, the spindle morphology remains unchanged with unaligned chromosomes (Fig. 1D and Fig. E). 8 h after GVBD, metaphase plates could be observed (Fig. 1 F) with the chromosomes perfectly aligned and a symmetrical arrangement of their arms. Anaphase (Fig. 1 G) and telophase (Fig. 1 H) figures were also present. Finally, we observed that the spindle length decreases 2–8 h after GVBD (reaching ∼50% of its initial length) before elongation at anaphase.
Figure 1.
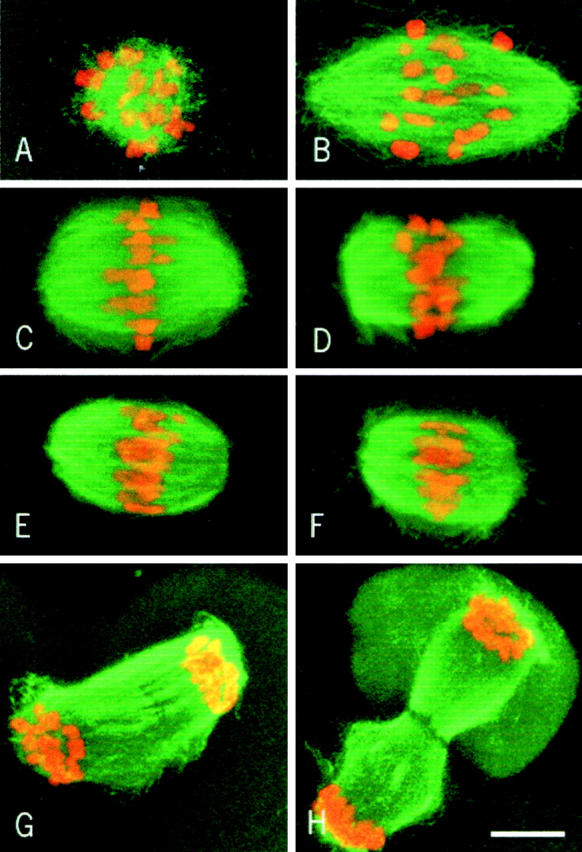
Kinetics of first meiotic spindle formation. Microtubules appear in green and chromosomes in red. (A) 1 h after GVBD. (B) 2 h after GVBD. (C) 4 h after GVBD. (D) 6 h after GVBD. (E–H) 8 h after GVBD. Note that some chromosomes are not aligned in E (late prometaphase), while all chromosomes are aligned on the metaphase plate in F. Bar, 10 μm.
Chromosome Dynamics during the First Meiotic M Phase
The immunofluorescence study of fixed material did not allow us to observe changes in dynamics of spindle morphology and chromosome position from 4 to 8 h after GVBD. The chromosomes are not sharply aligned during this period, suggesting that it corresponds to a long prometaphase. To address this point, chromosomes were stained with a vital dye and observed in vivo using a cooled CCD camera.
During the metaphase II arrest, the chromosomes are aligned on the metaphase plate and do not move (Fig. 2 A). In contrast, during the last 4 h of the first meiotic M phase, the chromosomes oscillate with a low amplitude around the equatorial plane of the spindle (Fig. 2 B). For example, the top right chromosome protruding from the main mass in Fig. 2 B, at 27 min (arrow) has moved back into it at 87 min, and out again at 105 min; the center right chromosome at 60 min (arrowhead) is in the main mass, out at 105 min, and in again at 117 min. They became aligned in metaphase for only a short period 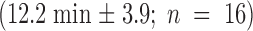 immediately before their segregation in anaphase (Fig. 2 B, at 195 min). Thus, during virtually all of the second half of the first meiotic M phase, the spindle appears to be maintained in a late prometaphase state.
immediately before their segregation in anaphase (Fig. 2 B, at 195 min). Thus, during virtually all of the second half of the first meiotic M phase, the spindle appears to be maintained in a late prometaphase state.
Figure 2.
Chromosome movements during the metaphase II arrest (A) and the first meiotic M phase (B). Pictures were taken every 3 min. Because the chromosomes did not move in metaphase II, one picture out of two is shown in A (6 min interval).
Chromatin–Microtubule Interactions during the First Meiotic M Phase
In mitosis, the accurate alignment of chromosomes on the metaphase plate is due to kinetochore–microtubule end interactions. To understand the peculiar congression of homologous chromosomes during the first meiotic M phase, we studied kinetochore–microtubule end interactions using EM.
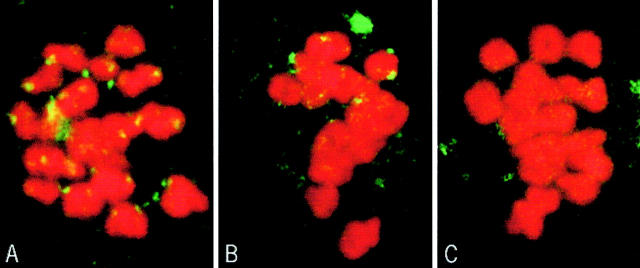
2 h after GVBD, no kinetochore–microtubule end interactions were observed (Fig. 3 A). Numerous microtubules interacted with the chromosome surface or penetrated within the chromosome (Fig. 3 A, arrows). A similar situation was observed 4 h after GVBD (not shown). 6 h after GVBD, microtubules interacted with the chromosome surface, but no longer penetrated chromosomes (Fig. 3 B). Some microtubules were present near the kinetochores, but insertion of microtubule ends in the kinetochore plate was not detected (Fig. 3 B, arrows). However, the kinetochores and adjacent chromatin appeared to be pulled out from the main chromatin mass. This likely is due to lateral interactions between some microtubules and the kinetochores, in the absence of stable kinetochore bundles. 8 h after GVBD, end interactions were not detected at some kinetochores (Fig. 3 C, arrows), but they were observed in others (Fig. 3 D, arrows), as in metaphase II-arrested oocytes (not shown). When a systematic analysis of microtubule end interactions with kinetochores was performed at 6 and 7 h after GVBD (Table ), none were observed at 6 h, the spindle areas facing the kinetochores being deprived of microtubules (Fig. 4A and Fig. B). A few microtubule ends were observed interacting with kinetochores at 7 h (Fig. 4 C, arrows), however, they were less numerous than at 8 h (compare Fig. 3 and Fig. 4C). All kinetochore regions observed were stretched towards the poles, suggesting that unstable lateral interactions with microtubules are able to position the kinetochores towards the poles.
Figure 3.
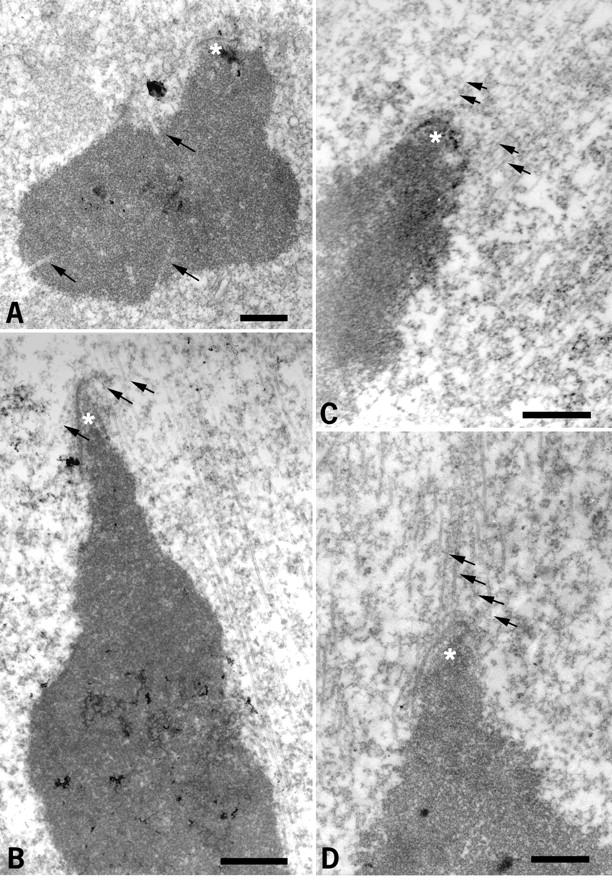
Study of chromatin and kinetochore–microtubule interactions during the first meiotic M phase by EM. (A) 2 h after GVBD. (B) 6 h after GVBD. (C and D) 8 h after GVBD. Asterisks, kinetochores; arrows, microtubules. Bars, 1 μm.
Table 1.
Electron Microscopic Analysis of the Setting Up of Kinetochore Fibers
| Time of oocyte fixation | Oocytes sectioned | Chromosomes sectioned | Number of kinetochores | |
|---|---|---|---|---|
| Without attached microtubule ends | With attached microtubule ends | |||
| h after GVBD | n | n | ||
| 6 | 5 | 69 | 23 | 0 ‡ |
| 7 | 10 | 131 | 10 | 22 ‡ |
Figure 4.
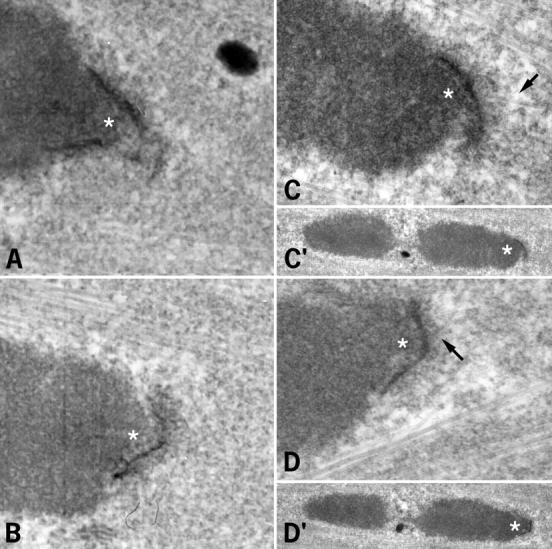
Study of kinetochore–microtubule end interactions 6 and 7 h after GVBD by EM. (A and B) 6 h after GVBD. (C and D) 7 h after GVBD. C and D correspond to two sections of the same kinetochore (C′ and D′ are the corresponding low magnification images). Asterisks, kinetochores; arrows, microtubules. Bar, 1 μm.
These data suggest that the long late prometaphase state is correlated with the lack of kinetochore fibers. During most of the first meiotic M phase, the microtubules interact directly with the surface of the chromosomes, and even penetrate within the chromatin, from 2 to 4 h after GVBD. These interactions could provide the forces leading to chromosome congression near the equatorial plane.
CLIP-170 Is Present on Kinetochores Until the End of Late Prometaphase
CLIP-170 is associated with kinetochores during prometaphase in somatic cells (Dujardin et al. 1998) until the formation of kinetochore fibers (Dujardin, D., and J. de Mey, manuscript in preparation). We first checked whether this was the case in mouse oocytes. In metaphase II-arrested oocytes, kinetochore fibers are present and no CLIP-170 staining was observed on the chromosomes (Fig. 5 A). After total depolymerization of microtubules by 10 μg/ml nocodazole, CLIP-170 staining was observed on kinetochores (Fig. 5 B). This staining disappeared rapidly when microtubules polymerized after nocodazole removal (Fig. 5C and Fig. D). Thus, in mouse oocytes, CLIP-170 behaves as it does in somatic cells: it is associated with kinetochores in the absence of kinetochore fibers.
Figure 5.
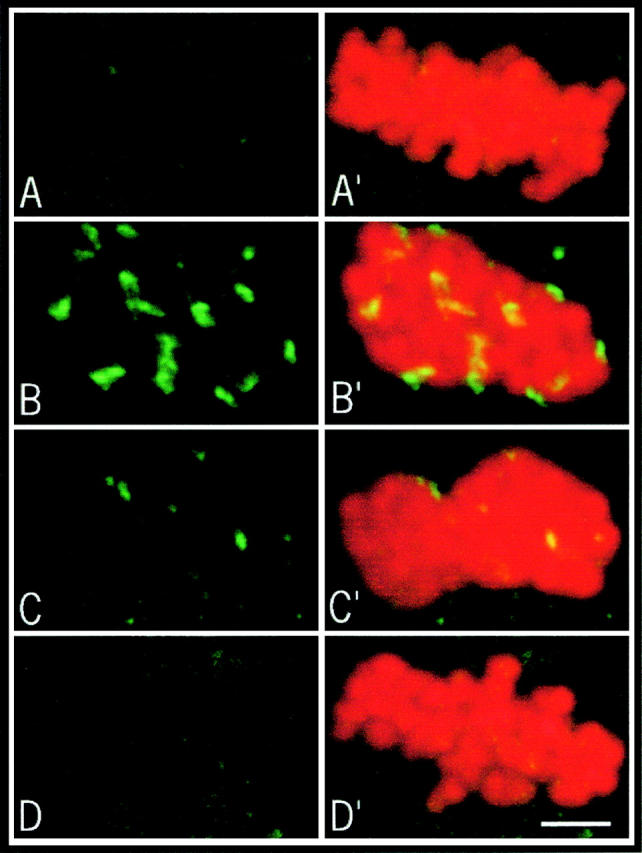
Characterization of CLIP-170 in mouse oocytes. CLIP-170 appears in green and chromosomes in red. 10–15 optical sections were merged for the final images. CLIP-170 staining during the metaphase II arrest (A). Oocytes were cultured in the presence of 10 μg/ml nocodazole for 1 h and fixed immediately (B), 15 min (C), and 30 min (D) after removal of the drug.
During the first meiotic M phase, CLIP-170 stains kinetochores already at GVBD and persists until 7 h after GVBD (Fig. 6, A–C). At this time, some kinetochores are no longer stained (Fig. 6 C). By 8 h after GVBD, the staining has disappeared from most kinetochores when chromosomes were still not aligned (Fig. 6 D), and completely absent at metaphase (Fig. 6 E). Thus, we can conclude that CLIP-170 remains on kinetochores until ∼7–8 h after GVBD and disappears just before the final alignment of the chromosomes. It is noteworthy that bivalents are correctly oriented (kinetochores of homologous chromosomes facing opposite poles) 5 h after GVBD, which suggests that lateral interactions between some microtubules and the kinetochores take place in the absence of stable kinetochore bundles. These data confirm our EM observations and show that the long late prometaphase state is correlated with the lack of kinetochore fibers.
Figure 6.
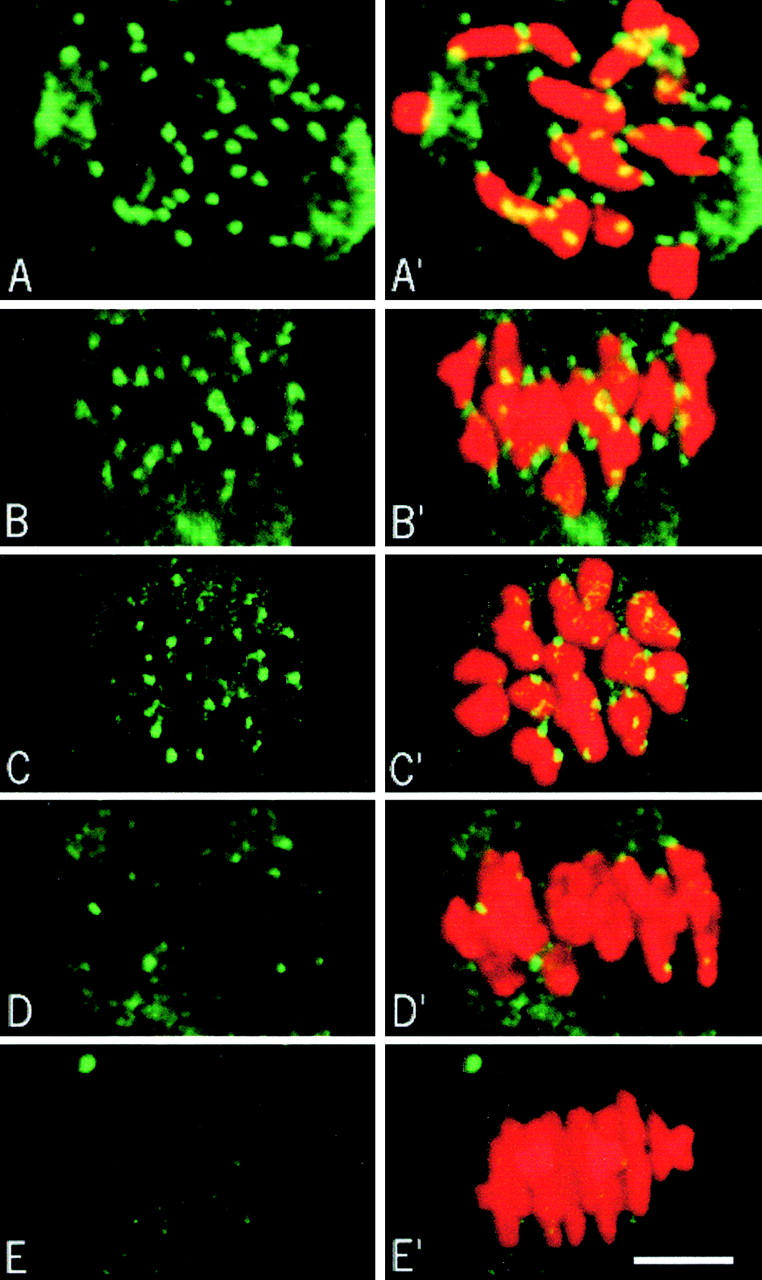
CLIP-170 staining during the first meiotic M phase. CLIP-170 appears in green and chromosomes in red. CLIP-170 on kinetochores appears yellow because of the merged green and red images. 10–15 optical sections were merged for the final images. (A) 2 h after GVBD, side view. (B) 5 h after GVBD, side view. (C) 7 h after GVBD, polar view. (D and E) 8 h after GVBD, side view. Bar, 10 μm.
The Continuous Presence of the Prometaphase Spindle Is Not Required for Completion of the First Meiotic M Phase
Our previous data show that, during most of the first meiotic M phase (6 out of 8 h), the chromosomes oscillate near the equatorial plane of a bipolar spindle. This observation led us to ask whether the persistence of this prometaphase structure was required for the assembly of a functional spindle and completion of the first meiotic M phase.
In mitosis, the duration of M phase is strongly correlated with the time devoted to spindle assembly and chromosome congression (Rieder et al. 1994). This dependence was confirmed in one-cell mouse embryos incubated after nuclear envelope breakdown for 1  or 2 h
or 2 h  in 10 μg/ml nocodazole. The drug was then removed to allow spindle reformation and the time of cell division was measured. A 1 h incubation in nocodazole delays cell division by 1 h (160 min vs. 100 min after nuclear envelope breakdown in control oocytes) and a 2 h incubation leads to a 2 h delay (210 min vs. 100 min after nuclear envelope breakdown).
in 10 μg/ml nocodazole. The drug was then removed to allow spindle reformation and the time of cell division was measured. A 1 h incubation in nocodazole delays cell division by 1 h (160 min vs. 100 min after nuclear envelope breakdown in control oocytes) and a 2 h incubation leads to a 2 h delay (210 min vs. 100 min after nuclear envelope breakdown).
A similar experiment was performed on oocytes during the first meiotic M phase. Starting 2 h after GVBD (when a bipolar spindle is already present), oocytes were incubated in nocodazole for increasing periods, varying from 2 to 6 h. Oocytes were then removed from the drug and the time of polar body extrusion (PBE) was measured. In all cases, the resulting metaphase II-arrested oocytes contained only monovalent chromosomes, as checked by propidium iodide staining. That the spindles reformed after nocodazole treatments led to the correct segregation of homologous chromosomes. Incubations in nocodazole for 2 or 3 h did not induce a delay in PBE. However, longer incubations (4, 5, and 6 h) resulted in significant delays (Table ).
Table 2.
Effects of Various Incubations in Nocodazole (10 μg/ml) on the Timing of Polar Body Extrusion
| Incubation in nocodazole | n | Time of PBE | Delay | Time required for PBE after nocodazole removal | Δ |
|---|---|---|---|---|---|
| h after GVBD | min after GVBD | min | min | ||
| Control | 92 | 483 ± 29 | — | — | |
| 2 to 4 | 75 | 495 ± 33 | 12 | 255 | |
| 2 to 5 | 78 | 503 ± 26 | 20 | 203 | |
| 4 to 5 (1 h incubation) | 73 | 505 ± 35 | 22 | 205 | 2 |
| 2 to 6 | 70 | 538 ± 29 | 50 | 178 | |
| 5 to 6 (1 h incubation) | 74 | 532 ± 34 | 49 | 172 | 6 |
| 2 to 7 | 88 | 584 ± 29 | 101 | 164 | |
| 6 to 7 (1 h incubation) | 57 | 577 ± 27 | 94 | 157 | 7 |
| 2 to 8 | 88 | 643 ± 21 | 160 | 163 | |
| 7 to 8 (1 h incubation) | 57 | 634 ± 36 | 151 | 154 | 9 |
To determine whether these delays were due to the duration of the incubation in nocodazole or to the time of drug removal, we measured the kinetics of PBE in groups of oocytes incubated for 1 h in nocodazole and removed from the drug 5, 6, 7, and 8 h after GVBD (Table ). The comparison of the results of the two experiments shows that the time of PBE depends only on the time of nocodazole removal, whatever the duration of nocodazole treatment.
Spindle destruction before 5 h after GVBD does not induce any significant delay in PBE; it continues to occur at ∼8 h after GVBD. Thus, the continuous presence of a prometaphase spindle is not required during the first meiotic M phase. This implies that the length of the first meiotic M phase is not strictly controlled by the microtubule network, but by some other unknown factor.
In contrast, nocodazole removal after 6 h after GVBD led to a linear increase in the time of PBE. However, polar bodies were extruded invariably 2.6 h after removal from the drug, probably corresponding to the minimum time required for spindle reformation and PBE. This observation suggests that the event controlling the length of the first meiotic M phase takes place between 6 and 8 h after GVBD.
Setting Up of Stable Kinetochore–Microtubule End Interactions Determines the Duration of the First Meiotic M Phase
To determine the nature of the event controlling the length of the first meiotic M phase, we took advantage of previous observations. We studied spindle reformation after a 2 h incubation in nocodazole and drug removal 8 h after GVBD (NZ/6h–8h; when this event is supposed to have occurred) or 5 h after GVBD (NZ/3h–5h; when the spindle would have been present for a few hours until this event takes place).
In both experiments, 20 min after drug removal a bipolar structure formed, although it was larger in the NZ/3h–5h group (Fig. 7A and Fig. E). 50 and 120 min after drug removal, the chromosomes were localized near the equatorial plane (Fig. 7B, Fig. C, Fig. F, and Fig. G). However, the spindle length did not change until metaphase in the NZ/6h–8h group (Fig. 7, E–G), while it decreased progressively until 120 min in the NZ/3h–5h group (Fig. 7 C). This late prometaphase spindle was maintained until 7.5 h after GVBD (Fig. 7 D), just before PBE. In the NZ/6h–8h group, the chromosomes seemed to congress progressively to the equatorial plane of the spindle (Fig. 7, E–G). PBE occurred 2.5 h after removal of the drug (Fig. 7 H). In the NZ/6h–8h group, nocodazole induced chromosome dispersal in some oocytes (∼1/3). After drug removal, a spindle reformed around each group of chromosomes and a polar body was extruded for each spindle. However, the only difference between the two groups was that the prometaphase spindle persisted for 3 h in the NZ/3h–5h group.
Figure 7.
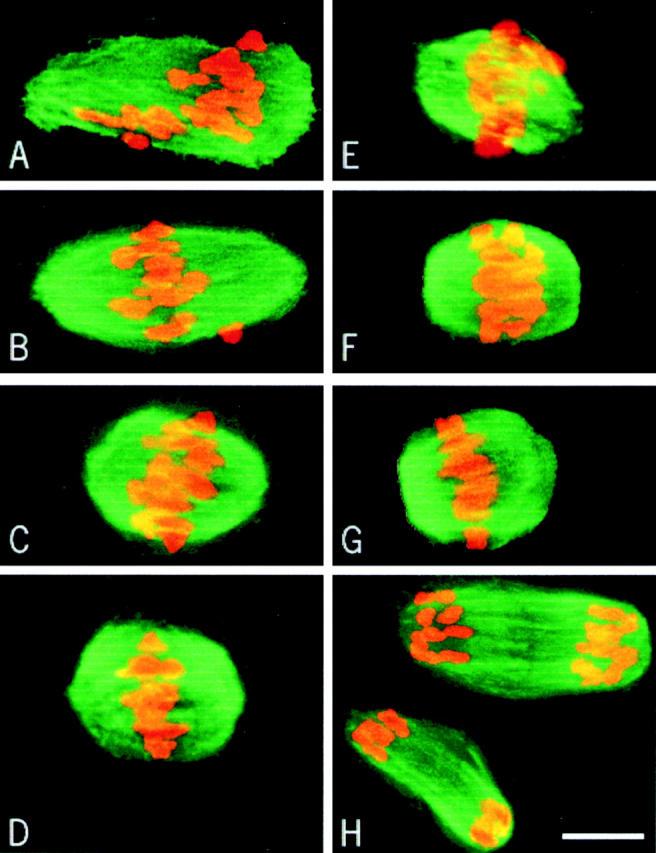
Spindle reformation after nocodazole treatment. Oocytes were cultured in the presence of 10 μg/ml nocodazole from 3 to 5 h (A–D) and from 6 to 8 h (E–H) after GVBD. Microtubules appear in green and chromosomes in red. Oocytes were fixed 20 (A and E), 50 (B and F), 120 (C and G), and 150 min (D and H) after removal of the drug.
This result was reinforced by live observation of chromosome movements after nocodazole treatment. In the NZ/3h–5h group, chromosomes kept oscillating near the equatorial plane until metaphase, as in untreated oocytes (not shown). In the NZ/6h–8h group, some individual chromosomes were observed moving towards the poles (Fig. 8, Fig. 010 Fig. 1 Fig. 2 Fig. 3 Fig. 4 Fig. 5 Fig. 6 min) and then congressing to the equatorial plane (Fig. 8, Fig. 6 Fig. 7 Fig. 8 Fig. 9 Fig. 10 min). Anaphase occurred after the alignment of the last chromosome (Fig. 8, 48 min).
Figure 8.
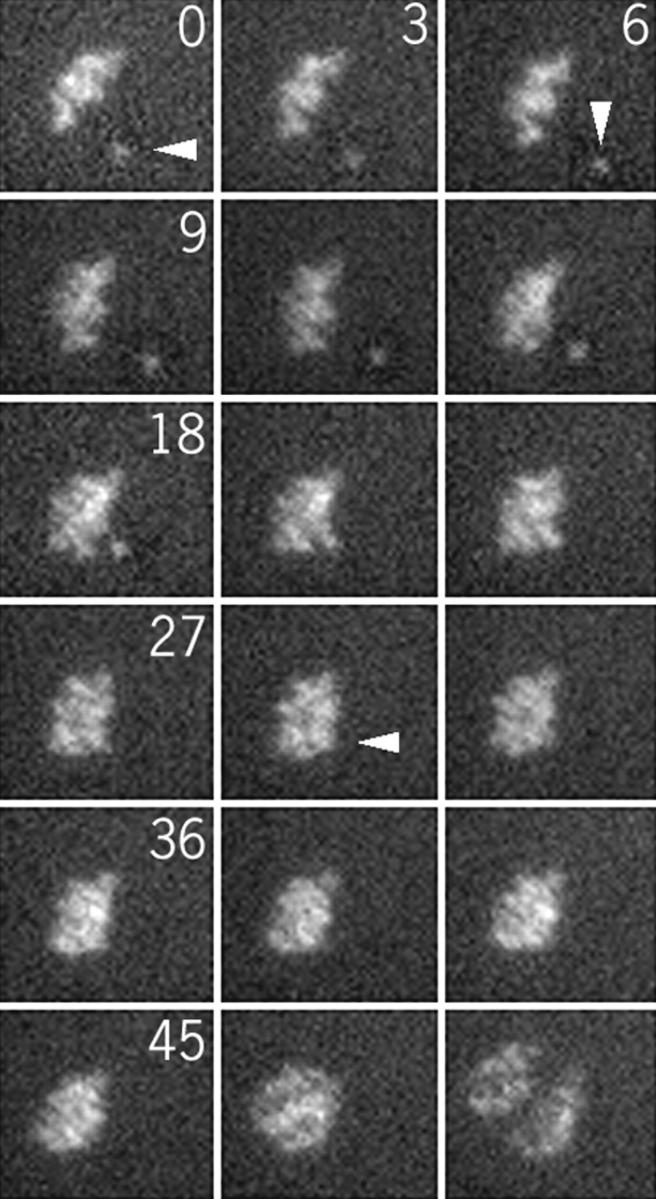
Chromosome movements after removal of the nocodazole 8 h after GVBD. Pictures were taken every 3 min.
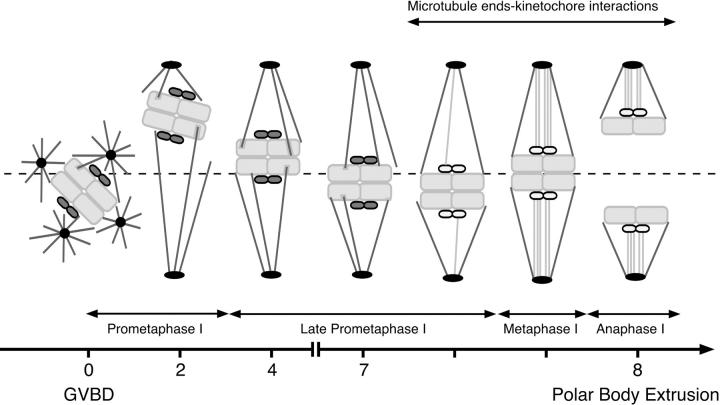
Figure 10.
Schematic representation of spindle formation during the first meiotic M phase.
Figure 9.
CLIP-170 staining after nocodazole treatment. CLIP-170 appears in green and chromosomes in red. 10–15 optical sections were merged for the final images. Oocytes were cultured in the presence of 10 μg/ml nocodazole from 3 to 5 h after GVBD and fixed 2 h after removal of the drug (A). Oocytes were cultured in the presence of 10 μg/ml nocodazole from 6 to 8 h after GVBD and fixed 15 (B) and 30 min (C) after removal of the drug.
Thus, the process of spindle reformation differs in the two groups. In the NZ/3h–5h, the process is very similar to the one observed in control oocytes during meiosis I, with the chromosomes oscillating during a long late prometaphase. In the NZ/6h–8h group, chromosomes behave as they do in mitosis. We observed that the kinetochore fibers (stable kinetochore–microtubule end interactions) are established only at the end of the first meiotic M phase, in contrast to mitosis, where they can form as soon as nuclear envelope breakdown. This led us to suspect that the setting up of these stable interactions between microtubule ends and kinetochore plates controls the duration of the first meiotic M phase. We studied the kinetochore–microtubule end interactions after nocodazole removal using CLIP-170 staining. In the NZ/3h–5h group, CLIP-170 was still observed on kinetochores 7 h after GVBD (Fig. 9 A) and disappeared ∼8 h after GVBD, as in nontreated oocytes (not shown). In the NZ/6h–8h oocytes, CLIP-170 disappeared from many kinetochores as soon as 15 min after removal of the drug (Fig. 9 B). The staining was completely abolished after 30 min (Fig. 9 C). These results show that the setting up of stable kinetochore–microtubule end interactions occurs invariably ∼8 h after GVBD, suggesting that this event controls the exit from meiosis I.
Protein Synthesis Is Not Required for the Setting Up of the Stable Kinetochore–Microtubule End Interactions
We tested whether the ability of kinetochores to interact with microtubules was dependent upon protein synthesis. Oocytes incubated in 10 μg/ml of the protein synthesis inhibitor, puromycin, for 5 h after GVBD, extruded their polar bodies at the same time as the control oocytes  . Thus, protein synthesis during the last 3 h of the first meiotic M phase is not required for the setting up of stable kinetochore–microtubule end interactions.
. Thus, protein synthesis during the last 3 h of the first meiotic M phase is not required for the setting up of stable kinetochore–microtubule end interactions.
Discussion
Our results support the following mechanism for first meiotic spindle assembly (Fig. 10). After entry into the first meiotic M phase, 2 h are required for the formation of a bipolar structure. For the next 2 h, the homologous chromosomes congress near the equatorial plane of the spindle. At that time, microtubules interact directly with the chromosomes' arms, but do not form kinetochore fibers. During the next 3 to 4 h, the chromosomes gather near the equatorial plane of the spindle. Unstable lateral interactions between microtubules and kinetochores may maintain a correct orientation of the sister kinetochores towards both poles. At 8 h after GVBD, the stable kinetochore–microtubule end interactions are set up, allowing the final alignment of the chromosomes on the metaphase plate. Metaphase lasts for a short time (∼12 min) and the metaphase–anaphase transition leads to the extrusion of the first polar body.
Formation of a Bipolar Spindle
In mitosis, the migration of the two centrosomes determines spindle bipolarity, even before nuclear envelope breakdown. Another component in establishing bipolarity is the presence of a kinetochore on each of the sister chromatids. They are located on opposite sides of the chromosome and stabilize microtubules nucleated by opposite poles, as soon as the cell enters prometaphase. In contrast, during the first meiotic M phase in mouse oocytes (and in other species, reviewed by Vernos and Karsenti 1995), bipolarity is not predefined. Oocytes are lacking centrioles and poles assemble progressively by the aggregation of numerous MTOCs. Here, we show that a bipolar spindle is also formed without stable kinetochore–microtubule end interactions.
First, we were not able to detect by EM any interaction between microtubule ends and kinetochore plates before 8 h after GVBD. Second, this observation was reinforced by the study of CLIP-170 in oocytes during the first meiotic M phase. Recently, Dujardin et al. 1998 reported that CLIP-170 associates with kinetochores during mitotic prometaphase until the formation of kinetochore fibers (Dujardin, D., and J. de Mey, manuscript in preparation). We first showed that this property of CLIP-170 was conserved during meiosis. In metaphase II-arrested oocytes, CLIP-170 is absent from kinetochores (Fig. 4 A). After a pulse of nocodazole and a transient depolymerization of the microtubule network, the CLIP-170 staining reappears and fades away when microtubules reform (Fig. 4B and Fig. C). During the first meiotic M phase, CLIP-170 is present on kinetochores when the bipolar spindle forms, showing that this process does not require any stable kinetochore–microtubule interaction.
Chromatin, per se, is involved in spindle formation, since it has been shown to have a microtubule stabilizing activity in vivo (Karsenti et al. 1984; Maro et al. 1986) and in vitro (Dogterom et al. 1996). This effect constrains microtubule polymerization near the chromosomes (Zhang and Nicklas 1995). However, the formation of a bipolar structure seems to be due to the properties of microtubules and associated factors only. We recently showed in mouse oocytes that normal bipolar spindles can form in cytoplasts deprived of chromosomes (Brunet et al. 1998). In this case, the formation of bipolar spindles is due to the setting up and stabilization of antiparallel interactions between microtubules nucleated from distinct MTOCs and the capacity of these MTOCs to aggregate. In Xenopus egg extracts containing centrioles, the formation of a bipolar spindle around nonspecific DNA (Heald et al. 1996) was shown to require cytoplasmic dynein for microtubule organization into poles (Heald et al. 1997). Other motors could be required for the organization of microtubules into antiparallel arrays.
Chromosome Congression during the First Meiotic M Phase Is a Two-step Process
Stable kinetochore–microtubule end interactions are essential for the congression of chromosomes to the equatorial plane of the spindle during mitotic prometaphase. In contrast, during the first meiotic M phase in mouse oocytes, chromosomes congress to the vicinity of the equatorial plane without such stable interactions. Using EM, we showed that end interactions between microtubules and the kinetochore plate, as in metaphase II-arrested oocytes, are only observed ∼8 h after GVBD. The fact that the bivalents are correctly oriented (kinetochores of homologous chromosomes facing opposite poles) during all the first meiotic M phase and the stretching of the kinetochores towards the pole suggests lateral interactions between some microtubules and kinetochores, in the absence of stable kinetochore bundles. The CLIP-170 staining disappears from the kinetochores between 7 and 8 h after GVBD, reinforcing the fact that stable kinetochore–microtubule end interactions are only established at the end of the first meiotic M phase, before the final alignment of chromosomes on the metaphase plate.
Previous studies have shown that direct interactions between microtubules and chromosomes are also involved in chromosome congression. First, a wind of microtubules exert some pushing forces on the chromosomes (Khodjakov et al. 1996). Second, similar forces are produced by several members of the kinesin family of microtubule motor proteins associated with chromatin, like Xklp1 during meiosis and mitosis (Vernos et al. 1995) or NOD during meiosis I in Drosophila oocytes (Theurkauf and Hawley 1992). During the first hours of the first meiotic M phase in mouse oocytes, numerous microtubules interact with the chromosomes arms and, more surprisingly, penetrate within them (Fig. 3 A).
Taken together, these observations suggest that during the long prometaphase of meiosis I, pushing forces, likely mediated by motor proteins, are exerted on the chromosomes and drive them to the vicinity of the equatorial plane of the spindle. Then, when kinetochore–microtubule end interactions are set up ∼7–8 h after GVBD, the chromosomes are sharply aligned on the metaphase plate by pushing and pulling forces exerted through the kinetochores.
The microinjection of an antikinetochore autoimmune serum in immature mouse oocytes impaired the formation of the metaphase I plate, but the microinjection of the same serum in cold-treated metaphase II oocytes did not interfere with the reformation of a metaphase II plate after warming (Simerly et al. 1990). However, the serum recognized the CENP-B protein that binds to centromeric sequences and seems to organize centromere satellite DNA into a higher order structure, which then directs centromere formation and kinetochore assembly (Goldberg et al. 1996; Iwahara et al. 1998). Such a role of CENP-B in kinetochore assembly, before or at the very beginning of M phase, may explain why microinjection of the serum did not interfere with metaphase plate formation when kinetochore–microtubule end interactions are possible because kinetochores are already formed. Moreover, we observed that the kinetochores are assembled already at the beginning of the first meiotic M phase, but are able to interact with microtubule ends only at the end of the first meiotic M phase.
Kinetochore Maturation Controls the Duration of the First Meiotic M Phase
In contrast to mitosis, spindle assembly does not determine the duration of the first meiotic M phase. Indeed, the continuous presence of the prometaphase spindle is not required for completion of the first meiotic metaphase. After spindle destruction by nocodazole, the minimum time needed for the formation of a functional spindle and PBE is 2.5 h. The study of the kinetochore–microtubule end interactions after nocodazole treatments leading to various delays in PBE shows that they are set up invariably ∼7–8 h after GVBD. Once they can be set up, the final alignment of the chromosomes occurs, followed by the metaphase/anaphase transition and extrusion of the polar body. These late events are likely under the control of the spindle assembly checkpoint, because after nocodazole-induced spindle disruption and drug removal PBE was delayed (Table ), anaphase did not occur before the alignment of the last chromosome on the metaphase plate (Fig. 7) and, when chromosomes were dispersed by the drug, spindles formed around each group of chromosomes, and polar bodies were synchronously extruded (Fig. 6 H).
The ability of the kinetochores to interact with microtubule ends is acquired at the end of the first meiotic M phase. This was confirmed in oocytes from two strains of mice, CBA/Kw and KE, which differ greatly in the timing of PBE, ∼7 and 11 h after GVBD, respectively (Polanski et al. 1998). In both strains, kinetochore fibers are observed just before PBE (not shown). This suggests that a slow and progressive maturation of the kinetochore controls the duration of the first meiotic M phase. Protein synthesis during the last 3 h of the first meiotic M phase is not required for this process, suggesting that it requires some post translational modification of kinetochore component(s), only achieved at the end of the first meiotic M phase.
During the first meiotic M phase, the activity of MPF (M phase promoting factor) increases slowly and reaches a plateau that is maintained for hours (Choi et al. 1992). We recently showed that cyclin B synthesis controls the duration of the first meiotic M phase through the raise in MPF activity, the plateau of maximum activity being constant (Polanski et al. 1998). The post-translational modification of kinetochores could be directly or indirectly under the control of this plateau of maximum MPF activity.
It is possible that some event(s) need(s) to be completed before the setting up of kinetochore fibers. Interestingly, chromatin appears not to be fully condensed during most of the first meiotic M phase, probably because the activation of MPF is slow and progressive. The lack of kinetochore fibers could prevent the chromosomes from being pulled apart by high intensity forces until condensation is sufficient to protect them from such tractions exerted on kinetochores.
Acknowledgments
We thank A.T.C. Carpenter, J.C. Courvalin, J. de Mey, and M.H. Verlhac for critical reading of the manuscript, and R. Schwartzmann for help with the illustrations.
This work was supported by grants from the Centre National de la Recherche Scientifique, Fondation pour la Recherche Médicale, and the Association pour la Recherche sur le Cancer (ARC) to B. Maro and by an ARC grant to J.Z. Kubiak. S. Brunet was the recipient of a fellowship from ARC.
Footnotes
1.used in this paper: CLIP-170, cytoplasmic linker protein 170; GV, germinal vesicle; GVBD, germinal vesicle break down; MTOCs, microtubule organizing centers; PBE, polar body extrusion
Stéphane Brunet's present address is Cell Biology and Biophysics Programme, EMBL, Heidelberg, Germany; Denis Dujardin's present address is University of Massachusetts Medical School, Worcester, MA; and Jacek Kubiak's present address is Biologie et Génétique du Développement, UPR 41, CNRS, Rennes, France.
References
- Brunet S., Polanski Z., Verlhac M.-H., Kubiak J.Z., Maro B. Bipolar meiotic spindle formation without chromatin. Curr. Biol. 1998;8:1231–1234. doi: 10.1016/s0960-9822(07)00516-7. [DOI] [PubMed] [Google Scholar]
- Choi T., Aoki F., Yamashita M., Nagahama Y., Kohmoto K. Direct activation of p34cdc2-protein kinase without preceding phosphorylation during meiotic cell cycle in mouse oocytes. Biomed. Res. 1992;13:423–427. [Google Scholar]
- Dogterom M., Felix M.A., Guet C.C., Leibler S. Influence of M-phase chromatin on the anisotropy of microtubule asters. J. Cell Biol. 1996;133:125–140. doi: 10.1083/jcb.133.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin D., Wacker U.I., Moreau A., Schroer T.A., Rickard J.E., De Mey J.R. Evidence for a role of CLIP-170 in the establishment of metaphase chromosome alignment. J. Cell Biol. 1998;141:849–862. doi: 10.1083/jcb.141.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg I.G., Sawhney H., Pluta A.F., Warburton P.E., Earnshaw W.C. Surprising deficiency of CENP-B binding sites in African green monkey alpha-satellite DNAimplications for CENP-B function at centromeres. Mol. Cell. Biol. 1996;16:5156–5168. doi: 10.1128/mcb.16.9.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R., Tournebize R., Blank T., Sandaltzopoulos R., Becker P., Hyman A., Karsenti E. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature. 1996;382:420–425. doi: 10.1038/382420a0. [DOI] [PubMed] [Google Scholar]
- Heald R., Tournebize R., Habermann A., Karsenti E., Hyman A. Spindle assembly in Xenopus egg extractsrespective roles of centrosomes and microtubule self-organization. J. Cell Biol. 1997;138:615–628. doi: 10.1083/jcb.138.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahara J., Kigawa T., Kitagawa K., Masumoto H., Okazaki T., Yokoyama S. A helix-turn-helix structure unit in human centromere protein B (CENP-B) EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:827–837. doi: 10.1093/emboj/17.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenti E., Newport J., Kirschner M. Respective roles of centrosomes and chromatin in the conversion of microtubule arrays from interphase to metaphase. J. Cell Biol. 1984;99:47s–54s. doi: 10.1083/jcb.99.1.47s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov A., Cole R.W., Bajer A.S., Rieder C.L. The force for poleward chromosome motion in Haemanthus cells acts along the length of the chromosome during metaphase but only at the kinetochore during anaphase. J. Cell Biol. 1996;132:1093–1104. doi: 10.1083/jcb.132.6.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin J.V., Wright B., Milstein C. Rat monoclonal antitubulin antibodies derived by using a new nonsecreting rat cell line. J. Cell Biol. 1982;93:576–582. doi: 10.1083/jcb.93.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubiak J.Z., Weber M., Géraud G., Maro B. Cell cycle modification during the transition between meiotic M-phases in mouse oocytes. J. Cell Sci. 1992;102:457–467. doi: 10.1242/jcs.102.3.457. [DOI] [PubMed] [Google Scholar]
- Maro B., Howlett S.K., Webb M. Non-spindle microtubule organizing centers in metaphase II-arrested mouse oocytes. J. Cell Biol. 1985;101:1665–1672. doi: 10.1083/jcb.101.5.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maro B., Johnson M.H., Webb M., Flach G. Mechanism of polar body formation in the mouse oocytean interaction between the chromosomes, the cytoskeleton and the plasma membrane. J. Embryol. Exp. Morphol. 1986;92:11–32. [PubMed] [Google Scholar]
- Polanski Z. In vivo and in vitro maturation rate of oocytes from two strains of mice. J. Reprod. Fertil. 1986;78:103–109. doi: 10.1530/jrf.0.0780103. [DOI] [PubMed] [Google Scholar]
- Polanski Z. Genetic background of the differences in timing of meiotic maturation in mouse oocytesa study using recombinant inbred strains. J. Reprod. Fertil. 1997;109:109–114. doi: 10.1530/jrf.0.1090109. [DOI] [PubMed] [Google Scholar]
- Polanski Z., Ledan E., Brunet S., Louvet S., Kubiak J.Z., Verlhac M.-H., Maro B. Cyclin synthesis controls the progression of meiotic maturation in mouse oocytes. Development. 1998;125:4989–4997. doi: 10.1242/dev.125.24.4989. [DOI] [PubMed] [Google Scholar]
- Rieder C.L., Salmon E.D. The vertebrate cell kinetochore and its roles during mitosis. Trends Cell Biol. 1998;8:310–318. doi: 10.1016/s0962-8924(98)01299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder C.L., Schultz A., Cole R., Sluder G. Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J. Cell Biol. 1994;127:1301–1310. doi: 10.1083/jcb.127.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly C., Balczon R., Brinkley B.R., Schatten G. Microinjected kinetochore antibodies interfere with chromosome movement in meiotic and mitotic mouse oocytes. J. Cell Biol. 1990;111:1491–1504. doi: 10.1083/jcb.111.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szöllösi D., Calarco P., Donahue R.P. Absence of centrioles in the first and second meiotic spindles of mouse oocytes. J. Cell Sci. 1972;11:521–541. doi: 10.1242/jcs.11.2.521. [DOI] [PubMed] [Google Scholar]
- Theurkauf W.E., Hawley R.S. Meiotic spindle assembly in Drosophila femalesbehavior of nonexchange chromosomes and the effects of mutations in the nod kinesin-like protein. J. Cell Biol. 1992;116:1167–1180. doi: 10.1083/jcb.116.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlhac M.-H., Kubiak J.Z., Clarke H.J., Maro B. Microtubule and chromatin behavior follow MAP kinase activity but not MPF activity during meiosis in mouse oocytes. Development. 1994;120:1017–1025. doi: 10.1242/dev.120.4.1017. [DOI] [PubMed] [Google Scholar]
- Vernos I., Karsenti E. Chromosomes take the lead in spindle assembly. Trends Cell Biol. 1995;5:297–301. doi: 10.1016/s0962-8924(00)89045-5. [DOI] [PubMed] [Google Scholar]
- Vernos I., Raats J., Hirano T., Heasman J., Karsenti E., Wylie C. Xklp1, a chromosomal Xenopus kinesin-like protein essential for spindle organization and chromosome positioning. Cell. 1995;81:117–127. doi: 10.1016/0092-8674(95)90376-3. [DOI] [PubMed] [Google Scholar]
- Whittingham D.G. Culture of mouse ova. J. Reprod. Fertil. Suppl. 1971;14:7–21. [PubMed] [Google Scholar]
- Zhang D.H., Nicklas R.B. The impact of chromosomes and centrosomes on spindle assembly as observed in living cells. J. Cell Biol. 1995;129:1287–1300. doi: 10.1083/jcb.129.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]