Abstract
During wound healing, fibroblasts are recruited from the surrounding tissue to accomplish repair. The requisite migration and proliferation of the fibroblasts is promoted by growth factors including those that activate the epidermal growth factor receptor (EGFR). Counterstimulatory factors in wound fluid are postulated to limit this response; among these factors is the ELR-negative CXC chemokine, interferon inducible protein-10 (IP-10). We report here that IP-10 inhibited EGF- and heparin-binding EGF-like growth factor–induced Hs68 human dermal fibroblast motility in a dose-dependent manner (to 52% and 44%, respectively, at 50 ng/ml IP-10), whereas IP-10 had no effect on either basal or EGFR-mediated mitogenesis (96 ± 15% at 50 ng/ml). These data demonstrate for the first time a counterstimulatory effect of IP-10 on a specific induced fibroblast response, EGFR-mediated motility.
To define the molecular basis of this negative transmodulation of EGFR signaling, we found that IP-10 did not adversely impact receptor or immediate postreceptor signaling as determined by tyrosyl phosphorylation of EGFR and two major downstream effectors phospholipase C-γ and erk mitogen-activated protein kinases. Morphological studies suggested which biophysical steps may be affected by demonstrating that IP-10 treatment resulted in an elongated cell morphology reminiscent of failure to detach the uropod; in support of this, IP-10 pretreatment inhibited EGF-induced cell detachment. These data suggested that calpain activity may be involved. The cell permeant agent, calpain inhibitor I, limited EGF-induced motility and de-adhesion similarly to IP-10. IP-10 also prevented EGF- induced calpain activation (reduced by 71 ± 7%). That this inhibition of EGF-induced calpain activity was secondary to IP-10 initiating a cAMP-protein kinase A-calpain cascade is supported by the following evidence: (a) the cell permeant analogue 8-(4-chlorophenylthio)-cAMP (CPT-cAMP) prevented EGF-induced calpain activity and motility; (b) other ELR-negative CXC chemokines, monokine induced by IFN-γ and platelet factor 4 that also generate cAMP, inhibited EGF-induced cell migration and calpain activation; and (c) the protein kinase A inhibitor Rp-8-Br-cAMPS abrogated IP-10 inhibition of cell migration, cell detachment, and calpain activation. Our findings provide a model by which IP-10 suppresses EGF-induced cell motility by inhibiting EGF-induced detachment of the trailing edges of motile cells.
Keywords: EGF receptor, cell motility, calpain, chemokine, fibroblasts
During normal skin wound healing, fibroblasts are recruited from the surrounding tissue (Hay 1993). Fibroblasts migrate into the granulation tissue and proliferate to close the wound. This repopulation, which involves both migratory and proliferative phases, is regulated by stimulatory and inhibitory cytokines as well as matrix constituents (Lawrence and Diegelmann 1994). Wound healing is a complex and highly orchestrated process regulated by these factors. This is best appreciated for factors that promote healing. Less well-defined are factors that act late to end wound repair and limit excessive scarring. These signals have been the focus of interventions targeted at improving wound repair. Much of these efforts have been ultimately unsuccessful. However, it is the interplay between pro- and counterstimulatory signals that needs to be understood for rational interventions to be designed.
Growth factors such as those that activate the EGF receptor (EGFR),1 including EGF, TGF-α, and heparin-binding EGF-like growth factor (HB-EGF), are present at many stages of wound healing and have strong effects on both cell proliferation and cell migration (Carpenter and Cohen 1978; Wells et al. 1998). EGFR-activating ligands are produced by or released from platelets during the initial clot, macrophages during the inflammatory phase, and fibroblasts during the reparative phase (Blotnick et al. 1994; Pan et al. 1995). That these EGFR ligands are present during all stages of wound repair suggests that they play important roles in wound repair (Kiritsy et al. 1993; Blotnick et al. 1994; Steenfos 1994). Activation of EGFR results in cell proliferation and migration as well as production of extracellular matrix constituents and processing enzymes (Gospodarowicz and Mescher 1977; Carpenter and Cohen 1978; Blay and Brown 1985; Barrandon and Green 1987; Colige et al. 1988; Chen et al. 1994b). All these attributes point to EGFR factors promoting wound healing. In support of this, EGF has been shown to improve animal models of wound healing (Breuing et al. 1997).
In the search for counterstimulatory factors, select chemokines have been investigated. ELR-negative CXC chemokines, such as interferon inducible protein-10 (IP-10) and platelet factor 4 (PF4), have been shown to inhibit endothelial cell proliferation and migration and angiogenesis (Luster et al. 1995; Strieter et al. 1995). IP-10 is produced late in wound healing, becoming evident 4 d after wounding (Engelhardt et al. 1998). However, IP-10 is a chemoattractant for monocytes and T lymphocytes. The effects of IP-10, if any, on dermal fibroblasts during wound healing are not known, although negative modulatory effects on other mesenchymal cells, endothelial cells, make IP-10 an attractive candidate for limiting wound healing.
Cell motility is a biophysical process that is actively controlled by specific, if yet incompletely defined, biochemical signaling cascades that result in dissociable biophysical phenomena (Wells et al. 1998). At least two specific biochemical cascades have been shown to be required for EGFR-mediated cell motility. The first identified, with phospholipase C-γ (PLC-γ) as the immediate postreceptor effector, leads to cytoskeletal reorganization and possibly contributes to lamellipod protrusion (Ware et al. 1998). The second pathway, via the erk mitogen-activated protein kinases (MAPKs), leads to partial de-adhesion from substratum (Xie et al. 1998). Recently, cell detachment at the rear of locomoting cells has been shown to be a rate-limiting step in fibroblast motility (Huttenlocher et al. 1997; Palecek et al. 1997). Regulated activation of the ubiquitous cytosolic protease, calpain, has been demonstrated to be important for this focal de-adhesion; if calpain is blocked, the result is an elongated but ultimately immobile cell (Huttenlocher et al. 1997; Palecek et al. 1998). We have evidence that this calpain-dependent event is modulated by EGFR signaling and required for EGFR-mediated de-adhesion and motility (Glading, A., P. Chang, D.A. Lauffenburger, and A. Wells, manuscript in preparation). Thus, there are specific biochemical signals and biophysical processes which may be negatively modulated to limit cell motility.
A counterstimulatory factor would be predicted to interfere with a specific subset of these biophysical processes. Therefore, we investigated whether specific processes were targeted by the putative counterstimulatory chemokine IP-10. The foregoing data suggested a testable model in which IP-10 negatively affects cell detachment at the rear to prevent cell motility and thereby limit wound repair. In this paper we report that IP-10 inhibits EGF-induced cell migration but not proliferation. We find that only EGFR-induced motility, but not basal motility that is signaled by adhesion receptors, is diminished by IP-10. This occurs at a postreceptor level since upstream EGFR signaling pathways are unaffected by IP-10 exposure. EGFR-mediated cell de-adhesion and rear detachment are significantly impaired in the face of IP-10 signaling. Furthermore, our data suggest that this occurs via a cAMP-dependent protein kinase A (PKA) attenuation of calpain activation, as similar biochemical and biophysical readouts can be mimicked by directly modulating these biochemical signals and mediators.
Materials and Methods
Reagents
Hs68 normal human diploid fibroblasts were purchased from American Type Culture Collection. Hs68 cells were used at passage 5–12. IP-10, monokine induced by IFN-γ (MIG), and PF4 were purchased from Peprotech. EGF was obtained from Collaborative Biomedical Products. HB-EGF and 8-(4-chlorophenylthio)-cAMP (CPT-cAMP) was purchased from Sigma Chemical Co. Calpain inhibitor I was purchased from Biomol. PDGF-BB, Rp-8-Br-cAMPS, and Rp-8-Br-cGMPS were purchased from Calbiochem. Plastic dishes for cell culture were purchased from Becton Dickinson.
In Vitro Wound Healing Assay
EGF-induced migration was assessed by the ability of the cells to move into an acellular area (Chen et al. 1994b). Hs68 cells were plated on 6-well plastic dishes and grown to confluence in DME with 10% FBS. After 48 h quiescent in the media with 0.1% dialyzed FBS, an area was denuded by a rubber policeman at the center of the plate. The cells were then treated with or without chemokines and EGF (1 nM) and incubated at 37°C. Photographs were taken at 0 h and 24 h, and the relative distance traveled by the cells at the acellular front was determined.
Cell Proliferation Assay
EGF-induced proliferation was determined by the incorporation of [3H]thymidine (Chen et al. 1996). Cells were grown to confluence in 12-well plates and quiesced for 48 h in DME with 0.1% dialyzed FBS and then incubated with the indicated concentration of IP-10 and EGF (1 nM) for 16 h. [3H]Thymidine (5 μCi/well) was added, and cells were incubated for a further 10 h. Cells were treated with 5% trichloroacetic acid for 30 min at 4°C and incorporated label solubilized by 1 N NaOH. Samples were analyzed by liquid scintillation counter (Beckman Fullerton).
cAMP Assay
Cells were plated in 10-cm culture plates and grown to confluence in DME with 10% FBS. Following the treatment of IP-10 for 2 or 4 h, ice cold extraction buffer (50% ethanol, 0.1 N HCl) were added and incubated on ice for 15 min. Extracts were lyophilized and resuspended in 100 μl of water. cAMP was quantitated using a cAMP assay kit (Amersham Life Science Inc.). After the extraction cells were lysed with 0.1 N NaOH and analyzed for protein content by Bradford protein assay.
Immunoblotting and Immunoprecipitation
Activation of EGFR, PLC-γ, and the erk MAPK were assessed by their tyrosyl-phosphorylation by immunoprecipitation and immunoblotting. Cells were treated with IP-10 (50 ng/ml) before EGF (1 nM) treatment. Cell lysates were separated on 7.5% SDS-PAGE and transferred to a PVDF membrane Immobilon-P (Millipore). Blots were probed by anti–phospho-erk-MAPK (New England Biolabs) or anti–calpain I or –calpain II (Biomol) antibodies before visualizing with AP-conjugated secondary antibodies followed by development with a colorimetric method (Promega).
For immunoprecipitations, cells (2 × 107) were treated with IP-10 and EGF as described above. Cell lysates were incubated overnight at 4°C with the indicated antibody, mixed monoclonal anti–PLC-γ1 (Upstate Biotechnology Inc.) or monoclonal anti-EGFR (Oncogene Science). Immunocomplexes were incubated with protein G–agarose and centrifuged. The pellets were washed three times with 20 nM Hepes buffer (pH 7.4) containing 10% glycerol, 0.1% Triton X-100, 500 mM sodium chloride, 1 mM sodium vanadate. Precipitated proteins were size-fractionated by SDS-PAGE and transferred to a PVDF membrane. Tyrosyl phosphorylation was determined by immunoblotting using the anti–phospho-tyrosine PY-20 (Transduction Laboratories). By diluting test specimens, we empirically found that we could detect difference in the signal strength on the order of 10%.
Morphological Analysis
Cells were plated in 2 ml in 6-well tissue culture plates with DME containing 0.1% dialyzed FBS at the concentration of 105 cells/ml. After 12 h of incubation at 37°C cells were treated with EGF (1 nM) and IP-10 (50 ng/ml) for another 24 h at 37°C. Cells were visualized by phase-contrast microscopy. Cell perimeter and cell surface area were analyzed by manually tracking cell edges on the computer-captured phase-contrast image using DIAS Dynamic image analyzing system (Solltech) and are expressed as a ratio of arbitrary units. Asymmetry index of nucleus localization was obtained by measuring the greatest cell length and the length between the nuclei and the tip of the longest projection, and calculating the deviation from equidistance (nucleus localization varies from central, a fraction of 0.5 and an index of 0, and at tip of a projection, for a fraction of 1.0 or an index of 100).
Adhesion Assay
Cell-substratum adhesiveness was quantitated using inverted centrifugation detachment. 24-well plates were coated with the human extracellular matrix Amgel (0.5 μg/ml) (Siegal et al. 1993) for 1 h at room temperature, after which they were blocked with 1% BSA for 60 min at room temperature. The plates were washed twice with PBS and used for the following experiment. Cells were plated at the concentration of 105 cells/ml with quiescent media (0.1% FBS) into Amgel coated plates and incubated for 12 h at 37°C. Plates were filled with DME with 1% BSA and 25 mM Hepes. Then plates were sealed with ELISA sealing tape (Corning) and centrifuged inverted for 5 min at 4,000 rpm at 37°C using Beckman CS6R plate centrifuge; 4,000 rpm (2,920 g) was chosen empirically as the force required to detach approximately half of EGF-treated cells. Before and after centrifugation, the amounts of cells on the plates were counted by phase-contrast microscopy.
Calpain Activity Assay
Cells were grown to confluence in 10-cm tissue culture plates and quiesced in DME with 0.1% dialyzed FBS for 48 h. After 4 h of treatment of IP-10 (50 ng/ml) with or without Rp-8-Br-cAMPS (50 μM), cells were treated with EGF (1 nM) and/or CPT-cAMP (20 μM) for 30 min. Cells were washed twice with ice cold PBS and lysed with cell lysis buffer (20 nM Hepes, pH 7.4, 10% glycerol, 0.1% Triton X-100, 500 mM sodium chloride, 1 mM sodium vanadate). After removing the cell debris by centrifugation, dichlorotriazinylamino-fluorescein–labeled microtubule-associated protein 2 (MAP2) (Cytoskeleton) (50 μg/ml) (Tompa et al. 1995) was added to the samples with either 0 or 10 mM free Ca2+ concentration and incubated for 3 min at 30°C. Samples were measured by Aminco-Bowman Series II spectrofluorometer (Spectronic Instruments Inc.) at excitation and emission wavelengths of 490 and 520 nm, respectively.
Levels of calpain I, calpain II, and calpastatin were assessed by immunoblotting using specific antibodies (Biomol).
Results
IP-10 Inhibits EGFR-mediated Cell Migration but Not Proliferation
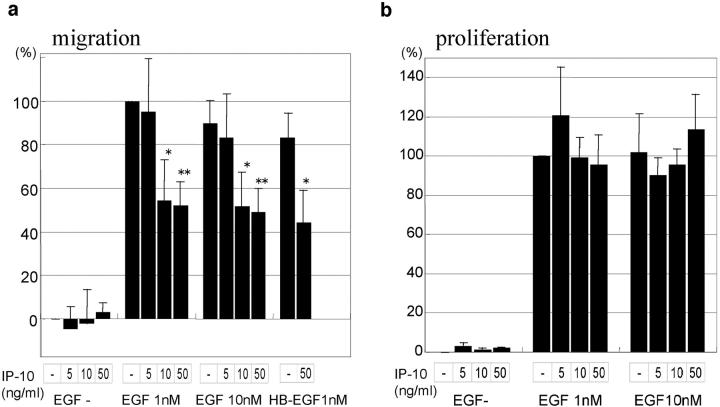
To determine whether the putative counterregulatory ELR-negative CXC chemokine IP-10 affects fibroblast functioning and responsiveness, we examined EGF-induced proliferation and migration in the presence of IP-10. Basal and EGF (1 nM)-induced cell migrative capacities were 452 ± 10 and 703 ± 26 μm/d, respectively. IP-10 was seen to inhibit EGF-induced cell migration (Fig. 1 a). IP-10 at 1 ng/ml had no effect, but 10 ng/ml and 50 ng/ml inhibited 1 nM EGF-induced cell migration 46% and 48%, respectively. This is not overcome by supersaturating doses of EGF, as IP-10 also inhibits 10 nM EGF-induced cell migration 43% (10 ng/ml) to 45% (50 ng/ml). On the other hand, no significant difference was found in basal cell migrative capacities which is signaled via adhesion receptors. These data suggest that IP-10 disrupts EGFR-mediated modulatory signals rather than the motility process per se.
Figure 1.
Effects of IP-10 on EGF-induced cell migration (a) and cell proliferation (b). Cells were grown to confluence and quiesced for 48 h in DME with 0.1% dialyzed FBS before treatment with or without IP-10 (1–50 ng/ml), EGF (1–10 nM), and/or HB-EGF (1 nM). Cell migration (a) and cell proliferation (b) assays were performed as described in Materials and Methods. The data are shown as the ratio to the 1 nM EGF-induced cell migrative or cell proliferative activity. The data are the mean ± SEM of at least three independent studies each performed in triplicate. Statistical analyses were performed by Student's t test as compared with EGF- or HB-EGF–induced cell migrative or cell proliferative capacity in the absence of IP-10: *P < 0.05, **P < 0.01.
IP-10 may diminish either EGFR signaling or specifically interrupt motility-enhancing pathways. To determine whether there was global abrogation of EGFR signaling, the effect of IP-10 on EGF-induced proliferation was examined (Fig. 1 b). The presence of IP-10 diminished neither basal nor EGF-induced thymidine incorporation suggesting that IP-10 modulatory signals target motility-specific pathways.
To ascertain whether IP-10 affected EGF as a ligand rather than EGFR-mediated signals, we tested the cell response to HB-EGF. HB-EGF–induced cell migration was also found to be inhibited up to 47% by IP-10 (Fig. 1 a). This was not unexpected as the pleiotropic nature of resultant cellular responses to EGF is thought to be due to intracellular signaling rather than multiple signals encoded in the ligand. Thus, these initial investigations pointed to a specific attenuation of the EGFR-mediated motility response.
IP-10 Does Not Inhibit EGFR, PLC-γ, or MAPK Phosphorylation
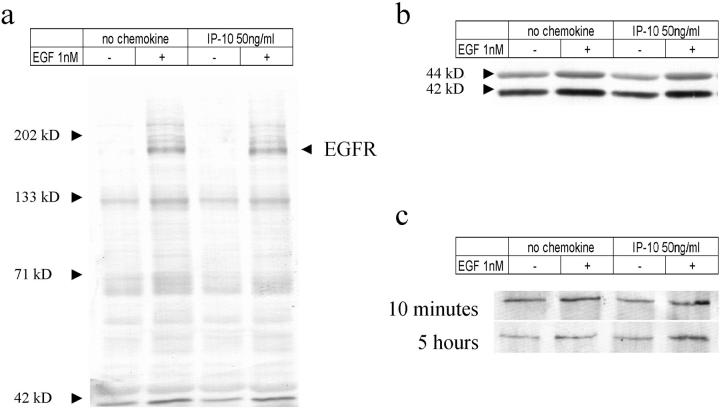
The fact that IP-10 blocks EGFR-mediated motility but not proliferation suggested that the point of signal disruption lies downstream of the receptor. This was confirmed by finding that IP-10 pretreatment had no effect on ligand activation of EGFR kinase as determined by whole cell tyrosyl-phosphorylation profiles in response to EGF (Fig. 2 a) (Chen et al. 1996). Even after 5 h of IP-10 exposure EGFR kinase was unaffected (data not shown).
Figure 2.
Tyrosine phosphorylation induced by EGF stimulation. Cells were grown to confluence and quiesced with the DME containing 0.1% dialyzed FBS for 48 h. (a and b) Cells then were treated with IP-10 (50 ng/ml) for 10 min before the 5 min of EGF (1 nM) stimulation. Cells were then lysed, and equal volumes of proteins were analyzed for phospho-tyrosine (a) and phospho-erk-MAPK (b) by immunoblotting. (c) For PLC-γ analyses, cells were treated with IP-10 (50 ng/ml) for 10 min or 5 h before the 5 min of EGF (1 nM) treatment. Cell lysates containing same amounts of proteins were immunoprecipitated with anti–PLC-γ antibody. Then immunoprecipitates were analyzed by 7.5% SDS-PAGE and immunoblotted with anti–phospho-tyrosine antibody PY-20. Representative blots of three independent experiments are shown.
Two divergent pathways have been shown to be required for EGFR-mediated motility, those involving PLC-γ (Chen et al. 1994b) and the erk family of MAPK (Xie et al. 1998). Therefore, we examined if the activation state of these mediators was adversely affected by IP-10 (Fig. 2b and Fig. c). Neither the basal nor the EGF-stimulated tyrosyl-phosphorylation of PLC-γ, nor dual phosphorylation of erk MAPK was diminished after either 10 min or 5 h of IP-10 exposure. These findings confirmed that IP-10 did not adversely impinge on EGFR signaling at the receptor or proximal postreceptor level.
IP-10 Exposure Results in an Elongated Cell Morphology
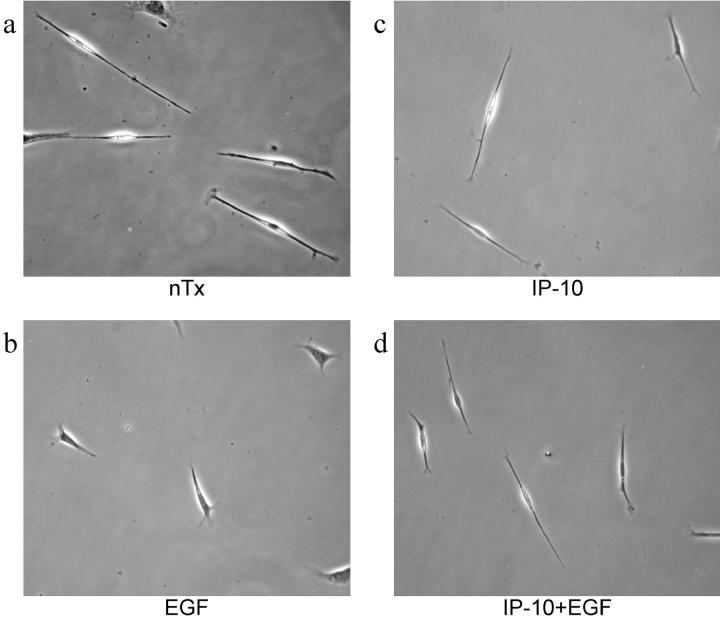
In the absence of effects on these signaling pathways, the target of signal attenuation is predicted to be downstream, and at or near the rate-limiting biophysical steps for motility. As an initial attempt to identify the affected biophysical process, cells were examined in the presence and absence of chemokine (Fig. 3). EGF-treated Hs68 cells presented a motile fusiform shape compared with control cells. IP-10 pretreatment prevented this conversion to a more contracted cell with shorter forward and rear projections. To determine if these morphological changes were significantly affected, two ratios were determined: extent of cell contraction and asymmetry of nuclear localization (Table ). Comparing the cell perimeter to surface contact area demonstrated that EGF-treated cells were significantly compacted compared with untreated cells, whereas IP-10 prevented this morphological change (P < 0.01), while not affecting basal cell morphometry. As this ratio may simply reflect centripetal contraction rather than motility-related changes, we also determined the asymmetry index of the nucleus localization; this should remain unchanged and central during retraction but be offset during locomotion. Again, EGF treatment resulted in a motile morphology which was abrogated by IP-10 pretreatment (P < 0.01). Thus, IP-10 inhibits EGF-induced cell compaction and results in elongated cell morphology, a phenotype reminiscent of cells that are unable to detach from substratum (Huttenlocher et al. 1997; Palecek et al. 1997).
Figure 3.
Morphology of EGF- and IP-10–treated cells. Cells were plated in 6-well tissue culture plates with DME containing 0.1% dialyzed FBS at the concentration of 105 cells/ml. After 12 h of incubation, cells were treated with EGF (1 nM) and IP-10 (50 ng/ml) for another 12 h, after which pictures were taken by phase-contrast microscopy. Pictures were representative fields from three independent experiments.
Table 1.
Hs68 Cellular Morphometry
| Treatment | Perimeter vs. area | Trend line | Asymmetry index |
|---|---|---|---|
| No tx | 3.66 ± 0.13 | 3.7223 | 18 ± 2 |
| EGF | 4.19 ± 0.14* | 4.6448 | 30 ± 2* |
| IP-10 | 3.51 ± 0.12 | 3.7940 | 21 ± 2 |
| IP-10 + EGF | 3.62 ± 0.13 | 3.6033 | 20 ± 2 |
Cells were plated at the concentration of 105 cell/ml and incubated in quiescent media for 24 h. Cells were treated with EGF (1 nM) and IP-10 (50 ng/ml) for 12 h. Phase-contrast images were captured at 0 and 12 h for image analysis. Perimeter and cell surface area were measured by manually tracking cell edges using DIAS image analyzing system (Solltech). Asymmetry indices were calculated as described in Materials and Methods. The data are the mean ± SEM (n = 100 cells from two experiments each analyzing 40–60 cells): * P < 0.01 compared to the other three conditions.
IP-10 Inhibits EGF-induced Cell Detachment and Cell Migration by Inhibiting Calpain Activation
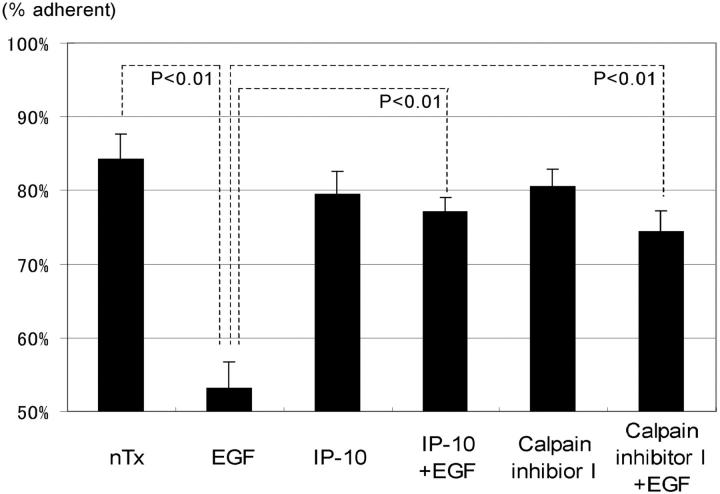
Morphological analyses suggested that EGF-induced cell detachment from substratum is inhibited by IP-10 signaling. Therefore, we assessed the effect of IP-10 on cell adhesion to the human extracellular matrix Amgel by the centrifugation detachment method. Subjecting the cells to 2,920 g for 5 min resulted in negligible removal of nontreated control Hs68 cells (84 ± 3% remaining) but about half of the EGF-treated cells (53 ± 4% remaining) (Fig. 4). IP-10 by itself slightly diminished cell adhesiveness (80 ± 3% remaining), but significantly diminished EGF-induced detachment (77 ± 2% remaining, P < 0.01 vs. EGF treatment).
Figure 4.
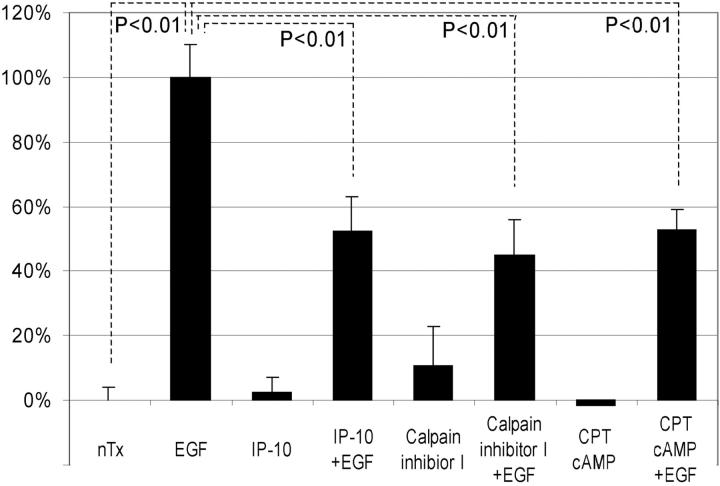
The effects of IP-10 and calpain inhibitor I on EGF-induced cell detachment. Cells were plated into the 24-well Amgel coated plates with quiescent media and incubated for 12 h. After pretreatment with or without IP-10 (50 ng/ml for 4 h) or calpain inhibitor I (1 ng/ml for 30 min), cells were treated with or without EGF (1 nM) for 30 min. Before and after the centrifugation, the numbers of the cells on the plates were counted under the phase-contrast microscope observation. The data are the mean ± SEM of three independent studies each performed in at least 18 wells. Statistical analyses were performed by Student's t test.
According to the morphological and cell detachment analyses, we predicted that EGF-induced focal adhesion disassembly and cell de-adhesion (Xie et al. 1998) are affected by IP-10. Therefore, we focused on biochemical events which affect focal adhesion disassembly, with calpain being a prime candidate due to its recent association with adhesion disruption and reorganization (Huttenlocher et al. 1997; Stewart et al. 1998). In addition, an ancillary study in our lab has found that EGF induces calpain activity in fibroblasts and that this is required for cell de-adhesion from substratum (Glading, A., P. Chang, D.A. Lauffenburger, and A. Wells, manuscript in preparation). In agreement with the hypothesis that calpain modulation leads to de-adhesion, the pharmacological inhibitor, calpain inhibitor I, also prevented EGF-induced detachment by 68% (74 ± 3% remaining). That this is linked to cell motility is shown by the fact that calpain inhibitor I also diminished EGF-induced, but not basal, motility (Fig. 5). Interestingly, this partial inhibition of EGF-induced cell motility would be expected from the report that de-adhesion of the trailing edge of cells is rate limiting only on highly adhesive surfaces (Palecek et al. 1997); thus, on a mixed substratum such as the one used, Amgel, one might predict only a partial effect of diminishing de-adhesion.
Figure 5.
The effects of calpain inhibitor I and CPT-cAMP on cell migration. Cells were grown to confluence and quiesced for 48 h in DME with 0.1% dialyzed FBS before treatment with or without IP-10 (1–50 ng/ml), EGF (1–10 nM), calpain inhibitor I (1 ng/ml), and/or CPT-cAMP (10 μM). Cell migration assays were performed as described in Materials and Methods. The data are shown as the ratio to the 1 nM EGF-induced cell migrative activity. The data are the mean ± SEM of at least three independent studies each performed in triplicate. Statistical analyses were performed by Student's t test.
A major question is how this inhibition may be signaled. IP-10 is a member of the subfamily of ELR-negative CXC chemokines which bind to and activate the CXCR3 receptor, leading to an increase in cAMP levels (Loetscher et al. 1996; Farber 1997). Therefore, we asked if this was the operative second messenger. We determined that IP-10 induced a slow rise of total cellular cAMP level up to approximately twofold at 4 h (1.97 ± 0.53-fold; P < 0.05), a response consistent with published reports on hematopoietic cells (Aronica et al. 1995). Thus, if cAMP was the initial second messenger, then a cAMP analogue, the cell permeable CPT-cAMP, should mimic the effects of IP-10. Similar to IP-10 itself, CPT-cAMP inhibits EGF-induced cell migration ∼50%, but not basal cell motility (Fig. 5). Another membrane permeant cAMP analogue, dibutyryl-cAMP, and the cAMP-generating agonist, forskolin, also reduced EGF-induced motility by about half (data not shown).
IP-10 Inhibits Calpain Activation and Cell Motility through cAMP-PKA-Calpain Signaling Pathway
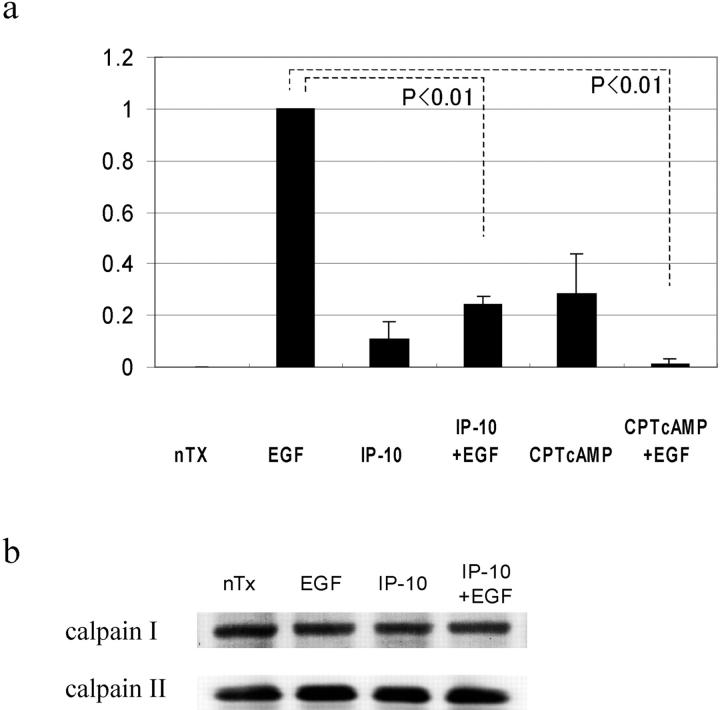
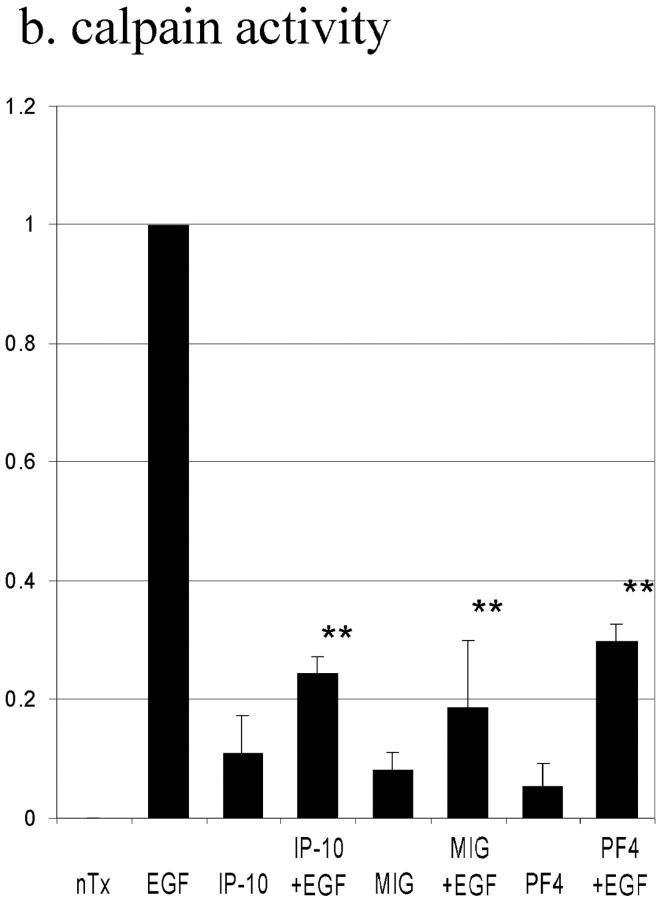
In parallel to its effect on EGF-induced cell adhesiveness and motility, IP-10 significantly inhibited EGF-induced calpain activity (by 71 ± 7%) (Fig. 6 a). If calpain activation was the point of signal convergence, then cAMP should also reduce calpain activity and de-adhesion. EGF-induced calpain activation in cells treated with CPT-cAMP was inhibited by 99 ± 2%. This decreased calpain activity was due to prevention of enzymatic activation and not a change in cellular levels of the calpain system as shown by relatively constant cellular levels of the calpains (Fig. 6 b) in the face of IP-10 treatment.
Figure 6.
EGF-induced calpain activation. (a) Cells were grown to confluence in 10-cm tissue culture plates and quiesced for 48 h with DME containing 0.1% dialyzed FBS. Cells were treated with IP-10 (50 ng/ml) for 4 h or CPT-cAMP (20 μM) for 30 min before EGF (1 nM) for 30 min. Calpain activities were analyzed by measuring the elevation in fluorescence intensity that occurs upon calpainolytic digestion of dichlorotriazinylamino-fluorescein–labeled MAP2. The data are shown as a ratio to EGF-induced calpain activation. The data are the mean ± SEM of at least three independent studies. Statistical analyses were performed by Student's t test. (b) Cells were grown to confluence in 6-well tissue culture plates and quiesced for 48 h. Cells were treated with IP-10 (50 ng/ml) for 4 h before EGF (1 nM) for 30 min. Cell lysates were separated by SDS-PAGE, transferred to Immobilon-P (Millipore), and immunoblotted with an anti–calpain I or –calpain II antibody (Biomol).
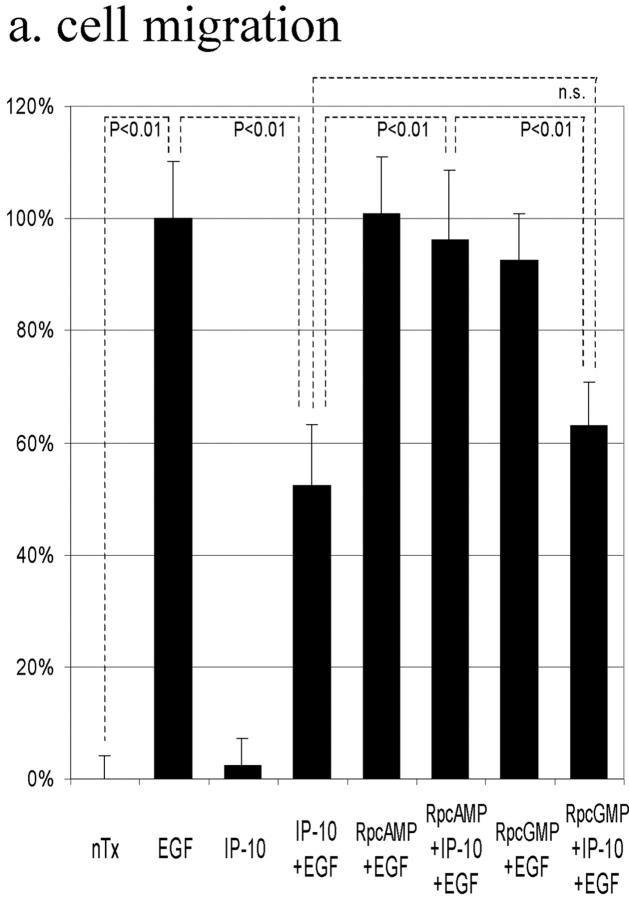
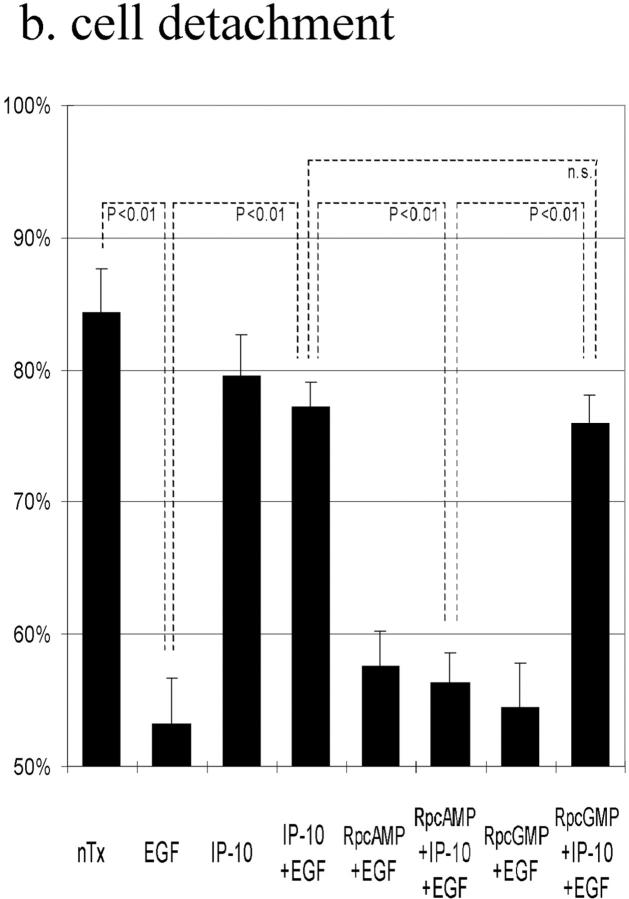
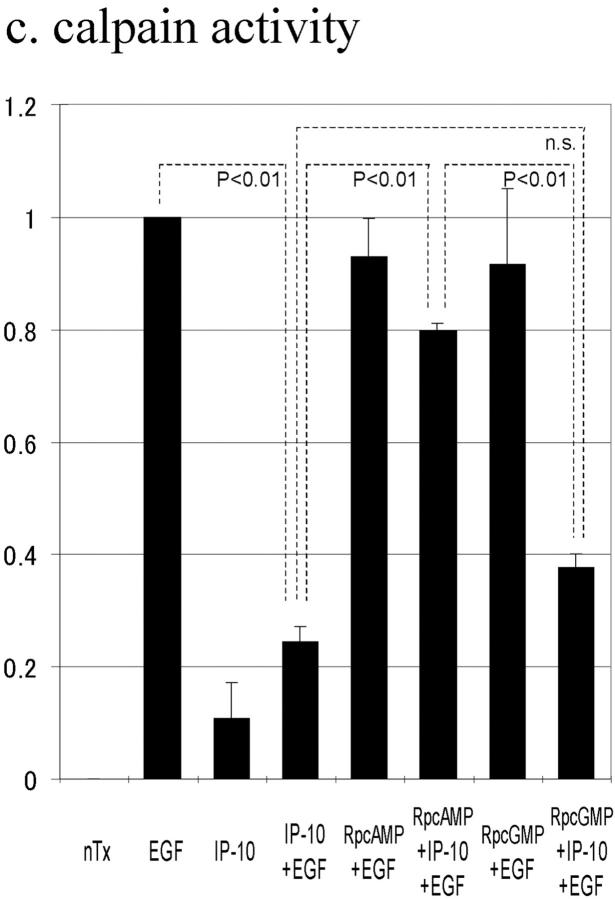
The above data suggested that IP-10 inhibits EGF-induced cell migration by inhibiting calpain activation through cAMP signaling. To determine whether cAMP-dependent PKA is involved in this IP-10 calpain signaling pathway, cell migration was performed in the presence of the cell permeant PKA inhibitor Rp-8-Br-cAMPS; the protein kinase G preferential inhibitor Rp-8-Br-cGMPS was used as a control. Rp-8-Br-cAMPS abrogated IP-10's inhibitory effect by 87%, but Rp-8-Br-cGMPS did not (Fig. 7 a). Rp-8-Br-cAMPS, but not Rp-8-Br-cGMPS, also prevented IP-10 from inhibiting EGF-induced detachment (Fig. 7 b). In support of our model that calpain activation is central to IP-10 inhibition of EGFR-mediated motility, the PKA preferential Rp-8-Br-cAMPS, but not the protein kinase G preferential inhibitor, abrogated the ability of IP-10 treatment to inhibit calpain activation almost completely (Fig. 7 c). In a parallel study, NR6 fibroblasts expressing full-length EGFR were stimulated to migrate in the presence of CPT-cAMP; time lapse videomicroscopy revealed that they were retarded in the ability to retract the trailing edge (data not shown), a cell behavior similar to that noted with IP-10 (Fig. 3).
Figure 7.
The effects of PKA inhibitor on IP-10 inhibition of EGF-induced cell migration (a), cell detachment (b), and calpain activation (c). (a) Cells were grown to confluence and quiesced for 48 h before treatment with or without IP-10 (50 ng/ml), Rp-8-Br-cAMPS (50 μM), Rp-8-Br-cGMPS (50 μM), and/or EGF (1 nM). Cell migration assays were performed as described in Materials and Methods. The data are shown as the ratio to the 1 nM EGF-induced cell migrative activity. The data are the mean ± SEM of more than three independent studies each performed in triplicate. Statistical analyses were performed by Student's t test. (b) Cells were plated into the 24-well Amgel coated plates with quiescent media and incubated for 12 h. After pretreatment with or without IP-10 (50 ng/ml), Rp-8-Br-cAMPS (50 μM), and/or Rp-8-Br-cGMPS (50 μM) for 4 h, cells were treated with or without EGF (1 nM) for 30 min. Before and after centrifugation, the numbers of the cells on the plates were counted under the phase-contrast microscope observation. The data are the mean ± SEM of three independent studies each performed in at least 18 wells. Statistical analyses were performed by Student's t test. (c) Cells were grown to confluence in 10-cm tissue culture plates and quiesced for 48 h. Cells were treated with or without IP-10 (50 ng/ml), Rp-8-Br-cAMPS (50 μM), and/or Rp-8-Br-cGMPS (50 μM) for 4 h before EGF (1 nM) for 30 min. Calpain activities were analyzed by measuring the elevation in fluorescence intensity that occurs upon calpainolytic digestion of dichlorotriazinylamino-fluorescein–labeled MAP2. The data are shown as a ratio to EGF-induced calpain activation. The data are the mean ± SEM of at least three independent studies. Statistical analyses were performed by Student's t test.
Other ELR-negative CXC Chemokines Also Increase Total Cellular cAMP Level and Inhibit EGF-induced Cell Migration by Inhibiting EGF-induced Calpain Activation
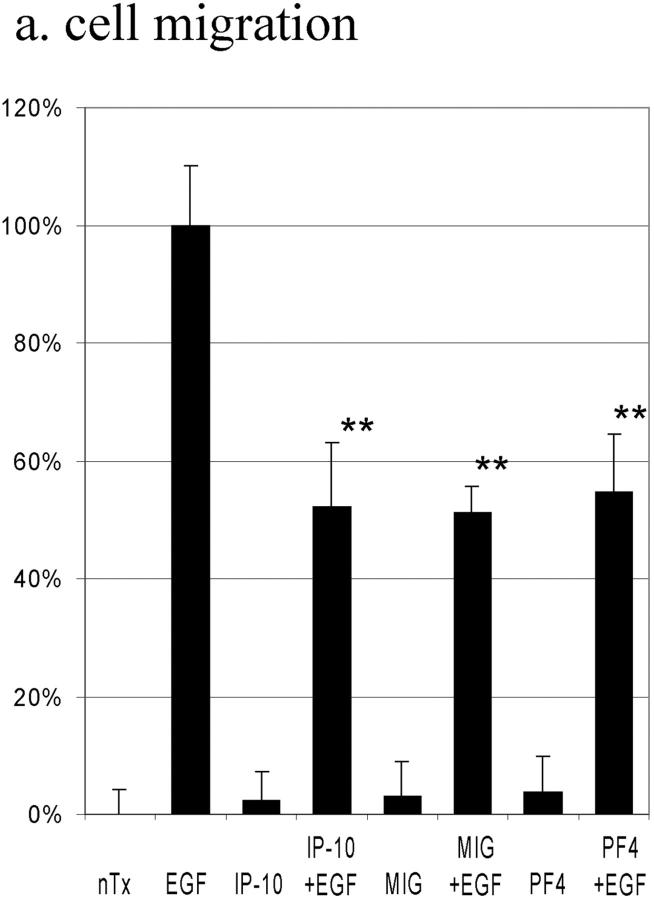
One of the predictions from our model of chemokine negative crossmodulation of growth factor receptor–mediated motility is that other chemokines that similarly lead to cAMP generation also would preferentially inhibit EGF-induced motility. The effects of two other ELR-negative CXC chemokines, MIG and PF4, were determined. These chemokines induced slow accumulation of intracellular cAMP (at 4 h: MIG, 1.92 ± 0.38-fold; PF4, 2.13 ± 0.49-fold) that was indistinguishable from IP-10. Both MIG (49 ± 4% inhibition) and PF4 (45 ± 10% inhibition) inhibited EGF-induced, but not basal motility (Fig. 8 a), and had little effect on thymidine incorporation (data not shown). As per the proposed model, both MIG and PF4 inhibited EGF-induced calpain activity (Fig. 8 b).
Figure 8.
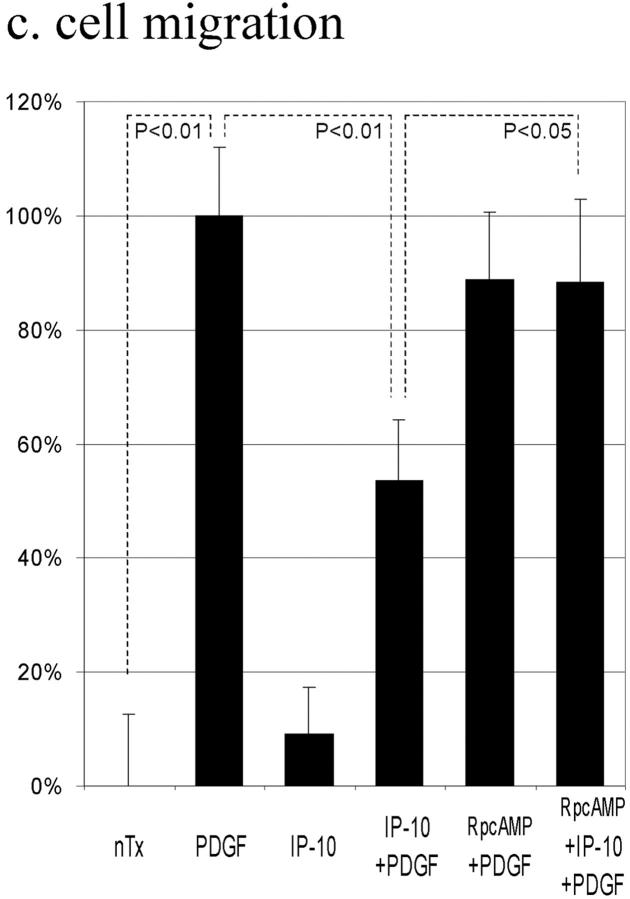
The effects of ELR-negative CXC chemokines on EGF-induced cell migration (a), EGF-induced calpain activation (b), and PDGF-induced motility (c). (a) Cells were grown to confluence and quiesced for 48 h before treatment with or with out IP-10 (50 ng/ml), MIG (100 ng/ml), PF4 (50 ng/ml), and/or EGF (1 nM). Cell migration assays were performed as described in Materials and Methods. The data are shown as the ratio to the 1 nM EGF-induced cell migrative activity. The data are the mean ± SEM of at least three independent studies each performed in triplicate. Statistical analyses were performed by Student's t test as compared with EGF-induced cell migrative capacity in the absence of CXC chemokines: **P < 0.01. (b) Cells were grown to confluence in 10-cm tissue culture plates and quiesced for 48 h. Cells were treated with or without IP-10 (50 ng/ml), MIG (100 ng/ml), and/or PF4 (50 ng/ml) for 4 h before EGF (1 nM) for 30 min. Calpain activities were analyzed by measuring the elevation in fluorescence intensity that occurs upon calpainolytic digestion of dichlorotriazinylamino-fluorescein–labeled MAP2. The data are shown as a ratio to EGF-induced calpain activation. The data are the mean ± SEM of at least three independent studies. Statistical analyses were performed by Student's t test as compared with EGF-induced calpain activation in the absence of CXC chemokines: **P < 0.01. (c) Cells were grown to confluence and quiesced for 48 h before treatment with or without IP-10 (50 ng/ml), Rp-8-Br-cAMPS (50 μM), and/or PDGF-BB (5 ng/ml). Cell migration assays were performed as described in Materials and Methods. The data are shown as the ratio to the 5 ng/ml PDGF-induced cell migrative activity. The data are the mean ± SEM of two independent studies each performed in triplicate. Statistical analyses were performed by Student's t test.
A second prediction would be that IP-10 would prevent motility induced by other growth factors. We examined PDGF-induced motility of the Hs68 cells. Similar to its effect on EGF-induced motility, IP-10 partially blocked PDGF-induced motility by 46 ± 11% (Fig. 8 c). That this is accomplished through the same signaling pathway as that which inhibits EGF-induced motility is demonstrated by IP-10 inhibition of PDGF-induced motility also being abrogated by Rp-8-Br-cAMPS (Fig. 8 c). These findings support the model that growth factor–induced motility depends, at least in part, on a calpain-mediated de-adhesion step which may be negatively impacted by chemokine generation of cAMP.
Discussion
We present here evidence that the counterstimulatory chemokine IP-10 affects dermal fibroblast cell responses to growth factors in addition to its known actions on hematopoietic and endothelial cells. We demonstrated that IP-10 inhibits EGFR-mediated motility specifically, likely via a cAMP/PKA-dependent inhibition of EGFR-mediated calpain activation. To our knowledge, this is the first report of the inhibitory effects of IP-10 on growth factor–induced fibroblast motility. These actions implicate a role for IP-10 in limiting fibroblast infiltration during wound healing in response to locally expressed growth factors.
We determined that IP-10 did not disrupt EGFR signaling at the ligand or receptor level. This was expected as (a) both EGF- and HB-EGF–induced motility was similarly inhibited; and (b) EGFR-mediated proliferation was unaffected by IP-10 pretreatment. As cellular response signaling diverges at the immediate postreceptor level with at least one pathway, via PLC-γ, being required for motility but not proliferation (Chen et al. 1994a,Chen et al. 1994b, Chen et al. 1996), it was reasonable to investigate whether IP-10 differentially abrogated signaling through the PLC-γ pathway. The activation status of this pathway and a second pathway required for both motility and mitogenesis, through erk MAPK, as mirrored by tyrosyl-phosphorylation, was investigated and found to be unaffected by IP-10 treatment. As the links between these and other postreceptor pathways and the biophysical events which actuate motility are still incompletely deciphered (Wells et al. 1998), the affected biophysical process was approached by morphometric analyses. IP-10 inhibited EGF-induced cell retraction and resulted in an elongated cell morphology reminiscent of failure to detach to the uropod. Rear detachment is necessary for migration (Chen 1981; Huttenlocher et al. 1997; Palecek et al. 1998). Taken together with our previous finding that EGF induces focal adhesion disassembly and cell de-adhesion from substratum (Xie et al. 1998), we predicted this process is negatively modulated by IP-10 treatment. 4-h treatment with IP-10 before EGF exposure reduced the EGF-induced cell detachment by over half (compared with IP-10 alone) in the centrifugation detachment assay. Interestingly, IP-10 alone caused a small but reproducible decrease in adhesiveness, though the predominant effect was to block the much greater EGF-induced de-adhesion. Thus, IP-10 does not globally increase adhesiveness to substratum but rather inhibits the EGF-induced modulation of cell-substratum interactions necessary for cell migration. This may be the basis for the differential outcome of IP-10 limiting EGF-induced motility but having no discernible effect on basal, haptokinetic motility.
IP-10 could limit EGFR-mediated detachment, and thus motility, by either anchoring the adhesion sites or disrupting signaling pathways leading to de-adhesion. We did not favor the former, as IP-10 has no discernible effect on basal cell motility or morphometry; alterations in these assays due to inhibition of basal haptokinesis would be expected if IP-10 directly strengthened adhesive sites (Huttenlocher et al. 1997; Palecek et al. 1997). Furthermore, IP-10 alone diminished cell adhesiveness, albeit slightly. Rather, we focused on signaling pathways downstream of MEK by which EGFR signaling decreases cell-substratum attachment (Xie et al. 1998). Calpain, which we have shown to be activated by EGFR signaling in related studies (Glading, A., P. Chang, D.A. Lauffenburger, and A. Wells, manuscript in preparation), has been implicated recently in the control of cell-substratum interactions (Huttenlocher et al. 1997; Stewart et al. 1998). Calpain localizes to focal adhesion and establishes associations with focal adhesion components including integrin cytoplasmic domains (Beckerle et al. 1987; Du et al. 1995; Cooray et al. 1996; Inomata et al. 1996). A reduction in calpain activity inhibits cell migration by decreasing the rate of cell detachment and stabilizing integrin-cytoskeletal linkages (Huttenlocher et al. 1997). To assess whether calpain activation may be the target of IP-10 signaling, we found that IP-10 treatment inhibited ∼70% of EGF-induced calpain activation. This inhibition is concordant with the inhibition of EGF-induced de-adhesion and motility. Treating the cells with a calpain inhibitor also resulted in EGF-induced motility and de-adhesion being reduced similarly to IP-10 treatment. As an aside, as calpain has been implicated in the formation of new adhesions and thus may be required for stable adhesion to substratum, it may be expected that negative regulation of calpain activity by IP-10 may lead to lessened adhesiveness even in the absence of EGF. This is what was noted by the centrifugation detachment assay.
The molecular bases of both the calpain activation by EGFR signaling and its disruption by IP-10 receptor signaling are unknown. As IP-10 has been reported to bind to CXCR3 and elevate cAMP levels (Aronica et al. 1995; Loetscher et al. 1996), we examined this possibility. IP-10 caused a slow rise in intracellular cAMP in human skin fibroblast Hs68, consistent with the previous report of hematopoietic cells (Aronica et al. 1995). The cell permeant cAMP analogue, CPT-cAMP, inhibited EGF-induced calpain activation almost completely. CPT-cAMP, dibutyryl-cAMP, and forskolin also inhibited EGF-induced cell migration similarly to IP-10. In addition, time lapse videomicroscopy of the murine WT NR6 fibroblasts stimulated to move by EGF clearly demonstrated significantly decreased motility in the presence of CPT-cAMP while showing that the presence of CPT-cAMP did not retard forward extension but limited the detachment of the cell tails; often cells were seen to recoil back to their original position (data not shown). Thus, the biophysical behavior of cells in the presence of increased cAMP mimicked that of cells treated with IP-10. The cell permeable cAMP-dependent PKA inhibitor Rp-8-Br-cAMPS, but not the PKG preferential inhibitor Rp-8-Br-cGMPS, abrogated IP-10's inhibitory effect on cell migration, cell detachment, and calpain activation. According to our results, IP-10 likely works by activating cAMP-dependent PKA. At this point we have not eliminated the possibility that cAMP-dependent PKA might also affect focal adhesions directly through phosphorylation of paxillin (Han and Rubin 1996) or affect other components of the adhesion apparatus (Brown et al. 1998). Thus, cAMP seems to be the operative second messenger for this IP-10 attenuation of EGFR signaling. How cAMP and PKA prevent EGF-induced activation of calpain is being explored. Initial data suggest that IP-10 prevents an EGFR-mediated dissociation of calpain from its endogenous inhibitor calpastatin (data not shown). This is compelling as calpain II, the isoform implicated as the target for EGF induction (Glading, A., P. Chang, D.A. Lauffenburger, and A. Wells, manuscript in preparation), presents an evolutionarily conserved PKA consensus site in domain III (Imajoh et al. 1988). However, several additional investigations, including molecular abrogation of putative kinase sites, will be required to determine the exact mechanism by which PKA attenuates growth factor–induced calpain activation.
There appears to be a discrepancy between IP-10's predominant inhibition of calpain activation and only partial inhibition of cell motility and de-adhesion. This difference may suggest that IP-10 inhibition of EGF-induced de-adhesion and motility is independent of suppression of calpain activation. However, this is unlikely, as exposure of the cells to calpain inhibitor I presented a similar partial inhibition of motility and de-adhesion. While we and others have not yet determined the exact mechanism or precise molecular targets by which calpain modulates cell-substratum adhesion (Huttenlocher et al. 1997; Byzova and Plow 1998; Stewart et al. 1998), these data strongly link calpain functioning to cell adhesion. The quantitative discrepancy between the extent of calpain inhibition and limiting de-adhesion likely highlights a situation in which calpain-mediated de-adhesion is only one of many modulated events involved in cell migration. Abrogation of calpain signaling in CHO cells did not completely prevent rear detachment, and in fact, this effect was barely noted on a substratum of low adhesiveness, suggesting that rear detachment may be accomplished in absence of calpain activation if the linkage with substratum is weak (Huttenlocher et al. 1997). Huttenlocher et al. 1997 concluded that rear detachment is one rate-limiting step in motility, but calpain activity becomes a predominant rate-limiting biochemical signal only when cells migrate over substrata of high adhesiveness. We analyze motility and de-adhesion on biologically complex matrices which contain both adhesive and antiadhesive signals (Siegal et al. 1993; Ware et al. 1998), and thus the Hs68 cells may be on substrata for which rear detachment can occur, at least partly, in the absence of calpain activation. Additionally, IP-10 may modulate cell adhesiveness via pathways in addition to calpain (Han and Rubin 1996); in fact, IP-10 itself slightly decreases adhesiveness, though a similar small decrease in adhesiveness is noted in the presence of calpain inhibitor I. In summary, the extent of inhibition of calpain is consistent with the observed lesser decrements in de-adhesion and motility.
Two predictions derive from this model in which calpain serves as a point of convergence for pro-motility signals and their counterregulatory signals. The first is that other extracellular signals that increase cAMP in a similar fashion to IP-10 should also decrease EGF-induced motility. We determined that the other ELR-negative CXC chemokines, MIG and PF4, also inhibited EGF-induced cell migration. These chemokines induced cAMP generation and inhibited EGF-induced calpain activation indistinguishably from IP-10. The second prediction is that these chemokines should abrogate motility signaled by other growth factors. Motility induced by another growth factor present during wound healing, PDGF, was partially limited by IP-10. That these two postulates are supported by experimental data strongly supports our model of IP-10 limiting growth factor–induced cell migration by inhibiting calpain activation.
In summary, we have defined for the first time a regulatory crosstalk between counterstimulatory chemokines and growth factor receptors. That this is specific for one cellular response to a pleiotropic signal highlights the closely orchestrated control of wound healing. One could easily envision a function for IP-10 in this respect; IP-10 would limit cell infiltration late in the reparative phase while leaving untouched other EGFR-mediated responses, such as production of matrix components and remodeling enzymes (Mawatari et al. 1989; Shima et al. 1993; Kondapaka et al. 1997; van der Zee et al. 1998). Furthermore, during the important phase of fibroblast-mediated wound contraction, IP-10–signaled inhibition of calpain activation would prevent breakdown of critical cell-matrix connections. A similar scenario could be invoked for MIG-mediated inhibition of calpain activation, as this chemokine also is generated late in wound healing (Engelhardt et al. 1998). Seemingly, PF4 presents a different situation in that is it released in large quantities from platelets during the earliest stage of wounding. However, this may be critical to appropriately targeting fibroblasts during wound healing. We have demonstrated that during chemokinesis in response to growth factors, cell locomotive speed is increased but persistence decreased so that dispersion is maximized (Ware et al. 1998). Thus, speed is intrinsic to the cell upon EGFR signaling, but localization would be dictated by external signals. PF4 could be one such external signal that limits motility as fibroblasts approach the initial platelet plug. As fibroblasts migrate into the wound site, they would accumulate nearest the platelet plug, but still be able to proliferate and perform other functions to replace the dermal tissue in response to the TGF-α and HB-EGF from platelets and macrophages, as well as other growth factors such as PDGF. As the wound healing progresses, the PF4 would be consumed and the motility block would be relieved with the fibroblasts then able to migrate to appropriate positions for the reparative phase of wound healing. While at present this is just one possible explanation for the specific counterregulatory effects of the ELR-negative CXC chemokines, it provides for a working model to test these cell behaviors and signaling pathways. Deciphering the precise molecular mechanisms will not only provide insight into the complex network of communications operational during organogenesis and repair, but also suggest targets for rational intervention.
Acknowledgments
We thank Tom Lincoln, Anna Huttenlocher, Doug Lauffenburger, Kathryn Drabik, Jeffry Chou, Philip Chang, Jareer Kassis, Jose Souto, Scott Swindle, and Hyung Kim for valuable suggestions.
This work was supported by National Institutes of Health, National Institute of General Medical Sciences grant GM54739.
Footnotes
1.used in this paper: CPT-cAMP, 8-(4-chlorophenylthio)-cAMP; EGFR, EGF receptor; HB-EGF, heparin-binding EGF-like growth factor; IP-10, interferon inducible protein-10; MAP2, microtubule-associated protein 2; MAPK, mitogen-activated protein kinase; MIG, monokine induced by IFN-γ; PF4, platelet factor 4; PKA, protein kinase A; PLC-γ, phospholipase C-γ
Hidenori Shiraha's and Angela Glading's present address is Department of Pathology, University of Pittsburgh, Pittsburgh, PA 15261.
References
- Aronica S.M., Mantel C., Gonin R., Marshall M.S., Sarris A., Cooper S., Hague N., Zhang X.F., Broxmeyer H.E. Interferon-inducible protein 10 and macrophage inflammatory protein-1α inhibit growth factor stimulation of Raf-1 kinase activity and protein synthesis in a human growth factor–dependent hematopoietic cell line. J. Biol. Chem. 1995;270:21998–22007. doi: 10.1074/jbc.270.37.21998. [DOI] [PubMed] [Google Scholar]
- Barrandon Y., Green H. Cell migration is essential for sustained growth of keratinocyte coloniesthe roles of transforming growth factor–α and epidermal growth factor. Cell. 1987;50:1131–1137. doi: 10.1016/0092-8674(87)90179-6. [DOI] [PubMed] [Google Scholar]
- Beckerle M.C., Burridge K., DeMartino G.N., Croall D.E. Colocalization of calcium-dependent protease II and one of its substrates at sites of cell adhesion. Cell. 1987;51:569–577. doi: 10.1016/0092-8674(87)90126-7. [DOI] [PubMed] [Google Scholar]
- Blay J., Brown K.D. Epidermal growth factor promotes the chemotactic migration of cultured rat intestinal epithelial cells. J. Cell. Physiol. 1985;124:107–112. doi: 10.1002/jcp.1041240117. [DOI] [PubMed] [Google Scholar]
- Blotnick S., Peoples G.E., Freeman M.R., Eberlein T.J., Klagsbrun M. T lymphocytes synthesize and export heparin-binding epidermal growth factor–like growth factor and basic fibroblast growth factor, mitogens for vascular cells and fibroblastsdifferential production and release by CD4+ and CD8+ T cells. Proc. Natl. Acad. Sci. USA. 1994;91:2890–2894. doi: 10.1073/pnas.91.8.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuing K., Andree C., Helo G., Slama J., Liu P.Y., Eriksson E. Growth factors in the repair of partial thickness porcine skin wounds. Plast. Reconstr. Surg. 1997;100:657–664. doi: 10.1097/00006534-199709000-00018. [DOI] [PubMed] [Google Scholar]
- Brown M.C., Perrotta J.A., Turner C.E. Serine and threonine phosphorylation of the paxillin LIM domains regulates paxillin focal adhesion localization and cell adhesion to fibronectin. Mol. Biol. Cell. 1998;9:1803–1816. doi: 10.1091/mbc.9.7.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byzova T.V., Plow E.F. Activation of αvβ3 on vascular cells controls recognition of prothrombin. J. Cell Biol. 1998;143:2081–2092. doi: 10.1083/jcb.143.7.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Human epidermal growth factorbinding of the polypeptide to human fibroblasts and stimulation of cell proliferation. Natl. Cancer Inst. Monogr. 1978;48:149–156. [PubMed] [Google Scholar]
- Chen P., Gupta K., Wells A. Cell movement elicited by epidermal growth factor receptor requires kinase and autophosphorylation but is separable from mitogenesis J. Cell Biol. 124 1994. 547 555a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Xie H., Sekar M.C., Gupta K., Wells A. Epidermal growth factor receptor-mediated cell motilityphospholipase C activity is required, but mitogen-activated protein kinase activity is not sufficient for induced cell movement J. Cell Biol. 127 1994. 847 857b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Xie H., Wells A. Mitogenic signaling from the EGF receptor is attenuated by a phospholipase C-γ/protein kinase C feedback mechanism. Mol. Biol. Cell. 1996;7:871–881. doi: 10.1091/mbc.7.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.T. Mechanism of retraction of the trailing edge during fibroblast movement. J. Cell Biol. 1981;90:187–200. doi: 10.1083/jcb.90.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colige A., Nusgens B., Lapiere C.M. Effect of EGF on human skin fibroblasts is modulated by the extracellular matrix Arch. Dermatol. Res. 280Suppl.1988. S42 S46 [PubMed] [Google Scholar]
- Cooray P., Yuan Y., Schoenwaelder S.M., Mitchell C.A., Salem H.H., Jackson S.P. Focal adhesion kinase (pp125FAK) cleavage and regulation by calpain. Biochem. J. 1996;318:41–47. doi: 10.1042/bj3180041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X., Saido T.C., Tsubuki S., Indig F.E., Williams M.J., Ginsberg M.H. Calpain cleavage of the cytoplasmic domain of the integrin β3 subunit. J. Biol. Chem. 1995;270:26146–26151. doi: 10.1074/jbc.270.44.26146. [DOI] [PubMed] [Google Scholar]
- Engelhardt E., Toksoy A., Goebeler M., Debus S., Brocker E.B., Gillitzer R. Chemokines IL-8, GROα, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am. J. Pathol. 1998;153:1849–1860. doi: 10.1016/s0002-9440(10)65699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber J.M. Mig and IP-10CXC chemokines that target lymphocytes. J. Leukoc. Biol. 1997;61:246–257. [PubMed] [Google Scholar]
- Gospodarowicz D., Mescher A.L. A comparison of the responses of cultured myoblasts and chondrocytes to fibroblast and epidermal growth factors. J. Cell. Physiol. 1977;93:117–127. doi: 10.1002/jcp.1040930115. [DOI] [PubMed] [Google Scholar]
- Han J.D., Rubin C.S. Regulation of cytoskeleton organization and paxillin dephosphorylation by cAMP Studies on murine Y 1adrenal cells. J. Biol. Chem. 2711996. 29211 29215 [DOI] [PubMed] [Google Scholar]
- Hay E.D. Extracellular matrix alters epithelial differentiation. Curr. Opin. Cell Biol. 1993;5:1029–1035. doi: 10.1016/0955-0674(93)90088-8. [DOI] [PubMed] [Google Scholar]
- Huttenlocher A., Palecek S.P., Lu Q., Zhang W., Mellgren R.L., Lauffenburger D.A., Ginsberg M.H., Horwitz A.F. Regulation of cell migration by the calcium-dependent protease calpain. J. Biol. Chem. 1997;272:32719–32722. doi: 10.1074/jbc.272.52.32719. [DOI] [PubMed] [Google Scholar]
- Imajoh S., Aoki K., Ohno S., Emori Y., Kawasaki H., Sugihara H., Suzuki K. Molecular cloning of the cDNA for the large subunit of the high–Ca2+-requiring form of human Ca 2+-activated neutral protease. Biochemistry. 1988;27:8122–8128. doi: 10.1021/bi00421a022. [DOI] [PubMed] [Google Scholar]
- Inomata M., Hayashi M., Ohno-Iwashita Y., Tsubuki S., Saido T.C., Kawashima S. Involvement of calpain in integrin-mediated signal transduction. Arch. Biochem. Biophys. 1996;328:129–134. doi: 10.1006/abbi.1996.0152. [DOI] [PubMed] [Google Scholar]
- Kiritsy C.P., Lynch A.B., Lynch S.E. Role of growth factors in cutaneous wound healing. Crit. Rev. Oral Biol. Med. 1993;4:729–760. doi: 10.1177/10454411930040050401. [DOI] [PubMed] [Google Scholar]
- Kondapaka S.B., Fridman R., Reddy K.B. Epidermal growth factor and amphiregulin up-regulate matrix metalloproteinase-9 (MMP-9) in human breast cancer cells. Int. J. Cancer. 1997;70:722–726. doi: 10.1002/(sici)1097-0215(19970317)70:6<722::aid-ijc15>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Lawrence W.T., Diegelmann R.F. Growth factors in wound healing. Clin. Dermatol. 1994;12:157–169. doi: 10.1016/0738-081x(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Loetscher M., Gerber B., Loetscher P., Jones S.A., Piali L., Clark-Lewis I., Baggiolini M., Moser B. Chemokine receptor specific for IP10 and MIGstructure, function, and expression in activated T-lymphocytes. J. Exp. Med. 1996;184:963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster A.D., Greenberg S.M., Leder P. The IP-10 chemokine binds to a specific cell surface heparan sulfate site shared with platelet factor 4 and inhibits endothelial cell proliferation. J. Exp. Med. 1995;182:219–231. doi: 10.1084/jem.182.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawatari M., Kohno K., Mizoguchi H., Matsuda T., Asoh K., Van Damme J., Welgus H.G., Kuwano M. Effects of tumor necrosis factor and epidermal growth factor on cell morphology, cell surface receptors, and the production of tissue inhibitor of metalloproteinases and IL-6 in human microvascular endothelial cells. J. Immunol. 1989;143:1619–1627. [PubMed] [Google Scholar]
- Palecek S.P., Loftus J.C., Ginsberg M.H., Lauffenburger D.A., Horwitz A.F. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- Palecek S.P., Huttenlocher A., Horwitz A.F., Lauffenburger D.A. Physical and biochemical regulation of integrin release during rear detachment of migrating cells. J. Cell Sci. 1998;111:929–940. doi: 10.1242/jcs.111.7.929. [DOI] [PubMed] [Google Scholar]
- Pan Z., Kravchenko V.V., Ye R.D. Platelet-activating factor stimulates transcription of the heparin-binding epidermal growth factor-like growth factor in monocytes. Correlation with an increased κB binding activity. J. Biol. Chem. 1995;270:7787–7790. doi: 10.1074/jbc.270.14.7787. [DOI] [PubMed] [Google Scholar]
- Shima I., Sasaguri Y., Kusukawa J., Nakano R., Yamana H., Fujita H., Kakegawa T., Morimatsu M. Production of matrix metalloproteinase 9 (92-kDa gelatinase) by human oesophageal squamous cell carcinoma in response to epidermal growth factor. Br. J. Cancer. 1993;67:721–727. doi: 10.1038/bjc.1993.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal G.P., Wang M.H., Rinehart C.A., Jr., Kennedy J.W., Goodly L.J., Miller Y., Kaufman D.G., Singh R.K. Development of a novel human extracellular matrix for quantitation of the invasiveness of human cells. Cancer Lett. 1993;69:123–132. doi: 10.1016/0304-3835(93)90164-5. [DOI] [PubMed] [Google Scholar]
- Steenfos H.H. Growth factors and wound healing. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1994;28:95–105. doi: 10.3109/02844319409071186. [DOI] [PubMed] [Google Scholar]
- Stewart M.P., McDowall A., Hogg N. LFA-1–mediated adhesion is regulated by cytoskeletal restraint and by a Ca2+-dependent protease, calpain. J. Cell Biol. 1998;140:699–707. doi: 10.1083/jcb.140.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieter R.M., Kunkel S.L., Arenberg D.A., Burdick M.D., Polverini P.J. Interferon γ-inducible protein 10 (IP-10), a member of the C-X-C chemokine family, is an inhibitor of angiogenesis. Biochem. Biophys. Res. Commun. 1995;210:51–57. doi: 10.1006/bbrc.1995.1626. [DOI] [PubMed] [Google Scholar]
- Tompa P., Schad E., Baki A., Alexa A., Batke J., Friedrich P. An ultrasensitive, continuous fluorometric assay for calpain activity. Anal. Biochem. 1995;228:287–293. doi: 10.1006/abio.1995.1352. [DOI] [PubMed] [Google Scholar]
- van der Zee E., Jansen I., Hoeben K., Beertsen W., Everts V. EGF and IL-1α modulate the release of collagenase, gelatinase and TIMP-1 as well as the release of calcium by rabbit calvarial bone explants. J. Periodontal Res. 1998;33:65–72. doi: 10.1111/j.1600-0765.1998.tb02293.x. [DOI] [PubMed] [Google Scholar]
- Ware M.F., Wells A., Lauffenburger D.A. Epidermal growth factor alters fibroblast migration speed and derectional persistence reciprocally and in a matrix-dependent manner. J. Cell Sci. 1998;111:2423–2432. doi: 10.1242/jcs.111.16.2423. [DOI] [PubMed] [Google Scholar]
- Wells A., Gupta K., Chang P., Swindle S., Glading A., Shiraha H. Epidermal growth factor receptor-mediated motility in fibroblasts. Microsc. Res. Tech. 1998;43:395–411. doi: 10.1002/(SICI)1097-0029(19981201)43:5<395::AID-JEMT6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Xie H., Pallero M.A., Gupta K., Chang P., Ware M.F., Witke W., Kwiatkowski D.J., Lauffenburger D.A., Murphy-Ullrich J.E., Wells A. EGF receptor regulation of cell motilityEGF induces disassembly of focal adhesions independently of the motility-associated PLC γ signaling pathway. J. Cell Sci. 1998;111:615–624. doi: 10.1242/jcs.111.5.615. [DOI] [PubMed] [Google Scholar]