Abstract
During telophase, Golgi cisternae are regenerated and stacked from a heterogeneous population of tubulovesicular clusters. A cell-free system that reconstructs these events has revealed that cisternal regrowth requires interplay between soluble factors and soluble N-ethylmaleimide (NEM)–sensitive fusion protein (NSF) attachment protein receptors (SNAREs) via two intersecting pathways controlled by the ATPases, p97 and NSF. Golgi reassembly stacking protein 65 (GRASP65), an NEM-sensitive membrane-bound component, is required for the stacking process. NSF-mediated cisternal regrowth requires a vesicle tethering protein, p115, which we now show operates through its two Golgi receptors, GM130 and giantin. p97-mediated cisternal regrowth is p115-independent, but we now demonstrate a role for p115, in conjunction with its receptors, in stacking p97 generated cisternae. Temporal analysis suggests that p115 plays a transient role in stacking that may be upstream of GRASP65-mediated stacking. These results implicate p115 and its receptors in the initial alignment and docking of single cisternae that may be an important prerequisite for stack formation.
Keywords: Golgi apparatus, mitosis, p115, tethering, stack
The Golgi apparatus exists as a series of flattened cisternal membranes that are tightly aligned in parallel to one another to form a stack. Transport vesicles are closely associated, often by fibrous attachments (Weidman et al. 1993; Orci et al. 1998) with the fenestrated cisternal rims at every layer of the stack. This unique architecture is highly conserved throughout eukaryotic evolution, and is even found in some stages of the life cycle of the diplomonad Giardia lamblia, one of the most primitive extant eukaryotes (Luján et al. 1995). In mammalian cells, the discrete Golgi stacks are linked to form a tight, juxtanuclear, usually pericentriolar, twisting ribbon bound on either face by extensive tubulovesicular networks (Rambourg and Clermont 1997). This unit receives virtually the entire output of de novo synthesized polypeptides from the ER at the cis-Golgi network, and functions to post-translationally modify them in the stack, sort them in the TGN, and shuttle them on to their appropriate, ultimate destinations (Mellman and Simons 1992).
To elucidate determinants that are important for the maintenance and establishment of this morphology, we have exploited the fact that during mammalian M phase, this elaborate structure is dramatically transformed into Golgi mini-stacks during prophase, and then a disseminated array of tubulovesicular clusters during metaphase (Lucocq et al. 1987; Shima et al. 1997, Shima et al. 1998), as part of the process that ensures Golgi apparatus inheritance through successive generations. These tubulovesicular clusters undergo variable amounts of further vesiculation (Lucocq et al. 1987; Jesch and Linstedt 1998), which may be cell type specific (Stanley et al. 1997), and are partitioned accurately between the two nascent daughter cells using the astral microtubules of the mitotic spindle (Shima et al. 1998) and not through redistribution to the ER (Jesch and Linstedt 1998; Shima et al. 1998; Farmaki et al. 1999). At telophase, these tubulovesicular clusters are remodeled to produce Golgi mini-stacks that then coalesce to reform the Golgi ribbon (Lucocq et al. 1989). In an attempt to delineate the molecular mechanisms responsible for some of these events, a cell-free system has been established that largely reconstructs the events of metaphase disassembly and telophase reassembly of Golgi mini-stacks (Misteli and Warren 1994; Rabouille et al. 1995a,Rabouille et al. 1995b).
From the cell-free system, mitotic disassembly appears to proceed via two independent, concurrent pathways. The coat protein I (COPI)1-dependent pathway proceeds as COPI vesicles continue to bud, but are unable to tether and therefore fuse with their target membrane due to a Cdc2 kinase-mediated event (Nakamura et al. 1997; Lowe et al. 1998). This pathway likely consumes the peripheral rims of cisternae and accounts for up to 65% of the total cisternal membrane (Misteli and Warren 1994). An ill-characterized COPI-independent pathway is thought to convert the flattened cisternal cores into a heterogeneous array of tubulovesicular profiles (Misteli and Warren 1995).
A possible molecular explanation for the accumulation of COPI vesicles at mitosis lies in the fact that the binding of p115, a vesicle tethering protein, to Golgi membranes is significantly inhibited at mitosis (Levine et al. 1996). p115 was originally identified as a high molecular weight component required for intra-Golgi membrane transport (Waters et al. 1992) and is capable of tethering COPI vesicles to Golgi membranes in vitro (Sönnichsen et al. 1998). The yeast homologue of p115, Uso1p, is required for tethering COPII vesicles at a prevesicle/target-soluble N-ethylmaleimide (NEM)–sensitive fusion protein (NSF) attachment protein (SNAP) receptor (SNARE) docking step (Sapperstein et al. 1996; Cao et al. 1998). Both Uso1p and p115 exist as myosin II shaped parallel homodimers, with two NH2-terminal globular heads, a coiled-coil tail, and a short acidic domain at the extreme COOH terminus (Sapperstein et al. 1995; Yamakawa et al. 1996).
It is postulated that p115 acts to cross-link the acceptor membrane to the incoming COPI vesicle by simultaneously binding its two receptors on Golgi membranes: GM130 on the acceptor membrane and giantin on the COPI vesicle (Sönnichsen et al. 1998). GM130 was first identified as a highly immunogenic component of a detergent-insoluble Golgi matrix (Nakamura et al. 1995). GM130 is an extensively coiled-coil, rod-like peripheral membrane protein, which may have some degree of flexibility, and is tightly anchored to the Golgi membrane at its COOH terminus by the NH2-terminally myristoylated Golgi reassembly stacking protein 65 (GRASP65; Barr et al. 1998). Giantin was first identified using an mAb raised against Golgi membranes (Linstedt and Hauri 1993). It is also predicted to be an extensively coiled-coil, rod-like protein, and is a type II Golgi membrane protein, with most of its mass projecting into the cytoplasm (Linstedt and Hauri 1993). In contrast to GM130, giantin is incorporated into in vitro-generated COPI vesicles in the presence of GTPγS at the prevailing donor membrane concentration, whereas GM130 is largely excluded (Sönnichsen et al. 1998). p115 is thought to link giantin on the COPI vesicle to GM130 on the acceptor membrane. This ternary complex is then a SNARE-independent tethering device and may be regulated by the direct phosphorylation of p115 by an as yet unidentified Golgi membrane-associated kinase (Sohda et al. 1998). Given the extended, flexible rod-like structure of these molecules, they are excellent candidates for the strings proposed to be important for processive intra-Golgi membrane transport (Orci et al. 1998).
At mitosis, the extreme basic NH2 terminus of GM130, comprising the p115 binding site (Nakamura et al. 1997), is directly phosphorylated by cyclin B-Cdc2 kinase on serine 25 (Lowe et al. 1998), with the effect of potently inhibiting p115 binding. The introduction of this phosphate moiety may break up an electrostatic interaction, since it is the extreme acidic COOH terminus of p115 that binds to the extreme basic NH2 terminus of GM130 (Nelson et al. 1998). So, although p115 can still bind giantin (Sönnichsen et al. 1998), it is no longer able to cross-link to GM130. As a result, COPI vesicles accumulate as they are unable to tether and so fuse, and intra-Golgi membrane transport is inhibited (Stuart et al. 1993; Misteli and Warren 1994; Nakamura et al. 1997).
This requirement for cyclin B-Cdc2 kinase activity for mitotic Golgi membrane fragmentation is in contrast to results obtained in a semipermeabilized cell system that requires MEK1 activity and not Cdc2 activity (Acharya et al. 1998). The fact that our cell-free system has no requirement for MEK1 for mitotic Golgi membrane fragmentation and is absolutely dependent on cyclin B-Cdc2 is given greater credence by the fact that MEK1 activity is not required for mitotic Golgi membrane fragmentation in vivo (Lowe et al. 1998).
As cells exit M phase, mitotic phosphorylations are reversed by protein phosphatases facilitating the reversion of mitotic Golgi fragments (MGF) to their original morphology. The first phase of reassembly entails the regeneration of single cisternae (Rabouille et al. 1995a). This event requires two members of the AAA ATPase family, NSF (and its soluble cofactors α-SNAP, γ-SNAP, and p115), and p97 (and its cofactor p47), which seem to contribute nonadditively to cisternal regrowth (Rabouille et al. 1995b, Rabouille et al. 1998) rather than sequentially, as is the case in reassembly from illimaquinone (IQ)-generated Golgi fragments (Acharya et al. 1995). The NSF pathway of cisternal regrowth utilizes the vesicle SNARE (v-SNARE), GOS-28 (Nagahama et al. 1996; Rabouille et al. 1998), and the Golgi membrane target SNARE (t-SNARE), syntaxin-5, and may be considered important for heterotypic fusion events between COPI vesicles and their target membranes. In contrast, the p97 pathway may operate solely through trans-syntaxin-5 t-SNARE/t-SNARE interactions (Patel et al. 1998; Rabouille et al. 1998), and therefore may be considered important for homotypic membrane fusion events. The two pathways intersect at the common determinant syntaxin-5, due to direct competition between p47 and α-SNAP for its binding (Rabouille et al. 1998), which might explain why they contribute nonadditively to cisternal regrowth.
As single cisternae begin to form, they align and dock to form stacks. So far, the only factor shown to be required for this event is an NEM-sensitive membrane-bound component (Barr et al. 1997). This factor was identified by using its alkylation by a biotinylated NEM analogue as a marker for its chromatographic behavior, and was found to be GRASP65, the receptor for GM130 (Barr et al. 1997). Soluble GRASP65 and affinity-purified antibodies raised against the region containing a conserved cysteine blocked the stacking process without affecting cisternal regrowth. GRASP65 is highly conserved, N-myristoylated, and is the major mitotic Golgi phosphoprotein (Barr et al. 1997). Precisely how GRASP65 functions to stack Golgi cisternae remains obscure.
It is possible to view cisternal stacking and COPI vesicle tethering as functionally equivalent processes. Both require agents that bring and hold membranes in close proximity. The intimacy of these two processes may be reflected by the intimacy of the interaction between GM130 and GRASP65 (Barr et al. 1998), the former being required for COPI vesicle tethering, and the latter for cisternal stacking. Both proteins are phosphorylated at mitosis (Barr et al. 1997; Lowe et al. 1998), when COPI vesicle tethering is inhibited and Golgi cisternae unstack. Given that p115 has the apparent capacity to cross-link membranes via GM130 and giantin, and that giantin is present at equal concentrations in Golgi cisternae, as it is in COPI vesicles (Sönnichsen et al. 1998), could p115 be involved in the tethering reaction that leads to stacking, as well as the tethering reaction that leads to COPI vesicle fusion? In other words, can p115 tether cisterna to cisterna, as well as COPI vesicle to cisterna?
Here we have examined whether p115 plays a direct role in stacking cisternae at the end of mitosis that is distinct from its role in membrane fusion. The results suggest that p115, in conjunction with giantin and GM130, is essential for cisternal stacking and NSF-mediated cisternal regrowth. p115 acts most potently at an early stage in the stacking reaction, upstream of GRASP65, and may facilitate the initial tethering of Golgi cisternae that is a prerequisite for stacking.
Materials and Methods
Materials
All reagents were of analytical grade or higher and purchased from Sigma Chemical Co., Boehringer Mannheim, or BDH Chemicals Ltd., unless otherwise stated. The following antibodies were used in this study: rabbit polyclonal antibodies NN15 against GM130 from N. Nakamura (Department of Molecular Biology, Kyushu University, Fukuoka, Japan); polyclonal antibodies against giantin from M. Renz (Department of Pharmacology, Basel University, Switzerland); mAbs 4H1 and 8A6 against p115 from G. Waters (Department of Molecular Biology, Princeton University, Princeton, NJ); mAbs against p97 from J.M. Peters (IMP, Vienna, Austria); mAb 2E5 against NSF from M. Tagaya (School of Life Science, Hachioji, Japan); rabbit polyclonal antibodies against α1,2-mannosidase I (Mann I) and GRASP65 from F. Barr (University of Glasgow, UK); polyclonal 1946 against α-SNAP from G. Stenbeck (UCL, London, UK); and rabbit polyclonal C-19 against rab6 (Santa Cruz).
Purification of Rat Liver Golgi Membranes
Rat liver Golgi membranes (RLG) were purified as in Hui et al. 1998. Purified membranes were assayed for β1,4-galactosyltransferase specific activity (Hui et al. 1998) and were purified 233 ± 17-fold 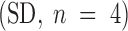 over rat liver homogenate.
over rat liver homogenate.
Mitotic and Interphase Cytosols
Mitotic and interphase cytosols were prepared from spinner HeLa cells (sHeLa), as in Sönnichsen et al. 1996. The mitotic index of the cells was typically 97–100%. The histone kinase activity of mitotic cytosol was 20–25-fold higher than interphase cytosol, as assayed in Lowe et al. 1998.
A 40% ammonium sulphate cut of rat liver cytosol was prepared as in Rabouille et al. 1995a. This cut was used for all reassembly reactions and will be referred to as rat liver cytosol.
Depletion of p115 from Rat Liver Cytosol
p115 was depleted from cytosol using either the mAb 4H1 or a biotinylated peptide comprising the NH2-terminal 73 amino acids of GM130 (N73pep), which binds p115 (Nakamura et al. 1997). 4H1 was coupled to Affigel-10 (Bio-Rad) to achieve 0.72 mg 4H1/ml resin. Beads were washed three times with KHM (60 mM KCl, 25 mM Hepes-KOH, pH 7.3, 5 mM magnesium acetate, 0.2 M sucrose, 1 mM glutathione) and made up as a 1:1 slurry in that buffer. 200 μl of slurry was dried using a Hamilton syringe, added to 800 μl rat liver cytosol (∼20 mg/ml), and incubated for 1 h with rotation at 4°C. The beads were then recovered by a pulse in a microfuge, the supernatant was removed, and added to fresh beads. This was repeated four times.
1 ml biotinylated N73pep (10 mg/ml in distilled water) was coupled to 2 ml Neutravidin beads (Pierce Chemical Co.). After coupling, beads were blocked with 10 mg/ml soybean trypsin inhibitor. Beads were then packed into a 0.7 × 10-cm Econo-column (Bio-Rad), and the column was equilibrated with 20 ml KHM. 2 ml rat liver cytosol was loaded onto the column and allowed to interact with the resin for 15 min. The column was then eluted with KHM.
In both cases, the mock depletions were made with the same blocked beads without antibody or peptide coupled. 20 μg cytosolic proteins were separated on a 7.5% SDS-polyacrylamide gel and transferred to nitrocellulose (Hybond C, Nycomed Amersham). Blots were probed with 8A6 to determine the extent of p115 depletion and processed as in Sönnichsen et al. 1996.
Protein Purification
p115 was purified as in Levine et al. 1996 and was 90–95% homogeneous, as judged by Coomassie blue staining with a protein concentration of 50–150 μg/ml, and was ∼4,000–5,000-fold purified over rat liver cytosol. Rat liver p97 and recombinant His-tagged p47 were purified as in Kondo et al. 1997. Recombinant His-tagged NSF was purified as in Whiteheart et al. 1994. Recombinant His-tagged α-SNAP and His-tagged γ-SNAP were prepared as in Rabouille et al. 1995b. NH2-terminally His-tagged GRASP65 was bacterially expressed and purified on a nickel-NTA-agarose column, followed by Superose-6 molecular sieving.
Reassembly Assay
The disassembly reaction was performed as in Rabouille et al. 1995a, except the incubation time was reduced to 20 min, and before recovery of the membranes, each reaction was underlaid with 125 μl MEB (10 mM MgCl2, 15 mM EGTA, 20 mM β-glycerophosphate, 0.2 M sucrose, 50 mM Tris-HCl, pH 7.3, 50 mM KCl, 2 mM ATP, 1 mM GTP, 1 mM glutathione) containing 0.5 M sucrose instead of 0.2 M sucrose (or just MEB for the purpose of comparison) and a 2 μl 2-M sucrose cushion. The membranes were recovered by centrifugation at 15,000 rpm (13.1 Kg av) for 25 min at 4°C in the horizontal rotor of the Eppendorf centrifuge and were termed MGF.
To assess the relative polypeptide composition of MGF isolated with or without the MEB/0.5-M sucrose cushion, the 2 μl 2-M sucrose cushion was omitted, and the resulting pellet solubilized in SDS-PAGE sample buffer, boiled for 3 min, and separated on 5–20% gradient SDS-polyacrylamide gels. The proteins in the gel were transferred to nitrocellulose (Hybond C, Nycomed Amersham) using a semi-dry blotter. Blots were processed as in Sönnichsen et al. 1996.
For reassembly, the MGF were gently resuspended (final concentrations 0.75–1 mg/ml) in rat liver cytosol (0.2–10 mg/ml final concentrations) in KHM buffer (with 2 mM ATP and 1 mM GTP) supplemented with a 10× ATP regeneration system (200 mM creatine phosphate, 10 mM ATP, 2 mg/ml creatine kinase, 0.2 mg/ml cytochalasin B). The final reaction volume was 20 μl. p115-depleted cytosol and p115-depleted cytosol with p115 added back were also used. p115 was estimated to be present at 3–4 ng/μg cytosol by Western analysis and was added back to this level. Cytosol was replaced by the purified components NSF, α-SNAP, γ-SNAP, and/or p97, p47, as in Rabouille et al. 1998. 0–30 ng/μl p115 (final concentration) was titrated into this system. Incubations were carried out for up to a maximum of 120 min at 37°C.
For EM, reactions were fixed, processed, and sectioned as in Rabouille et al. 1995a.
For Western blotting, completed reactions were made up to 120 μl with ice-cold KHM and membranes were recovered by centrifugation at 15,000 rpm (13.1 Kg av) for 30 min at 4°C in the horizontal rotor of the Eppendorf centrifuge. The resulting pellet was solubilized in SDS-PAGE sample buffer and processed as for MGF.
In some experiments, the MGF were pretreated with 1 μl anti-GM130 NN15 and/or 1 μl antigiantin for 15 min on ice before resuspension in the purified component reassembly system. The reaction was then allowed to proceed for 60 min at 37°C.
In some experiments, the MGF were pretreated for 15 min on ice with N73pep (0–80 μM) or soluble GRASP65 (0–75 ng/μl) and the complete purified reassembly reaction mix (i.e., NSF, α-SNAP, γ-SNAP, p115, p97, and p47). The reaction was then allowed to proceed at 37°C for 60 min. The effect of N73pep and soluble GRASP65 treatment was also assessed on starting RLG. RLG at 0.75 mg/ml were treated with N73pep (80 μM) or soluble GRASP65 (75 ng/μl) in KHM (with 2 mM ATP and 1 mM GTP) buffer and an ATP regeneration system in a final volume of 20 μl, and incubated for 15 min on ice or 60 min at 37°C. They were then fixed and processed for EM.
To assess the temporal sensitivity of reassembly to N73pep and soluble GRASP65, the complete purified reassembly reaction was allowed to proceed for increasing time at 37°C. At various times, the reaction was transferred to ice and fixed and processed for EM or treated with KHM, 80 μM N73pep, or 75 ng/μl soluble GRASP65 for 15 min on ice. They were then reincubated at 37°C for a total time of 60 min.
Stereology
Stereological definitions were as in Rabouille et al. 1995a, except that a stacked region of a cisterna was described as two or more cisternae that are aligned in parallel and separated by no more than 15 nm. The relative proportion of each category of membrane was determined as in Rabouille et al. 1995a and the percentage cisternal regrowth as in Nakamura et al. 1997. The length of cisternae and the number of cisternae per stack was determined as in Misteli and Warren 1994.
Results
MGF Isolated through a 0.5-M Sucrose Cushion Require Cytosolic Components for Cisternal Regrowth and Stacking
To test whether p115 played a role in cisternal stacking during reassembly, we needed to alter the reassembly assay to make it dependent on added soluble factors. Previously, MGF reassembled into stacked cisternae in buffer alone to the same extent as when cytosol was added, suggesting that everything required for correct reassembly and cisternal stacking was present on the MGF (Rabouille et al. 1995b). This background fusion and stacking activity was abolished by treating the MGF with the sulfhydryl modifying reagent, NEM (Rabouille et al. 1995b). The irreversible chemical modifications rendered by NEM precluded study of cisternal stacking as it abolished correct GRASP65 function (Barr et al. 1997). To study the importance of soluble factors for cisternal stacking, we needed to remove this background reassembly competence without NEM-treating the membranes. This was achieved by removing any cytosolic contaminants from the MGF by isolating them through a 0.5-M sucrose cushion, enabling assessment of the relative importance of cytosolic factors, and in particular, p115 in cisternal stacking and cisternal regrowth.
Highly purified RLG were incubated with mitotic sHeLa cytosol for 20 min at 37°C and membranes were reisolated by centrifugation in the presence or absence of a 0.5-M sucrose cushion. These membranes were termed MGF and were morphologically similar, whether the 0.5-M cushion was present or not. The percentage total membrane present as Golgi cisternae fell from 77% in RLG to 31% in either set of MGF (Table ). The most dramatic loss was from stacked Golgi cisternae, which fell from 53% in RLG to <1% in the MGF (Table ). The 47% loss of membrane from cisternal membranes was accounted for by a concomitant 30–35% increase in tubules and 10–15% increase in vesicles (data not shown). The mean cross-sectional length of cisternae diminished dramatically by ∼70% during the mitotic incubation. The mean cisternal length fell from 1.1 μm in RLG to 0.33–0.35 μm in the two sets of MGF (Table ). These MGF do not differ significantly in morphology from those used in previous studies of reassembly (Rabouille et al. 1995a,Rabouille et al. 1995b, Rabouille et al. 1998; Barr et al. 1997).
Table 1.
Stereological Profile of RLG, MGF, and Reassembled Golgi Membranes
| Membranes | Incubation | Membrane in cisternae | Membrane in stacked regions of cisternae | Mean cisternal length | Mean number of cisternae per stack |
|---|---|---|---|---|---|
| % | % | μm | |||
| RLG | − | 77 ± 2.2 | 53 ± 2.1 | 1.1 ± 0.13 | 2.8 ± 0.12 |
| MGF − cushion | − | 31 ± 2.1 | 0.9 ± 0.5 | 0.35 ± 0.03 | |
| MGF + cushion | − | 31 ± 0.9 | 0.8 ± 0.5 | 0.33 ± 0.04 | |
| MGF − cushion | Buffer | 50 ± 1.5 | 7.2 ± 0.9 | 0.98 ± 0.13 | 2.3 ± 0.15 |
| MGF + cushion | Buffer | 36 ± 2.1 | 0.8 ± 0.6 | 0.38 ± 0.05 | |
| MGF − cushion | Cytosol | 62 ± 1.5 | 25 ± 2.7 | 1.2 ± 0.16 | 2.6 ± 0.16 |
| MGF + cushion | Cytosol | 60 ± 1.1 | 23 ± 2.9 | 1.3 ± 0.15 | 2.6 ± 0.09 |
| MGF + cushion | p97/p47 | 69 ± 3 | 1.7 ± 1.3 | 1.4 ± 0.17 | |
| MGF + cushion | NSF/SNAPs/p115 | 70 ± 2.3 | 22 ± 0.1 | 0.79 ± 0.06 | 3.1 ± 0.19 |
| MGF + cushion | p97/p47 and NSF/SNAPs/p115 | 69 ± 3.3 | 25 ± 5 | 1.3 ± 0.13 | 2.5 ± 0.11 |
The stereological characteristics of RLG, MGF, and MGF reassembled under various conditions as described in Materials and Methods are presented. The parameters were determined as described in Materials and Methods, and the results are presented as the mean ± SEM.
The MGF isolated without the 0.5-M sucrose cushion were fusion competent when incubated in KHM buffer alone for 60 min at 37°C (Table ), as previously reported (Rabouille et al. 1995b). The percentage total membrane present as cisternae increased from 31 to 50% and the percentage total membrane present as stacked regions of cisternae from <1 to 7% (Table ). These stacks contained between two and three cisternae (Table ). The mean cross-sectional length of these reassembled cisternae was 0.98 μm (Table ). This was not the case for MGF isolated through the 0.5-M sucrose cushion, where the percentage total membrane present as cisternae and the mean cross-sectional length of cisternae showed no significant increase (Table ).
However, both sets of MGF were fusion competent when incubated in rat liver cytosol (10 mg/ml) for 60 min at 37°C (Table ). The percentage total membrane as cisternae rising from 31 to ∼60% for both sets of MGF, and the percentage total membrane present as stacked regions of cisternae from <1 to 20–25% (Table ). The mean cross-sectional length increased to 1.3 μm in cisternae reassembled from MGF isolated with the 0.5-M sucrose cushion and 1.2 μm in cisternae reassembled from MGF isolated without the 0.5-M sucrose cushion (Table ).
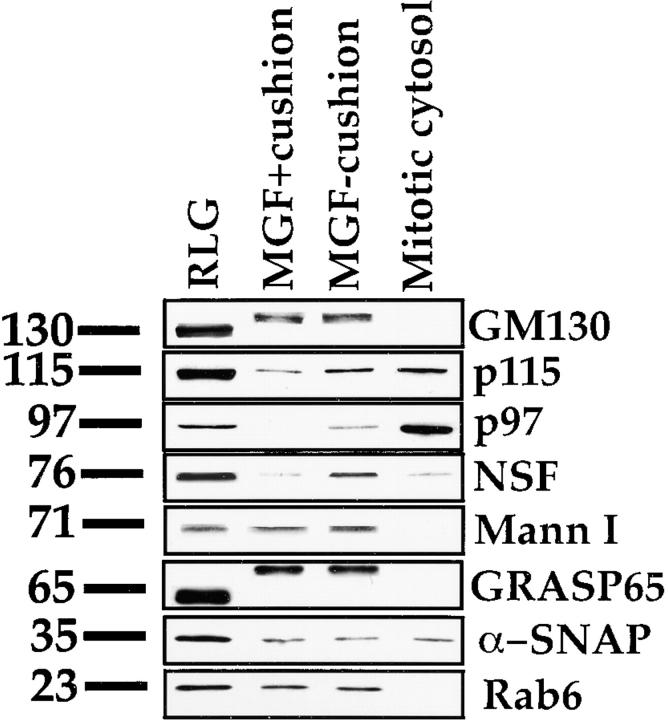
Analysis of the polypeptide composition of the two sets of fragments revealed that the MGF isolated through the 0.5-M cushion were significantly less contaminated with cytosolic factors (data not shown). In fact, the MGF isolated through the 0.5-M cushion contained 65% less protein (data not shown). Western analysis (Fig. 1) revealed MGF isolated with or without the 0.5-M sucrose cushion contained similar amounts of Mann I, GM130, and GRASP65 (Fig. 1). Therefore, the 0.5-M cushion was not affecting the amount of membranes that were recovered. However, when the MGF are compared with starting RLG, virtually all the Mann I was recovered, but only 40–50% of the GM130 and GRASP65 appeared to be recovered. This may be due to a decrease in the reactivity of the antibodies against mitotically phosphorylated GM130 and GRASP65. MGF isolated with or without the 0.5-M sucrose cushion had similar levels of rab6 and α-SNAP.
Figure 1.
Western analysis of MGF isolated with or without a 0.5-M sucrose cushion. 10 μg RLG, MGF derived from 10 μg RLG isolated with or without a 0.5-M sucrose cushion, and 10 μg sHeLa mitotic cytosol (1% of input) were fractionated by SDS-PAGE, transferred to nitrocellulose, and probed for GM130, p115, p97, NSF, Mann I, GRASP65, α-SNAP, and rab6 using specific antibodies. Molecular weights in kD are shown on the left. Note the shift in molecular weight of GM130 and GRASP65 in the MGF owing to mitotic phosphorylation of these two proteins.
The presence of the 0.5-M sucrose cushion reduced the MGF p115 levels four- to fivefold, and NSF and p97 levels 20–25-fold. The insertion of a 0.5-M sucrose layer separates the mitotic cytosol (of which 1% of total input is shown in the far right lane), which rests on top of this layer, from the MGF, which sediment through this layer on to the underlying 2-M sucrose cushion. This reduces the risk of collecting contaminating cytosolic proteins on collection of the MGF. As the membranes enter the 0.5-M sucrose layer, there may also be some differential removal of the p115, p97, and NSF that are still loosely bound to the membranes. The reduced levels of p115, NSF, and p97 may explain why these MGF are fusion incompetent in buffer alone. This is also consistent with previous observations that NEM treatment or 0.25-M KCl extraction of MGF isolated without a 0.5-M sucrose cushion renders them fusion-incompetent in buffer alone (Rabouille et al. 1995b).
p115 Is Essential for the Post-mitotic Stacking of Reassenbling Golgi Cisternae
To assess p115 function in the reassembly process, rat liver cytosol, p115-depleted cytosol, and p115-depleted cytosol with purified p115 added back were titrated into the reassembly assay. p115 was depleted >95% from rat liver cytosol using either the mAb 4H1 or N73pep (Fig. 2 A). p115 was purified to near homogeneity from rat liver cytosol to add back to this depleted cytosol. MGF were resuspended in cytosol of increasing concentrations and incubated for 60 min at 37°C.
Figure 2.
Effect of interphase cytosol depleted of p115 on the reassembly process. (A) Rat liver cytosol was depleted of p115, as described in Materials and Methods, using either the anti-p115 mAb antibody 4H1 or the N73pep. 20 μg of cytosol was fractionated by SDS-PAGE using a 7.5% gel, transferred to nitrocellulose, and probed with the anti-p115 mAb 8A6. (B–J) MGF isolated through a 0.5-M sucrose cushion were incubated for 60 min at 37°C with increasing concentrations of either rat liver cytosol (B–D), p115-depleted cytosol (E–G), or p115-depleted cytosol supplemented with purified rat liver p115 (H–J), fixed, and processed for EM. Representative fields are shown. Note the presence of stacks (arrowheads) in B–D and H–J, but only single cisternae (arrows) in E–G. The reduced number of cisternae in E suggests poor reassembly at this concentration of p115-depleted cytosol. Note that the cisternae formed often have a wrinkled appearance (asterisks in G) in p115-depleted cytosol and are often blunt-ended with few associated vesicles (compare asterisks in F and I), in contrast to when p115 is present. Bar, 0.5 μm.
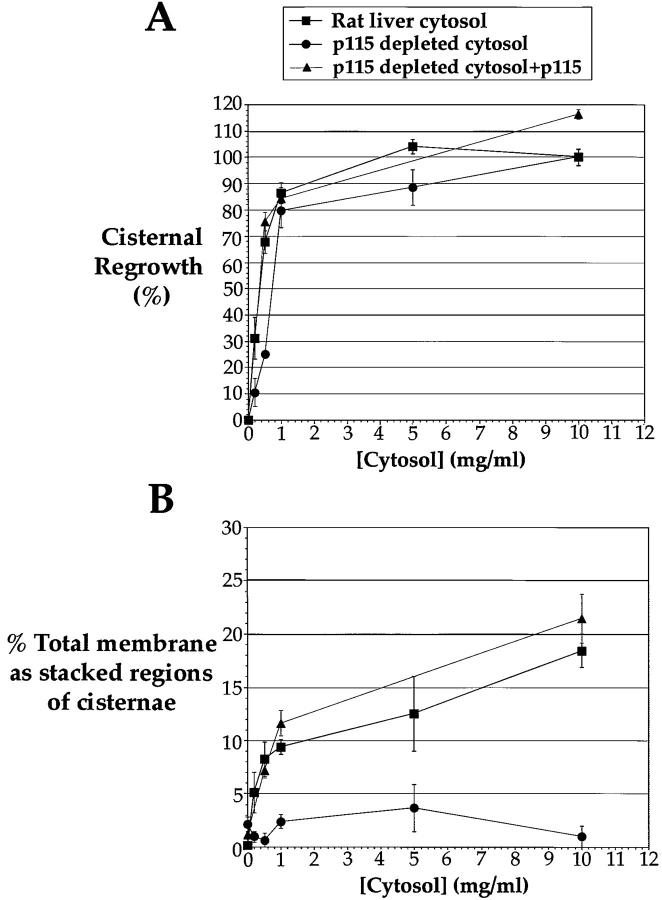
In rat liver cytosol, cisternal regrowth was near maximal at 1 mg/ml (Fig. 2, B–D, and 3 A) and the same was true for the mock depleted cytosols (data not shown). At cytosol concentrations below 1 mg/ml, the p115-depleted cytosol supported threefold less cisternal regrowth (Fig. 2 E and 3 A). This inhibition was reversed by adding purified p115 back to the depleted cytosol (Fig. 2 H and 3 A). Therefore, this loss of activity was due to p115 activity and not the activity of another factor that may have been codepleted from the cytosol by an interaction with p115. However, at cytosol concentrations of 1 mg/ml and above, p115-depleted cytosol supported full cisternal regrowth (Fig. 2F and Fig. G, and Fig. 3 A). Therefore, p115 is not essential for this process, or a p115-independent pathway of cisternal regrowth is operating. We favor the latter explanation because two nonadditive pathways of cisternal regrowth controlled by NSF and p97 have been described previously (Rabouille et al. 1995b, Rabouille et al. 1998). The p97 pathway has no requirement for p115 for cisternal regrowth, and is presumably responsible for the complete cisternal regrowth activity of p115-depleted cytosol. A hint that this may be true comes from the morphology of the cisternae reassembled in p115-depleted cytosol, in that they are often blunt-ended with few associated vesicles (compare asterisks in Fig. 2F and Fig. I). This is the characteristic phenotype for p97 reassembled cisternae (Rabouille et al. 1995b).
Figure 3.
Quantitation of Golgi membrane reassembly in rat liver cytosol, p115-depleted cytosol, and p115-depleted cytosol supplemented with p115. MGF isolated through a 0.5-M sucrose cushion were incubated for 60 min at 37°C with increasing concentrations of either rat liver cytosol, p115-depleted cytosol, or p115-depleted cytosol supplemented with purified rat liver p115, fixed and processed for EM, and quantitated as described in Materials and Methods. (A) The percentage cisternal regrowth ± SEM  for each cytosol concentration tested. (B) The percentage total membrane present as stacked regions of cisternae ± SEM
for each cytosol concentration tested. (B) The percentage total membrane present as stacked regions of cisternae ± SEM  for each cytosol concentration tested.
for each cytosol concentration tested.
The stacking process in rat liver cytosol displayed distinct properties to cisternal regrowth in that the number of stacks were still increasing at the highest cytosol concentration tested (10 mg/ml; Fig. 2 D and 3 B). This asymmetry may be due to an imbalance in factors required for cisternal regrowth and stacking. This mirrors the disassembly process in that low concentrations of mitotic cytosol are sufficient to inhibit transport (Stuart et al. 1993), yet do not affect Golgi structure significantly (Misteli and Warren 1994).
The most striking effect on reassembly in p115-depleted cytosol at all concentrations tested was the virtual complete absence of stacked Golgi structures at the end of the incubation (Fig. 2, E–G, and 3 B). This effect could be reversed by adding purified p115 back to the depleted cytosol (Fig. 2, H–J, and 3 B), again suggesting that p115 itself was the active component, and not that another factor had been codepleted. Cisternal regrowth and stacking are thus separable processes. The single cisternae formed in the absence of p115 had a more wrinkled, corrugated appearance (asterisks in Fig. 2 G), suggesting an involvement of p115 in a membrane smoothing event during the reassembly process. This effect was again reversed by supplementing the depleted cytosol with purified p115. These effects of p115 depletion on reassembly were identical if sHeLa interphase cytosol was used instead of rat liver cytosol (data not shown).
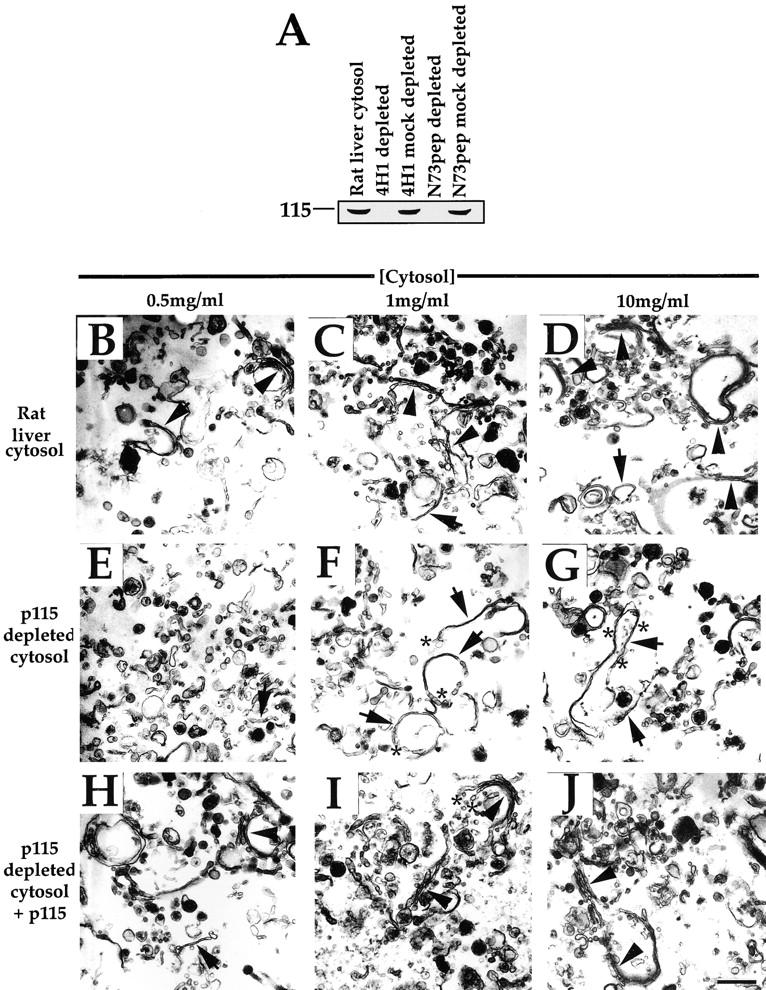
Kinetic analysis revealed the reassembly reaction was complete for both cisternal regrowth and stacking after 60 min in rat liver cytosol (10 mg/ml; Fig. 4 I). The first intermediates that formed quickly during the first 15 min of the incubation were single cisternae (Fig. 4A and Fig. B), frequently with tubular networks at their rims (asterisks in Fig. 4 B). By 15 min, these intermediates had begun to dock and align to form the beginnings of stacked Golgi structure (arrowheads in Fig. 4 B). The lag in the formation of stacked structures (Fig. 4 I) therefore may be considered due to the need to form single cisternae first. By 45 min, this process was well advanced and Golgi stacks with two or more cisternae per stack were prevalent and these discrete stacks were becoming linked via tubular networks (Fig. 4 C). By 60–120 min, these linkages had been made, the tubular networks were less apparent, and long cisternal stacks were the end product, which often adopted an approximate closed concentric circular morphology (compare Fig. 2 D and 4 D).
Figure 4.
Kinetic analysis of Golgi membrane reassembly in rat liver cytosol and p115-depleted cytosol. (A–H) MGF isolated through a 0.5-M sucrose cushion were incubated for increasing time at 37°C with either rat liver cytosol (A–D) or p115-depleted cytosol (E–H) with the cytosol concentration set at 10 mg/ml, fixed and processed for EM, and quantitated as described in Materials and Methods. Representative fields are shown. In rat liver cytosol, note that the first intermediate formed is the single cisterna after 5 min (arrows in A). By 15 min, these single cisternae had grown in length, had tubular networks associated with their rims (asterisks in B), and had begun to align and dock to form stacks (arrowheads in B). Many discrete stacks had formed by 45 min (arrowheads in C), which had joined up by 120 min (arrowheads in D). In p115-depleted cytosol, single cisternae were again present after 5 min (arrows in E), and had increased in length by 15 min (arrows in F), but were often blunt-ended (asterisk in F) and were not stacked. At 45 min, these cisternae remained blunt-ended (asterisk in G) and unstacked. By 120 min, some stacks of blunt-ended cisternae had begun to form (arrowhead in H), but many single cisternae remained (arrows in H). Bar, 0.5 μm. (I) Quantitation of the time course. The percentage total membrane present as cisternae ± SEM  and stacked regions of cisternae ± SEM
and stacked regions of cisternae ± SEM  are presented for each cytosol at every time point tested. (J) MGF were reassembled in p115-depleted cytosol and supplemented with p115 at different times (time of addition of p115). The reaction was allowed to proceed for a total time of 120 min at 37°C. Samples were then fixed and processed for EM, and the percentage total membrane as stacked regions of cisternae ± SEM
are presented for each cytosol at every time point tested. (J) MGF were reassembled in p115-depleted cytosol and supplemented with p115 at different times (time of addition of p115). The reaction was allowed to proceed for a total time of 120 min at 37°C. Samples were then fixed and processed for EM, and the percentage total membrane as stacked regions of cisternae ± SEM  are presented for each time point tested.
are presented for each time point tested.
In p115-depleted cytosol, single cisternae formed at the start of the reaction, although with a reduced initial rate (Fig. 4I and Fig. E). Once again these cisternae were blunt-ended, indicating the p97 pathway of reassembly may be dominant (asterisks in Fig. 4F and Fig. G). By 15 min, these single cisternae were still well separated (Fig. 4 F), and even after 45–60 min, single, blunt-ended cisternae were the major reaction product (Fig. 4G and Fig. I). However, after 120 min, even though no more cisternal regrowth occurred, these single cisternae did begin to align and form stacks (Fig. 4H and Fig. I). Even then, the level of stacking only reached ∼50% of that of rat liver cytosol (Fig. 4 I), and the intercisternal distance between adjacent cisternae of the stack seemed more variable (compare Fig. 4C and Fig. H). We conclude that the absence of p115 severely retards both the initial rate and overall extent of the stacking of Golgi cisternae during the reassembly reaction.
Was the time at which p115 added back to the depleted cytosol crucial to reverse the effect on stacking? To assess this, reassembly was conducted in p115-depleted cytosol and p115 was added back to the reaction at 15, 30, or 60 min and the reaction was allowed to proceed for 120 min. If added within the first 30 min, the p115 was able to restore stacking activity to the cytosol. However, if added at 60 min, the p115 only slightly stimulated stacking (Fig. 4 J). This suggests that p115 must be present as cisternae are reassembling for it to fulfil its stacking function. Once cisternae have formed completely, it seems p115 is no longer able to stimulate stacking.
The p97 Pathway Generates Only Single Cisternae in the Absence of p115
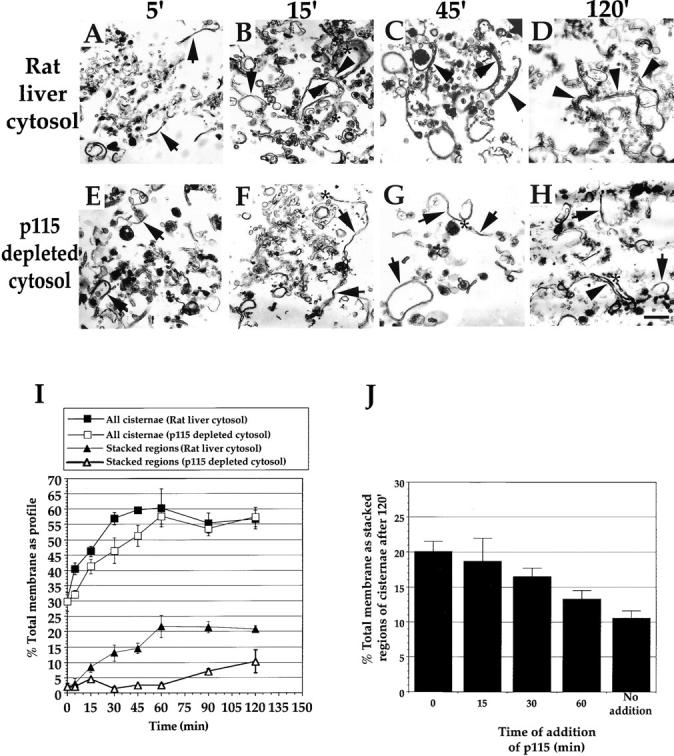
To more finely discern the role played by p115 in the p97 and NSF pathways of reassembly, and to corroborate the above findings, we moved to the purified system. p115 was titrated into the p97, NSF, and NSF/p97 catalyzed reassembly reactions. The amount of cisternal regrowth using these systems was moderately better than that achieved in rat liver cytosol. The NSF, p97, and NSF/p97 combined reactions generated ∼70% total membrane present as cisternae from 31% in MGF after a 60 min incubation at 37°C (Table ).
Titration of p115 into the p97 reaction revealed that this fusion pathway was insensitive to added p115 (Fig. 5 A). However, only single cisternae with a mean cisternal cross-sectional length of 1.4 μm formed in the absence of p115 (Table ). These single cisternae had a wrinkled appearance reminiscent of those formed in p115-depleted cytosol (compare Fig. 2 G and 5 A). Upon addition of p115, these cisternae had a smoother appearance and formed stacks (Fig. 5 A). However, the stacks formed rarely had more than two cisternae per stack.
Figure 5.
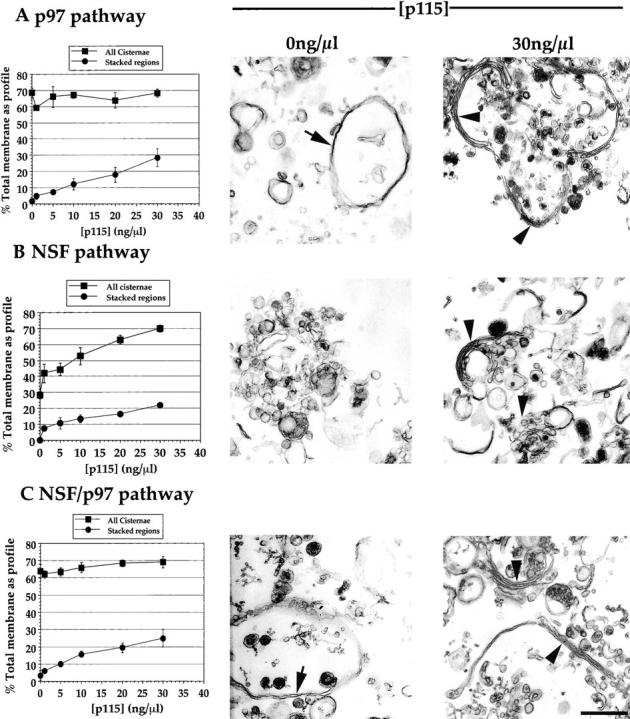
Titration of p115 into the p97, NSF, and NSF/p97 catalyzed reassembly reactions. MGF isolated through a 0.5-M sucrose cushion were incubated for 60 min at 37°C with: A, p97 (70 ng/μl) and p47 (37.5 ng/μl); B, NSF (100 ng/μl), α-SNAP (25 ng/μl) and γ-SNAP (25 ng/μl); or C, all these components combined with from 0–30 ng/μl p115. Samples were fixed and processed for EM, and then quantitated for percentage total membrane as cisternae ± SEM  and percentage total membrane present as stacked regions of cisternae ± SEM
and percentage total membrane present as stacked regions of cisternae ± SEM  . Results are displayed in graphic form at the left and representative fields of the two extreme p115 concentrations are positioned adjacent. Note the long single, wrinkled cisternae formed by the p97 pathway in the absence of p115 (arrow in A) and the long stacks of two cisternae with maximum p115 (arrowheads in A). Note the virtual absence of cisternae for the NSF pathway in the absence of p115 (B) and the short stacks of three or more cisternae with maximum p115 (arrowheads in B). Note the long, wrinkled single cisternae formed by the NSF/p97 pathway in the absence of p115 (arrow in C) and the stacks of two or three cisternae with maximum p115 (arrowheads in C). Bar, 0.5 μm.
. Results are displayed in graphic form at the left and representative fields of the two extreme p115 concentrations are positioned adjacent. Note the long single, wrinkled cisternae formed by the p97 pathway in the absence of p115 (arrow in A) and the long stacks of two cisternae with maximum p115 (arrowheads in A). Note the virtual absence of cisternae for the NSF pathway in the absence of p115 (B) and the short stacks of three or more cisternae with maximum p115 (arrowheads in B). Note the long, wrinkled single cisternae formed by the NSF/p97 pathway in the absence of p115 (arrow in C) and the stacks of two or three cisternae with maximum p115 (arrowheads in C). Bar, 0.5 μm.
Titration of p115 into the NSF reaction revealed that p115 was required for both cisternal regrowth and stacking (Fig. 5 B). Both processes were saturating, but still rising at the maximum p115 concentration tested (30 ng/μl). The reassembling cisternae formed stacks that usually had three or more cisternae per stack (Table ). The cisternae formed had a mean cross-sectional length of 0.79 μm (Table ), which is considerably shorter than those formed by the p97 pathway. A Mann-Whitney test revealed the NSF and p97 cisternal length distributions to be significantly different in location, with 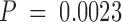 .
.
When p115 was titrated into the combined NSF/p97 reaction, cisternal regrowth was insensitive to added p115 (Fig. 5 C). However, stacking still required p115. In the absence of added p115, only wrinkled single cisternae were formed. On addition of p115, the cisternae formed were smooth and formed stacks with two or three cisternae per stack, similar to the number formed in rat liver cytosol (Table ). The mean cisternal cross-sectional length was 1.3 μm and this value was similar to that achieved in rat liver cytosol (Table ). A Mann-Whitney test revealed that these two distributions were not significantly different in location. This provides more correlational evidence that the p97 and NSF pathways operate to reform cisternae in rat liver cytosol.
The p115 Stacking Event Requires GM130 and Giantin
Previously, it has been shown that p115 binds to GM130 and giantin on Golgi membranes, and that these interactions are important for COPI vesicle tethering in vitro (Sönnichsen et al. 1998). To test whether these two molecules were required for the p115 stacking function, MGF were pretreated with anti-GM130 (NN15) and/or antigiantin antibodies. The MGF were then resuspended for reassembly via the NSF, p97 (including p115 so stacks will form), or NSF/p97 combined pathway using the purified components.
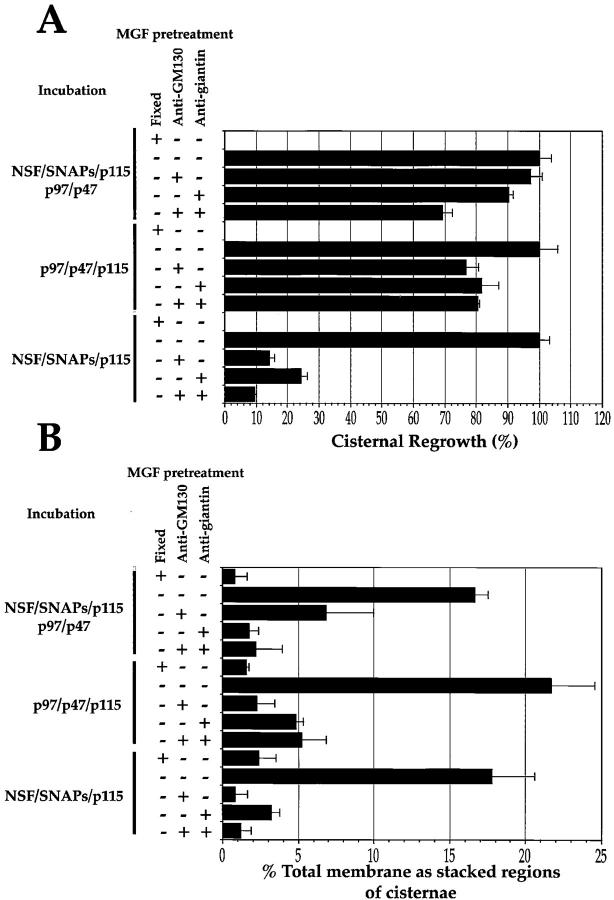
When the MGF were resuspended for the p97 or NSF/p97 combined pathway, cisternal regrowth was unaffected by antigiantin and/or anti-GM130 (Fig. 6 A). In contrast to this, the stacking process was severely inhibited in these two pathways (Fig. 6 B) and the preimmune sera had no effect on this process (data not shown). Thus, p115 stacking function requires p115 interactions with GM130 and giantin. Furthermore, this indicates that p115 may be able to tether cisterna to cisterna, as well as COPI vesicle to cisterna.
Figure 6.
Effect of antibodies against giantin and GM130 on the reassembly process using pure components. MGF isolated through a 0.5-M sucrose cushion were either fixed and processed for EM, held on ice for 15 min, or preincubated on ice for 15 min with 1 μl of anti-GM130 NN15 serum, antigiantin serum, or a combination of both. The pretreated MGF were then incubated for 60 min at 37°C with: p97 (70 ng/μl); p47 (37.5 ng/μl) and p115 (30 ng/μl); or NSF (100 ng/μl), α-SNAP (25 ng/μl), γ-SNAP (25 ng/μl) and p115 (30 ng/μl); or all these components combined. Samples were fixed and processed for EM, and the percentage cisternal regrowth ± SEM 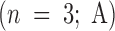 and the percentage total membrane present as stacked regions of cisternae ± SEM
and the percentage total membrane present as stacked regions of cisternae ± SEM 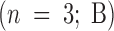 was determined.
was determined.
When MGF were pretreated with antigiantin or anti-GM130 and resuspended for the NSF pathway, both cisternal regrowth and, as a consequence, stacking were inhibited (Fig. 6A and Fig. B), and the preimmune sera had no effect (data not shown). That cisternal regrowth is inhibited strongly suggests that the interaction between GM130, p115, and giantin is essential for NSF-mediated Golgi membrane fusion. This may be due to an inhibition of COPI vesicle tethering to Golgi membranes (Sönnichsen et al. 1998).
p115 Is Required Before GRASP65 at an Early Stage in Stack Formation
An NEM-sensitive membrane-bound component of MGF is essential for the stacking reaction during reassembly. This factor was identified as GRASP65, a highly conserved, N-myristoylated protein that exists as a tight complex with GM130 on the membrane (Barr et al. 1997). A soluble, nonmyristoylated recombinant form of GRASP65 inhibited stacking, but not cisternal regrowth, in the cytosol based reassembly (Barr et al. 1997). This soluble GRASP65 was titrated into the NSF/p97 combined pathway of reassembly and was able to inhibit stacking potently without affecting cisternal regrowth (Fig. 7 A). This was also true in the p97-catalyzed and NSF-catalyzed reactions (data not shown). NEM-treated soluble GRASP65 had no effect on stacking or cisternal regrowth. Soluble GRASP65 prequenched with anti-GRASP65 antibodies also had no effect (data not shown). Soluble GRASP65 may then inhibit the stacking process by interacting with a Golgi membrane-bound factor.
Figure 7.
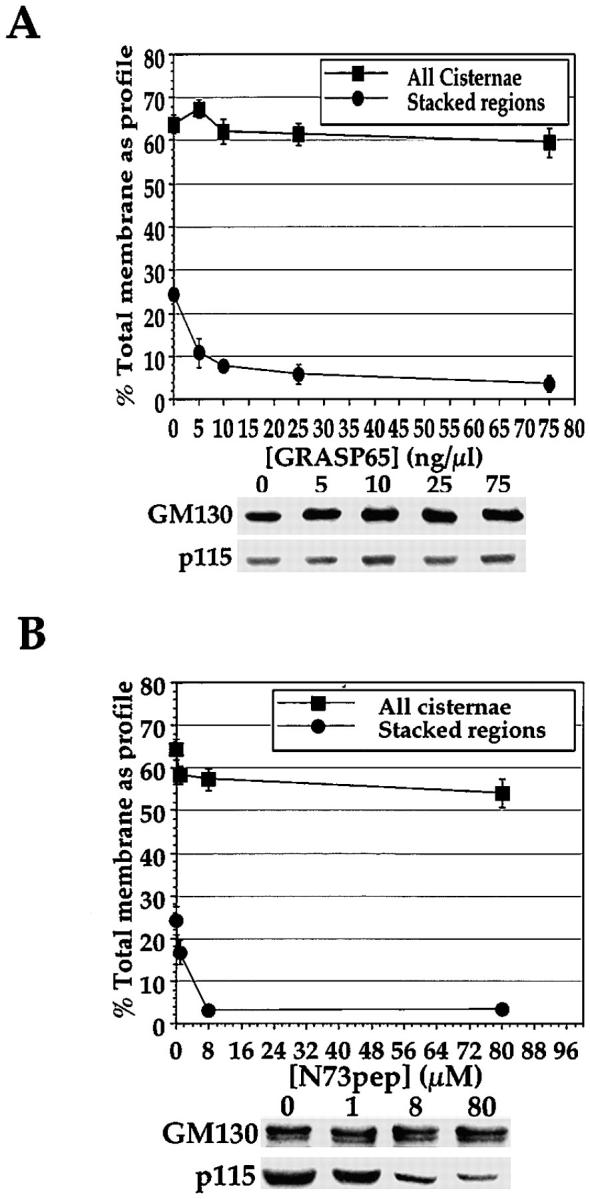
Effect of soluble GRASP65 and N73pep on the reassembly process using pure components. MGF isolated through a 0.5-M sucrose cushion were preincubated for 15 min on ice with increasing amounts of soluble GRASP65 (A) or N73pep (B). The pretreated MGF were then incubated for 60 min at 37°C with p97 (70 ng/μl), p47 (37.5 ng/μl), p115 (30 ng/μl), NSF (100 ng/μl), α-SNAP (25 ng/μl), and γ-SNAP (25 ng/μl). Samples were either fixed and processed for EM and quantitated, with the percentage total membrane present as cisternae ± SEM  and as stacked regions of cisternae ± SEM
and as stacked regions of cisternae ± SEM  shown; or the membranes were recovered and fractionated by SDS-PAGE using a 7.5% gel, transferred to nitrocellulose, and probed for GM130 and p115 using specific antibodies (shown below graphs in A and B).
shown; or the membranes were recovered and fractionated by SDS-PAGE using a 7.5% gel, transferred to nitrocellulose, and probed for GM130 and p115 using specific antibodies (shown below graphs in A and B).
The soluble GRASP65 did not act to remove GM130 from the membranes (Fig. 7 A), which is consistent with the fact that the complex between GM130 and GRASP65 is stable and can only be reconstituted if both proteins are cotranslated (Barr et al. 1998). Similarly, soluble GRASP65 did not appear to interfere with p115 rebinding to reassembling Golgi membranes (Fig. 7 A) or inhibit NSF-catalyzed cisternal regrowth, for which p115 is essential (data not shown and Fig. 5 B), and presumably does not affect p115 function. The effect of soluble GRASP65 on the stacking reaction therefore would not appear to be due to disruption of the endogenous GRASP65–GM130 interaction, and thus is not affecting the stacking reaction by preventing p115 function. It may be that the soluble GRASP65 competes with the endogenous GRASP65 for other interactions that help promote stacking by anchoring cisternae to each other.
N73pep was also titrated into this assay, and in agreement with the effect of anti-GM130, potently inhibited stacking, but not cisternal regrowth (Fig. 7 B). N73pep clearly inhibited the rebinding of p115 to reassembling Golgi membranes (Fig. 7 B). The S25D N73pep mutant, which binds p115 with a much lower affinity, had no effect on either process (data not shown).
To assess the temporal sensitivity of the stacking reaction to these two inhibitors, MGF were incubated in the NSF/p97-purified reaction for increasing time at 37°C. At various time points (time of addition, t; Fig. 8, A–D) the reaction was transferred to ice and either fixed with 2% glutaraldehyde and processed for EM, or treated with buffer (KHM, the GRASP65 and N73pep solvent), N73pep, or soluble GRASP65, and then reincubated at 37°C for a total time of 60 min.
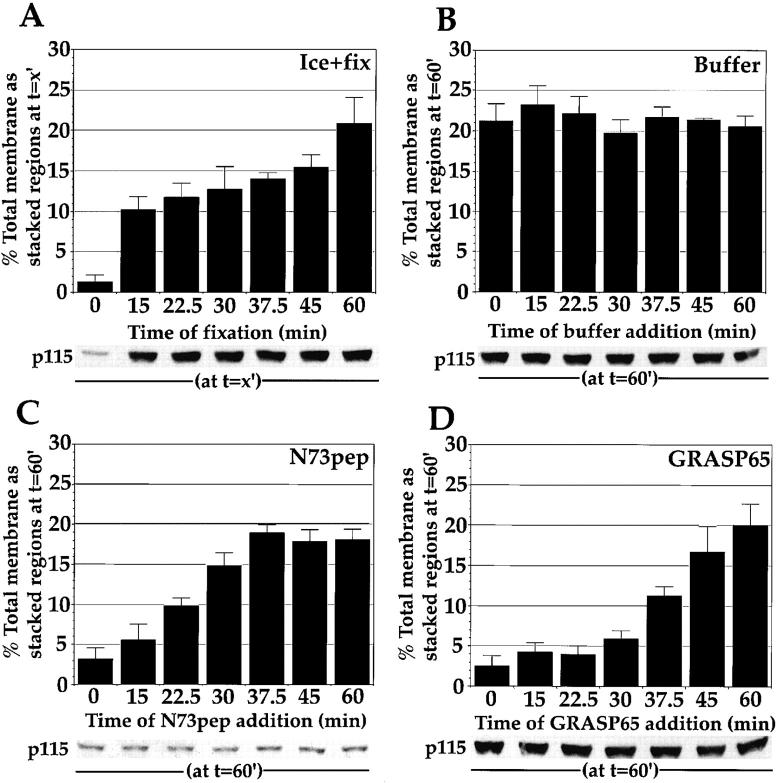
Figure 8.
Temporal sensitivity of stacking reaction to soluble GRASP65 and N73pep. MGF isolated through a 0.5-M sucrose cushion were incubated for the indicated time (time of addition) at 37°C with p97 (70 ng/μl), p47 (37.5 ng/μl), p115 (30 ng/μl), NSF (100 ng/μl), α-SNAP (25 ng/μl), and γ-SNAP (25 ng/μl). At this time, the reactions were transferred to ice and either fixed with 2% glutaraldehyde and processed for EM (A) and the percentage total membrane present as stacked regions of cisternae ± SEM  was determined, or the amount of p115 bound to the membranes at each time point was determined (shown below graph in A). Alternatively, reactions were transferred to ice and supplemented with buffer (B), 80 μM N73pep (C), or 75 ng/μl GRASP65 (D). After 15 min on ice, buffer-, N73pep-, and GRASP65-treated samples were transferred to 37°C and incubated for a total time of 60 min. Samples were fixed and processed for EM, quantitated, and the percentage total membrane present at the end of the incubation as stacked regions of cisternae ± SEM
was determined, or the amount of p115 bound to the membranes at each time point was determined (shown below graph in A). Alternatively, reactions were transferred to ice and supplemented with buffer (B), 80 μM N73pep (C), or 75 ng/μl GRASP65 (D). After 15 min on ice, buffer-, N73pep-, and GRASP65-treated samples were transferred to 37°C and incubated for a total time of 60 min. Samples were fixed and processed for EM, quantitated, and the percentage total membrane present at the end of the incubation as stacked regions of cisternae ± SEM  was determined. The amount of p115 bound to Golgi membranes at the end of the incubation was also determined by Western blotting (shown below each graph).
was determined. The amount of p115 bound to Golgi membranes at the end of the incubation was also determined by Western blotting (shown below each graph).
The time course for the reassembly of stacked regions of cisternae in the NSF/p97 reaction displayed similar characteristics to the rat liver cytosol catalyzed reaction (compare Fig. 8 A and 4 I). p115 rebound rapidly to the reassembling Golgi membranes (Fig. 8 A).
Addition of KHM buffer had no effect on the stacking reaction or on the amount of p115 bound to the Golgi membranes at the end of the incubation (Fig. 8 B). This suggests that the buffer, and transferring the reaction to ice, was not detrimental to the process.
The stacking process was sensitive to N73pep for the first 15 min of the reaction (Fig. 8 C). When added at 15 min, the time point when cisternae begin to dock and align (Fig. 4 B), the N73pep actually unstacked those stacks that had formed, suggesting that p115 was mediating this event (compare Fig. 8A and Fig. C). At time points later than 15 min, the reassembled stacks became resistant to added N73pep and normal stacking was able to proceed. Stacked RLG are also unaffected by N73pep treatment (Table ), suggesting that this is a shared property of reassembled Golgi stacks and starting stacked RLG. At all time points tested, N73pep was able to significantly remove bound p115 from the membranes, such that, at the end of the incubation, only 15% of the p115 was bound, as compared with control reactions (Fig. 8 C). Thus, it was not that N73pep could no longer remove p115 from the membranes at later time points. The requirement for p115 appears to be a transient event required for the initial docking and alignment of newly formed single cisternae.
Table 2.
Effect of GM130-N73pep and Soluble GRASP65 Treatment on RLG
| Incubation | Total membrane as stacked regions of cisternae | Mean number of cisternae per stack |
|---|---|---|
| None | 48 ± 3.2 | 3.0 ± 0.18 |
| N73pep 15 min on ice | 46 ± 2.9 | 2.9 ± 0.13 |
| N73pep 60 min at 37°C | 41 ± 5.9 | 2.8 ± 0.1 |
| GRASP65 15 min on ice | 43 ± 4.1 | 2.8 ± 0.18 |
| GRASP65 60 min at 37°C | 40 ± 1.9 | 2.9 ± 0.17 |
RLG at 0.75 mg/ml were treated with N73pep (80 μM) or soluble GRASP65 (75 ng/μl) in KHM (with 2 mM ATP and 1 mM GTP) buffer and an ATP regeneration system in a final volume of 20 μl, and incubated for 15 min on ice or 60 min at 37°C, fixed, and processed for EM. The parameters were determined as described in Materials and Methods and are presented as the mean ± SEM (n = 2).
The stacking process was sensitive to soluble GRASP65 for the first 30 min (Fig. 8 D). Soluble GRASP65 acted to unstack Golgi cisternae that had formed before this point (compare Fig. 8A and Fig. D). However, beyond 30 min, the reassembled stacks became resistant to soluble GRASP65, which is also a property of the starting stacked RLG (Table ). Soluble GRASP65 treatment of starting RLG did not disrupt their stacked structure. Neither the percentage total membrane as stacked regions of cisternae nor the number of cisternae per stack were affected (Table ). At no time point did soluble GRASP65 affect p115 binding: the amount bound at the end of the incubation remained constant (Fig. 8 D). That the reassembled stacks remain sensitive to soluble GRASP65 longer than they do to N73pep suggests that GRASP65 may act downstream of p115 in the stacking pathway, raising the possibility that the stacking reaction proceeds by an initial p115-dependent tethering step that is followed by a GRASP65-dependent stacking step.
Discussion
We have employed a modified cell-free assay to more closely assess p115 function in cisternal regrowth and stacking. In this assay, the MGF are isolated through a 0.5-M sucrose cushion that renders them incompetent for cisternal regrowth in the absence of added soluble factors, due to the virtual absence of the membrane fusion ATPases, NSF and p97. Previously, treatment of the MGF with the cysteine alkylating reagent NEM inactivated any residual NSF or p97, thus ensuring dependence on added soluble factors (Rabouille et al. 1995b). However, this treatment inhibited the stacking of the Golgi cisternae that reformed, presumably due to modification of a conserved cysteine on GRASP65 (Barr et al. 1997). The obviation of MGF NEM-treatment enabled the study of cisternal regrowth and stacking simultaneously in a membrane polypeptide environment devoid of alkylated cysteines. Using this system, we have found a novel, essential role for p115 in the stacking of reassembling Golgi cisternae and NSF-mediated cisternal regrowth.
Several lines of evidence strongly suggest a requirement for p115 in the stacking of reassembling Golgi cisternae. Firstly, p115-depleted cytosol supports full cisternal regrowth at cytosol concentrations above 1 mg/ml, but not cisternal stacking, suggesting that these are separable processes. Cisternal stacking is restored by addition of purified p115 to the depleted cytosol. Reassembly conducted in p115-depleted cytosol at maximum concentration for periods of well over 1 h did support some stacking, but the initial rate and overall extent of stacking were severely retarded. In the reassembly assay conducted with purified fusion components, NSF-dependent reassembly required p115 for stacking and cisternal regrowth. While in the p97-dependent reassembly, p115 was required for stacking, but not cisternal regrowth. Similarly, when the NSF/p97 pathways were combined, p115 was only required for cisternal stacking, as was the case in the reassembly conducted in p115-depleted cytosol. Thus, we conclude that p115 is able to tether cisterna to cisterna, as well as COPI vesicle to cisterna, and in so doing, plays a role in cisternal stacking.
That p115 functions in both the NSF (for membrane fusion and stacking) and p97 (stacking only) pathways suggests that this may be another point where these pathways intersect and are modulated. Syntaxin 5 is also a common component of the two pathways, and may explain why they contribute nonadditively to cisternal regrowth (Rabouille et al. 1998). A modulatory role for p115 is perhaps reflected by the different cisternal morphologies the two pathways produce. A clear continuum exists whereby at one extreme, the p97 pathway in the absence of p115 only generates long single cisternae, whereas the NSF pathway in the presence of p115 generates stacks with three or more short cisternae. When both pathways are combined, the result was approximately intermediate, with no really long cisternae forming, and stacks with only two to three cisternae per stack. Why the NSF pathway generates stacks with more cisternae per stack is not yet clear.
The difference in cisternal length produced by the NSF and p97 pathways was not detected when MGF were pretreated with NEM (Rabouille et al. 1995b). This may reflect an NEM sensitivity of membrane-bound components required for the p97 pathway. These may be involved in the tethering step of the reaction, since p97-mediated fusion seems to be independent of p115 and has no known tethering molecules. In addition, these cisternal length differences may reflect the mode of fusion p97 and NSF catalyze. That the p97-generated cisternae are longer suggests that it may be acting to fuse cisterna to cisterna in a homotypic fashion. In contrast, the NSF pathway may be acting to fuse COPI vesicle to its target membrane, a heterotypic fusion event, generating numerous short cisternae that are less able to fuse homotypically.
Therefore, our working hypothesis is that the NSF pathway reconstitutes the Golgi rims while the p97 pathway reconstitutes the cisternal cores, as has been suggested before (Rabouille et al. 1995b, Rabouille et al. 1998). We are currently attempting to verify this model. This model is in contrast to another system in which IQ has been used to disassemble the Golgi apparatus into small fragments. After the removal of IQ, the reassembly pathway has been shown to involve the sequential action of NSF followed by p97 (Acharya et al. 1995). This sequence does not lend itself to the simple rebuilding of cores by p97 and the rims by NSF. However, this sequence may be dictated by the fact that the fragments were generated by an IQ-specific mechanism and not by a mitotic process, so, although the end-products (stacked cisternae) are the same, the route of reassembly may well be different.
Another feature of cisternae reassembled in the absence of p115 is their frequent wrinkled, corrugated morphology. This suggests p115 is required for a membrane-smoothing event during the reassembly process. Analogy may be drawn to the post-mitotic reassembly of the nuclear envelope. In a cell-free system that utilizes Xenopus egg extracts and scanning EM to visualize nuclear envelope assembly (Wiese et al. 1997), once membrane fusion has created a fully enclosed nuclear envelope the membrane at first appears wrinkled. The envelope is then smoothed by a process that requires active transport by nuclear pore complexes, and may be due to the uptake of soluble lamins and reassembly of the nuclear lamina on the nucleoplasmic face. It may be that the 15% of Golgi membrane-bound p115 molecules that are resistant to salt extraction are deeply enmeshed in the Golgi matrix (Nakamura et al. 1995). The incorporation of this p115 back into this matrix at the end of mitosis may be responsible for the cisternal membrane-smoothing event. It is conceivable that the reformation of the Golgi matrix on the cytoplasmic face of the Golgi membrane causes a concomitant increase in membrane tension, and thus results in membrane smoothing. Perhaps p115 acts by establishing cis-interactions between GM130 and giantin, or by forming homooligomeric structures. In fact, one may compare p115 to the A-type lamins because both are released in a soluble state at mitosis (Gerace and Blobel 1980) are extensively coiled coil dimers. p115 also bears significant resemblance to the nuclear matrix protein, NuMA, which is capable of self assembling into homooligomeric structures (Harborth et al. 1999).
p115-mediated stacking requires both receptors for p115 on Golgi membranes, giantin and GM130. Pretreatment of MGF with antibodies against GM130 and/or giantin precluded cisternal stacking, as well as NSF-mediated cisternal regrowth. Previously, the GM130–p115–giantin complex had been implicated in tethering COPI vesicles that had been isolated in the presence of GTPγS to Golgi membranes (Sönnichsen et al. 1998). Since the vesicles could not uncoat, it could not be proven that this tethered intermediate reflected a bona fide intermediate in the transport reaction. However, the fact that antigiantin and anti-GM130 block COPI vesicle tethering and NSF-mediated membrane fusion suggests that the GM130–p115–giantin complex does act in COPI vesicle tethering that then leads to NSF-mediated membrane fusion.
The fact that GM130 largely appears to be excluded from COPI vesicles, and the relative effects of preblocking COPI vesicles or Golgi membranes with antigiantin or anti-GM130 antibodies on subsequent COPI vesicle tethering, suggested that the tether was made up of giantin on the COPI vesicle linked to GM130 on the target membrane via p115 (Sönnichsen et al. 1998). The closest explanation for p115 action in cisternal stacking is that it proceeds through this same heteroternary complex. This is supported by the fact that preincubation of MGF with either anti-GM130 or antigiantin alone precludes stacking, suggesting that stacking cannot be operating through GM130–p115–GM130 or giantin–p115–giantin cross-bridges alone.
Many of the factors required for the reassembly assay have a predominantly cis-Golgi membrane localization (e.g., GM130 [Nakamura et al. 1995], GRASP65 [Barr et al., 1997], and p115 [Nelson et al., 1998]). Giantin represents an exception because it is located around the periphery of stacks (Shima et al. 1997). One explanation for this apparent cis-bias is that our RLG preparation is enriched for cis-medial markers relative to trans-markers (Hui et al. 1998), and therefore the reassembly assay may be biased towards isolating cis-Golgi membrane acting factors. Alternatively, factors that are necessary to establish the stacked Golgi structure may be concentrated at the cis-face as part of the biogenetic process that is constantly occurring in interphase cells, as proposed by the cisternal maturation model of Golgi membrane transport (Pelham 1998).
Whether the cisternal maturation model or the vesicular transport model is true, both models have a requirement for the transfer of COPI vesicles between successive layers of the stack, even though the directionality/content of these vesicles may vary between models (Pelham 1998) and awaits in vivo confirmation, at least for Golgi enzymes, if not cargo (Orci et al. 1997). That p115 plays a role in the establishment of the Golgi stack indicates that the mechanism of intra-Golgi membrane transport may be hard-wired into the structure of the stack. One might envisage the existence of a continuum of the giantin–p115–GM130 heteroternary complex. Whereby, at the cisternal rim, this complex links COPI vesicle to cisterna, and on moving towards the cisternal core, links cisterna to cisterna. In this way, a COPI vesicle may already be linked to its acceptor cisterna before budding is completed. Intra-Golgi membrane transport would then proceed by transfer of COPI vesicles that are pretethered to their acceptor membrane, rather than release of COPI vesicles by the donor membrane, followed by capture by the acceptor membrane. This would increase the efficiency of the reaction by eliminating the reliance on a vesicle meeting its target membrane by random collision, and reduce the chance of losing the vesicle in the surrounding cytoplasm. Since giantin is most likely to enter the budding COPI vesicle, the orientation of the complex might even help determine the next cisterna with which the COPI vesicle is to fuse.
Previously, it has been shown that GRASP65, which anchors the COOH terminus of GM130 to the Golgi membrane, is involved in the stacking process (Barr et al. 1997). How p115 and GRASP65 functions relate to promote cisternal stacking during reassembly was assessed by determining the temporal sensitivity of the stacking reaction to agents that specifically interfere with p115 (N73pep) and GRASP65 (soluble, nonmyristoylated GRASP65) function. The soluble GRASP65 neither removed GM130 from the Golgi membrane, prevented p115 rebinding to reassembling Golgi membranes, nor inhibited NSF-mediated cisternal regrowth. Therefore, it seems unlikely that soluble GRASP65 is acting to disrupt the endogenous GRASP65–GM130 interaction and thus does not interfere with stacking by preventing p115 function. Rather, the soluble GRASP65 is more likely to be competing with the endogenous GRASP65 for other interactions. In the combined NSF/p97 reassembly system, both N73pep and soluble GRASP65 potently inhibited stacking without affecting cisternal regrowth. The stacking reaction remained sensitive to soluble GRASP65 for longer than it did to N73pep, suggesting that p115 acts upstream of GRASP65 in cisternal stacking.
The 15 min time point of reassembly, where N73pep has its most potent effects, is the stage when single cisternae begin to dock and align to form stacks (Rabouille et al. 1995a). p115 may be required for this initial meeting of the cisternal membranes and then pass on the stacking function proper to another machinery, which likely involves GRASP65. Therefore, the giantin–p115–GM130 complex would not be essential for steady-state stacking per se, and this is consistent with the Golgi stack's resistance to N73pep which removes ∼85% of p115.
Precisely how GRASP65 acts to stack cisternae and precisely how soluble GRASP65 interferes with this reaction remains obscure. One possibility is that the oligomeric state of GRASP65 may be important for anchoring cisternae together. GRASP65 appears to be either a dimer or a trimer (Barr et al. 1998). It may be that GRASP65 monomers insert their myristoyl groups into opposite membranes of adjacent cisternae, so holding them together. Soluble GRASP65 may then prevent the endogenous GRASP65 from interacting with itself and in so doing, form inactive oligomers. Alternatively, there may be as yet unidentified GRASP65 interacting molecules which are titrated out by the soluble GRASP65 that help to promote stacking. It will be important to determine the precise higher order structure of the GM130–GRASP65 complex, and to elucidate other GRASP65-interacting Golgi molecules before a molecular mechanism of stacking can be established.
Analogy may be drawn to the proposed mechanism of vesicular transport, where p115 acts at an early stage in tethering the COPI vesicle to its acceptor membrane, and then hands it over to the SNAREs to complete the fusion step. Similarly, in cisternal stacking, p115 may act at an early stage in tethering cisternal membranes together, and then hand over to another set of molecules that complete the stacking reaction. GRASP65 is an excellent candidate for one of these downstream factors. The stacking reaction has also been shown to have a microcystin-sensitive component (Rabouille et al. 1995a). The identification and the point of action of which will prove revealing.
Comparison of COPI vesicle production by the Golgi apparatus under interphase and mitotic conditions reveals an apparent capacity to generate twice as many COPI vesicles at mitosis with the same content (Sönnichsen et al. 1996). This suggests the Golgi stack may be seen as a capacitor for COPI vesicle flow. At mitosis, the Golgi stack is opened up, eventually disappears, and more COPI vesicles form, as compared with the closed stack during interphase. This may be due to more Golgi rim being available for COPI vesicles to bud from, such that, as more rim is available, COPI vesicle flux increases and vice versa. The amount of rim available for COPI vesicle formation may be determined by how much of the giantin–p115–GM130 complex is sequestered, tethering cisterna to cisterna. This complex is abolished during mitosis by Cdc2-mediated phosphorylation of GM130, and may be disrupted by direct phosphorylation of p115 during interphase (Sohda et al. 1998). The ratio of complex tethering cisterna to cisterna and cisterna-COPI vesicle may then be tailored to suit the COPI vesicle flow needs of the cell. That this complex is essential for establishing stacked structure after mitosis suggests that it may also act to stabilize stacked architecture at steady-state, and in so doing, couple the stacked structure to processive intra-Golgi COPI vesicle flow. The proposed function p115 in processive intra-Golgi membrane transport and post-mitotic cisternal stacking does not preclude p115 from having other functions, such as COPII vesicle tethering on the intermediate compartment, where it also has been localized (Nelson et al. 1998). Our current focus is to characterize more finely the interphase dynamics of the tethering complex and to determine whether p115 fulfils the same function in stacking at the end of mitosis in vivo.
Acknowledgments
We would like to thank Francis Barr, Barbara Dirac-Svejstrup, and Martin Lowe for many constructive comments and support; Hemmo Meyer for p97, Joyce Müller for NSF, Hisao Kondo for p47, Francis Barr for soluble GRASP65, G. Waters, N. Nakamura, M. Tagaya, G. Stenbeck, F. Barr, M. Renz, and J.M. Peters for antibodies, and Rose Watson and Eija Jämsä for help with EM.
James Shorter was supported by a predoctoral research fellowship from the Imperial Cancer Research Fund.
Footnotes
1.used in this paper: COPI, coat protein I; GRASP65, Golgi reassembly stacking protein 65; IQ, illimaquinone; Mann I, α1,2-mannosidase I; MGF, mitotic Golgi fragments; N73pep, NH2-terminal 73 amino acids of GM130 peptide; NEM, N-ethylmaleimide; NSF, NEM-sensitive fusion protein; RLG, rat liver Golgi membranes; sHeLa, spinner HeLa cells; SNAP, soluble NSF attachment protein; SNARE, SNAP receptor; t, target; v, vesicle
Dr. Warren's current address is Department of Cell Biology, SHM, C441, Yale University School of Medicine, 33 Cedar St., PO Box 208002, New Haven, CT 06520-8002. Tel.: (203) 785-5058. Fax: (203) 785-4301. E-mail: graham.warren@yale.edu
References
- Acharya U., Jacobs R., Peters J.M., Watson N., Farquhar M.G., Malhotra V. The formation of Golgi stacks from vesiculated Golgi membranes requires two distinct fusion events. Cell. 1995;82:895–904. doi: 10.1016/0092-8674(95)90269-4. [DOI] [PubMed] [Google Scholar]
- Acharya U., Mallabiabarrena A., Acharya J.K., Malhotra V. Signaling via mitogen-activated protein-kinase (MEK 1) is required for Golgi fragmentation during mitosis. Cell. 1998;92:183–192. doi: 10.1016/s0092-8674(00)80913-7. [DOI] [PubMed] [Google Scholar]
- Barr F.A., Puype M., Vandekerckhove J., Warren G. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell. 1997;91:253–262. doi: 10.1016/s0092-8674(00)80407-9. [DOI] [PubMed] [Google Scholar]
- Barr F.A., Nakamura N., Warren G. Mapping the interaction between GRASP65 and GM130, components of a protein complex involved in the stacking of Golgi cisternae. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:3258–3268. doi: 10.1093/emboj/17.12.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., Ballew N., Barlowe C. Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:2156–2165. doi: 10.1093/emboj/17.8.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmaki T., Ponnambalam S., Prescott A.R., Clausen H., Tang B.L., Hong W., Lucocq J.M. Forward and retrograde trafficking in mitotic animal cells. J. Cell Sci. 1999;112:589–600. doi: 10.1242/jcs.112.5.589. [DOI] [PubMed] [Google Scholar]
- Gerace L., Blobel G. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell. 1980;19:277–287. doi: 10.1016/0092-8674(80)90409-2. [DOI] [PubMed] [Google Scholar]
- Harborth J., Wang J., Gueth-Hallonet C., Weber K., Osborn M. Self assembly of NuMAmultiarm oligomers as structural units of a nuclear lattice. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:1689–1700. doi: 10.1093/emboj/18.6.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui N., Nakamura N., Slusarewicz P., Warren G. Purification of rat liver Golgi stacks Celis J. Cell BiologyA Laboratory Handbook 1998. Academic Press; San Diego, CA: 46–55.. Vol. 2 [Google Scholar]
- Jesch A., Linstedt A.D. The Golgi and endoplasmic reticulum remain independent during mitosis in HeLa cells. Mol. Biol. Cell. 1998;9:623–635. doi: 10.1091/mbc.9.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H., Rabouille C., Newman R., Levine T.P., Pappin D., Freemont P., Warren G. p47 is a cofactor for p97-mediated membrane fusion. Nature. 1997;388:75–78. doi: 10.1038/40411. [DOI] [PubMed] [Google Scholar]
- Levine T.P., Rabouille C., Kieckbusch R.H., Warren G. Binding of the vesicle docking protein p115 to Golgi membranes is inhibited under mitotic conditions. J. Biol. Chem. 1996;271:17304–17311. doi: 10.1074/jbc.271.29.17304. [DOI] [PubMed] [Google Scholar]
- Linstedt A.D., Hauri H.P. Giantin, a novel conserved Golgi membrane protein containing a cytoplasmic domain of at least 350 kDa. Mol. Biol. Cell. 1993;4:679–693. doi: 10.1091/mbc.4.7.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe M., Rabouille C., Nakamura N., Watson R., Jackman M., Jämsä E., Rahman D., Pappin D.J., Warren G. Cdc2 kinase directly phosphorylates the cis-Golgi matrix protein GM130 and is required for Golgi fragmentation in mitosis. Cell. 1998;94:783–793. doi: 10.1016/s0092-8674(00)81737-7. [DOI] [PubMed] [Google Scholar]
- Lucocq J.M., Pryde J.G., Berger E.G., Warren G. A mitotic form of the Golgi apparatus in HeLa cells. J. Cell Biol. 1987;104:865–874. doi: 10.1083/jcb.104.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucocq J.M., Berger E.G., Warren G. Mitotic Golgi fragments in HeLa cells and their role in the reassembly pathway. J. Cell Biol. 1989;109:463–474. doi: 10.1083/jcb.109.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luján H.D., Marotta A., Mowatt M.R., Sciaky N., Lippincott-Schwartz J., Nash T.E. Developmental induction of Golgi structure and function in the primitive eukaryote Giardia lamblia . J. Biol. Chem. 1995;270:4612–4618. doi: 10.1074/jbc.270.9.4612. [DOI] [PubMed] [Google Scholar]
- Mellman I., Simons K. The Golgi complexin vitro veritas? Cell. 1992;68:829–840. doi: 10.1016/0092-8674(92)90027-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misteli T., Warren G. COP-coated vesicles are involved in the mitotic fragmentation of Golgi stacks in a cell-free system. J. Cell Biol. 1994;125:269–282. doi: 10.1083/jcb.125.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misteli T., Warren G. A role for tubular networks and a COPI-independent pathway in the mitotic fragmentation of Golgi stacks in a cell-free system. J. Cell Biol. 1995;130:1027–1039. doi: 10.1083/jcb.130.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahama M., Orci L., Ravazzola M., Amherdt M., Lacomis L., Tempst P., Rothman J.E., Söllner T.H. A v-SNARE implicated in intra-Golgi transport. J. Cell Biol. 1996;133:507–516. doi: 10.1083/jcb.133.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N., Rabouille C., Watson R., Nilsson T., Hui N., Slusarewicz P., Kreis T.E., Warren G. Characterization of a cis-Golgi matrix protein, GM130. J. Cell Biol. 1995;131:1715–1726. doi: 10.1083/jcb.131.6.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N., Lowe M., Levine T.P., Rabouille C., Warren G. The vesicle docking protein p115 binds GM130, a cis-Golgi matrix protein, in a mitotically regulated manner. Cell. 1997;89:445–455. doi: 10.1016/s0092-8674(00)80225-1. [DOI] [PubMed] [Google Scholar]
- Nelson D.S., Alvarez C., Gao Y.S., Garcia-Mata R., Fialkowski E., Sztul E. The membrane transport factor TAP/p115 cycles between the Golgi and earlier secretory compartments and contains distinct domains required for its localization and function. J. Cell Biol. 1998;143:319–331. doi: 10.1083/jcb.143.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Stamnes M., Ravazzola M., Amherdt M., Perrelet A., Söllner T.H., Rothman J.E. Bidirectional transport by distinct populations of COPI-coated vesicles. Cell. 1997;90:335–349. doi: 10.1016/s0092-8674(00)80341-4. [DOI] [PubMed] [Google Scholar]
- Orci L., Perrelet A., Rothman J.E. Vesicles on stringsmorphological evidence for processive transport within the Golgi stack. Proc. Natl. Acad. Sci. USA. 1998;95:2279–2283. doi: 10.1073/pnas.95.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S.K., Indig F.E., Olivieri N., Levine N.D., Latterich M. Organelle membrane fusiona novel function for the syntaxin homolog Ufe1p in ER membrane fusion. Cell. 1998;92:611–620. doi: 10.1016/s0092-8674(00)81129-0. [DOI] [PubMed] [Google Scholar]
- Pelham H.R.B. Getting through the Golgi-complex. Trends Cell Biol. 1998;8:45–49. doi: 10.1016/s0962-8924(97)01185-9. [DOI] [PubMed] [Google Scholar]
- Rabouille C., Misteli T., Watson R., Warren G. Reassembly of Golgi stacks from mitotic Golgi fragments in a cell-free system J. Cell Biol. 129 1995. 605 618a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabouille C., Levine T.P., Peters J.M., Warren G. An NSF-like ATPase, p97, and NSF mediate cisternal regrowth from mitotic Golgi fragments Cell. 82 1995. 905 914b [DOI] [PubMed] [Google Scholar]
- Rabouille C., Kondo H., Newman R., Hui N., Freemont P., Warren G. Syntaxin 5 is a common component of the NSF- and p97-mediated reassembly pathways of Golgi cisternae from mitotic Golgi fragments in vitro. Cell. 1998;92:603–610. doi: 10.1016/s0092-8674(00)81128-9. [DOI] [PubMed] [Google Scholar]
- Rambourg A., Clermont Y. Three-dimensional structure of the Golgi apparatus in mammalian cells. In The Golgi Apparatus. E.G. Berger and J. Roth, editors. Birkhäuser Verlag, Basel. 1997;37–61 [Google Scholar]
- Sapperstein S.K., Walter D.M., Grosvenor A.R., Heuser J.E., Waters M.G. p115 is a general vesicular transport factor related to the yeast endoplasmic reticulum to Golgi transport factor Uso1p. Proc. Natl. Acad. Sci. USA. 1995;92:522–526. doi: 10.1073/pnas.92.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapperstein S.K., Lupashin V.V., Schmitt H.D., Waters M.G. Assembly of the ER to Golgi SNARE complex requires Uso1p. J. Cell Biol. 1996;132:755–767. doi: 10.1083/jcb.132.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima D.T., Haldar K., Pepperkok R., Watson R., Warren G. Partitioning of the Golgi apparatus during mitosis in living HeLa cells. J. Cell Biol. 1997;137:1211–1228. doi: 10.1083/jcb.137.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima D.T., Cabrera-Poch N., Pepperkok R., Warren G. An ordered inheritance strategy for the Golgi apparatusvisualization of mitotic disassembly reveals a role for the mitotic spindle. J. Cell Biol. 1998;141:955–966. doi: 10.1083/jcb.141.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohda M., Misumi Y., Yano A., Takami N., Ikehara Y. Phosphorylation of the vesicle docking protein p115 regulates its association with the Golgi membrane. J. Biol. Chem. 1998;273:5385–5388. doi: 10.1074/jbc.273.9.5385. [DOI] [PubMed] [Google Scholar]
- Sönnichsen B., Watson R., Clausen H., Misteli T., Warren G. Sorting by COPI-coated vesicles under interphase and mitotic conditions. J. Cell Biol. 1996;134:1411–1425. doi: 10.1083/jcb.134.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sönnichsen B., Lowe M., Levine T., Jämsä E., Dirac-Svejstrup B., Warren G. A role for giantin in docking COPI vesicles to Golgi membranes. J. Cell Biol. 1998;140:1013–1021. doi: 10.1083/jcb.140.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley H., Botas J., Malhotra V. The mechanism of Golgi segregation during mitosis is cell type-specific. Proc. Natl. Acad. Sci. USA. 1997;94:14467–14470. doi: 10.1073/pnas.94.26.14467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart R.A., Mackay D., Adamczewski J., Warren G. Inhibition of intra-Golgi transport in vitro by mitotic kinase. J. Biol. Chem. 1993;268:4050–4054. [PubMed] [Google Scholar]
- Waters M.G., Clary D.O., Rothman J.E. A novel 115-kD peripheral membrane protein is required for intercisternal transport in the Golgi stack. J. Cell Biol. 1992;118:1015–1026. doi: 10.1083/jcb.118.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidman P., Roth R., Heuser J. Golgi membrane dynamics imaged by freeze-etch electron microscopyviews of different membrane coatings involved in tubulation versus vesiculation. Cell. 1993;75:123–133. [PubMed] [Google Scholar]
- Whiteheart S.W., Rossnagel K., Buhrow S.A., Brunner M., Jaenicke R., Rothman J.E. N-ethylmaleimide–sensitive fusion proteina trimeric ATPase whose hydrolysis of ATP is required for membrane fusion. J. Cell Biol. 1994;126:945–954. doi: 10.1083/jcb.126.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese C., Goldberg M.W., Allen T.D., Wilson K.L. Nuclear envelope assembly in Xenopus extracts visualized by scanning EM reveals a transport-dependent “envelope smoothing” event. J. Cell Sci. 1997;110:1489–1502. doi: 10.1242/jcs.110.13.1489. [DOI] [PubMed] [Google Scholar]
- Yamakawa H., Seog D.H., Yoda K., Yamasaki M., Wakabayashi T. Uso1 protein is a dimer with 2 globular heads and a long coiled-coil tail. J. Struct. Biol. 1996;116:356–365. doi: 10.1006/jsbi.1996.0053. [DOI] [PubMed] [Google Scholar]