Abstract
Although chemotherapy for breast cancer can increase inflammation, few studies have examined predictors of this phenomenon. This study examined potential contributions of demographics, disease characteristics, and treatment regimens to markers of inflammation in response to chemotherapy for breast cancer. Thirty-five women with stage I - III-A breast cancer (mean age 50 years) were studied prior to cycle 1 and prior to cycle 4 of anthracycline-based chemotherapy. Circulating levels of inflammatory markers with high relevance to breast cancer were examined, including C-reactive protein (CRP), tumor necrosis factor-α (TNF-α), Interleukin-1 receptor antagonist (IL1-RA), vascular endothelial growth factor (VEGF), soluble intercellular adhesion molecule-1 (sICAM-1), Interleukin- (IL-6), soluble P-selectin (sP-selectin), and von Willebrand factor (vWf). Chemotherapy was associated with elevations in VEGF (p≤0.01), sICAM-1 (p≤0.01), sP-selectin (p≤0.02) and vWf (p≤0.05). Multiple regression analysis controlling for age and body mass index (BMI) showed that higher post-chemotherapy levels of inflammation were consistently related to higher pre-chemotherapy levels of inflammation (p’s ≤0.05) as well as to certain disease characteristics. Post-chemotherapy IL-6 levels were higher in patients who had larger tumors (p≤0.05) while post-chemotherapy VEGF levels were higher in patients who had smaller tumors (p≤0.05). Post-chemotherapy sP-selectin levels were highest in women who had received epirubicin, cytoxan, 5fluorouracil chemotherapy (p≤0.01). These findings indicate that chemotherapy treatment can be associated with elevations in certain markers of inflammation, particularly markers of endothelial and platelet activation. Inflammation in response to chemotherapy is most significantly related to inflammation that existed prior to chemotherapy but also potentially to treatment regimen and to certain disease characteristics.
Keywords: breast cancer, chemotherapy, inflammation, CRP, TNF-α, IL1-RA, VEGF, sICAM-1, IL-6, sP-selectin, vWf
Introduction
Inflammation is common in breast cancer, being associated with both cancer onset and progression (Lithgow and Covington, 2005). There are several important peripheral markers of inflammation in breast cancer, each providing unique insight into inflammatory processes, including VEGF, sICAM-1, IL-6, TNF-α, IL-1, CRP, vWf, and sP-selectin.
VEGF is an angiogenic cytokine which stimulates the formation of new blood vessels necessary for tumor growth and metastasis (Boudreau and Myers, 2003). Circulating levels of VEGF are elevated in breast cancer and are an independent predictor of poorer survival (Nishimura et al., 2003; Wu et al., 2002). Expression of the adhesion molecule ICAM-1, which aids in host immune surveillance of tumor cells (Ogawa et al., 1998; Shirai et al., 2003), has been implicated in tumor progression and metastases, with greater expression being associated with low growth potential, negative lymph node involvement, and good prognosis (O’Hanlon et al., 2002; Vasse et al., 2001). In contrast, the soluble form of ICAM-1 (sICAM-1) is elevated in breast cancer (Klein et al., 1995; Merendino et al., 2001; O’Hanlon et al., 2002) and is predictive of poorer response to chemotherapy and poorer prognosis (O’Hanlon et al., 2002; Zhang and Adachi, 1999). sICAM-1 reflects generalized inflammation (Blann et al., 2002) and exerts immunomodulatory effects such as suppression of natural killer cell and T-cell function and inhibition of leukocyte binding to target cells, including formation of natural killer cell/tumor cell conjugates (Cho et al., 2000; Kaihara et al., 1998).
Several components of the acute-phase reaction are also elevated in breast cancer, including the inflammatory cytokines IL-6, TNF-α and IL-1, and CRP (Blann et al., 2002; O’Hanlon et al., 2002; Tsavaris et al., 2002). IL-6 has tumor-inhibiting and tumor-promoting effects in breast cancer (Knupfer and Preiss, 2006). High serum levels of IL-6 in breast cancer are independent predictors of poor survival (Bachelot et al., 2003). TNF-α is an independent predictor of progression free survival in metastatic breast cancer patients who receive chemotherapy (Bozcuk et al., 2004). IL-1α and IL-1β are secreted by macrophages and monocytes, stimulate breast cancer proliferation, and along with IL-1RA, are found in breast cancer tissue (Nicolini et al., 2006; Singer et al., 2003). Elevated serum levels of IL-1β correlate with breast cancer recurrence (Mettler et al., 2004). IL-1RA levels are higher in older breast cancer patients whose cancer lack estrogen receptors (Fuksiewicz et al., 2006).
The acute-phase reactant CRP is produced by the liver in response to inflammation. Elevated CRP levels are predictive of poor survival in metastatic breast cancer (Albuquerque et al., 1995). In addition, elevated CRP levels correlate with sICAM-1, providing evidence that elevated sICAM-1 levels reflect the acute phase response and generalized inflammation (Blann et al., 2002).
von Willebrand factor (vWf), which is found in the Weibel-Palade bodies of secretory organelles of endothelial cells and platelets and plays a key role in hemostasis, is elevated in breast cancer (Blann et al., 2002; Blann et al., 2001). Elevated vWf levels do not appear to reflect platelet activation but rather endothelial activation and/or damage (Blann et al., 2002; Blann et al., 2001). Soluble P-selectin (sP-selectin), on the other hand, does reflect platelet activation in breast cancer (Blann et al., 2002; Fox et al., 1995). Platelet P-selectin expression supports the formation of blood platelet – tumor cell complexes in the circulation and facilitates metastases (Blann et al., 2001).
Chemotherapy for breast cancer can lead to further increases in these inflammatory mediators, or in some cases, decreases. Three cycles of standard anthracycline-based chemotherapy in stage I-III-B patients leads to an increase in circulating levels of sICAM-1 and VEGF (Mills et al., 2004).
Chemotherapy with the taxanes paclitaxel and docetaxel increases IL-6 levels but decreases IL-1 and TNF-α levels, with more pronounced effects with docetaxel (Tsavaris et al., 2002). Three courses of neoadjuvant chemotherapy for non-inflammatory stage III-B breast cancer leads to a decrease in TNF-α levels, an effect which is greater in patients with complete versus partial responses (Berberoglu et al., 2004). Single agent paclitaxel therapy but not fluorouracil plus doxorubicin and cyclophosphamide chemotherapy increases IL-6 levels (Pusztai et al., 2004).
Although there are several markers of disease that are associated with inflammation in breast cancer (Fuksiewicz et al., 2006), few studies have prospectively examined disease and treatment factors that might be related to change in inflammation in response to chemotherapy for breast cancer. Kummel et al. (Kummel et al., 2006) examined circulating levels of VEGF (VEGF-A) and VEGF-D (a lymphangiogenic growth factor) (Yamazaki and Morita, 2006), prior to and following adjuvant chemotherapy in patients with positive node breast cancer. There was a significant reduction in VEGF levels, but only in patients who had large tumors and in patients with a positive hormone receptor status.
The primary aims of this study were to examine the effects of chemotherapy for breast cancer on measures of inflammation and to test for potential predictors of inflammation in response to chemotherapy. Our measures of inflammation included a panel of eight circulating markers known to have clinical relevance in breast cancer. We hypothesized that four of them - sICAM-1, VEGF, sP-selecting, and vWF - would be increased in response to chemotherapy. For potential predictors of inflammation, we tested a panel of demographic, breast cancer, and treatment regimen characteristics.
Methods
Women with breast cancer were recruited from the UCSD Moores Cancer Center and from oncologists in the San Diego area. Those diagnosed with stage I through III-A breast cancer and referred for adjuvant anthracycline-based chemotherapy were invited to participate. Exclusion criteria included pregnancy, women undergoing bone marrow transplants, metastatic breast cancer, confounding underlying medical illnesses including renal failure, significant pre-existing anemia, and other physical or psychological impairments which would limit participation. The study was approved by the UCSD Human Research Protection Program and the UCSD Cancer Center Human Research Protection Program of Participating Oncologists.
Breast cancer disease staging was performed by the referring medical oncologist (typically utilizing the American Joint Committee on Cancer Staging Manual 5th Edition). Patients received adjuvant anthracycline-based chemotherapy after clinical staging and definitive surgical treatment with either lumpectomy or mastectomy and axillary staging with sentinel node biopsy or axillary lymph node dissection according to institutional standards. The individual anthracycline regimens in this study included standard clinical doses of adriamycin and cyclophosphamide alone, adriamycin and cyclophosphamide with taxanes, and epirubicin, cytoxan, 5fluorouracil chemotherapy (Mills, P. J., B. Parker, et al. (2004).
The 35 women who completed this study were obtained from a larger pool of 128 women who initially requested information about a study on the effects of chemotherapy for breast cancer on disturbed sleep and fatigue. Of these, 19 were not interested in participating, 17 were not eligible to participate, and 6 dropped out before study completion. Of the remaining 86 participants, 35 completed this sub-study designed to examine the effects of chemotherapy on inflammation. Each participant was studied prior to and following three 3-week cycles of adjuvant anthracycline-based chemotherapy. Nine of the women were treated with hormones prior to chemotherapy. None of the women had radiation therapy before the chemotherapy.
Blood Sampling and Assays
Blood samples were collected at the breast cancer clinic between the hours of 10:00 am and 2:00 pm immediately prior to the administration of cycle 1 (Pre-C1) of chemotherapy and immediately prior to the start of cycle 4 of chemotherapy (Pre-C4). Whole blood was preserved with either EDTA (sICAM-1, VEGF, sP-selectin, TNF-α, IL-1-RA, IL-6, and CRP) or sodium citrate (vWF). Following centrifugation, plasma or serum was stored at −80° C until assay. sICAM-1, VEGF, sP-selectin, TNF- α, IL1-RA, and IL-6 were determined in duplicate by commercial ELISA with internal controls (R&D Systems, Mpls, MN) (Mills et al., 2004; von Kanel et al., 2001). The precision of the assays were as follows: intra-assay CV’s for sICAM-1, VEGF, sP-selectin, TNF- α, IL1-RA, IL-6 were 3.9%, 5.0%, 7.8%, 8.1%, 3.5%, and 2.3%, respectively; inter-assay CV’s were 6.0%, 6.6%, 4.7%, 4.3% 5.1%, and 4.6%, respectively. Sensitivity values were < 0.35 ng/ml, < 5.0 pg/ml, < 0.5 ng/ml, <0.18 pg/ml, <10 pg/ml, and <0.72 pg/ml, respectively. vWf was determined in duplicate by commercial ELISA with internal controls (Diagnostica Stago Inc., Parsippany, New Jersey). The intra-assay CV for vWF was 2.6%, the inter-assay CV was 4.6%, and the sensitivity was 1.0%. CRP levels were determined in duplicate by the high sensitivity Denka-Seiken assay with internal controls (Roberts et al., 2001). The intra-assay CV for CRP was < 1.0%, the inter-assay CV was 1.6%, and the sensitivity was < 0.05 mg/l. Both samples (i.e. Pre-C1 and Pre-C4) from a given patient were assayed together.
Data Analysis
Prior to chemotherapy, simple correlation analyses and one-way ANOVA’s were run examining relationships among each inflammatory marker at Pre-C1 and the demographic (age and BMI) and breast cancer disease characteristics (including cancer type (infiltrating lobular carcinoma, infiltrating ductal carcinoma or infiltrating mixed carcinoma), estrogen receptor expression, progesterone receptor expression, human epidermal growth factor receptor-2 (Her-2) expression, tumor size and presence or absence of positive nodes).
For the primary aims of the study, in order to test whether there was a significant pre to post chemotherapy change in the overall inflammatory marker panel, while adjusting for multiple comparisons, we ran a MANOVA that included all eight inflammatory markers as dependent variables. This analysis takes into account correlations among the different markers. The MANOVA was significant and hence, following Fisher’s least significant difference paradigm (Miller, 1981), we conducted separate one-way repeated measures analyses of variance for each marker. This individual analysis served to tease out the potential chemotherapy treatment effects for each inflammatory marker. Multiple linear regression analyses were then run to determine the extent to which variables independently predicted the levels of each inflammatory marker at Pre-C4. Potential predictor variables were entered into the regression model in separate blocks. Age and BMI were force entered into the first block. The level of the respective inflammatory marker prior to chemotherapy was force entered into the second block. Factors related to breast cancer diagnosis as described above were entered into the third block (disease stage was not entered as a separate potential predictor variable because factors that determine stage, i.e., tumor size and lymph node status, were entered into the model). Treatment information, including chemotherapy regimen (adriamycin and cyclophosphamide alone, adriamycin and cyclophosphamide with taxanes, and epirubicin, cytoxan, 5fluorouracil chemotherapy) was entered into the fourth block. Among the 7 patients in the adriamycin and cyclophosphamide with taxanes group, 1 patient had received taxol and 6 received taxotere. Among the 17 patients in the mastectomy group, 2 received a double mastectomy and 15 a mastectomy. Data were analyzed using the SPSS 14.0 for Windows (SPSS Inc., Chicago, IL) and presented as means ± SD. The level of statistical significance was set at p≤.05 (two-tailed). Borderline significance (p ≤ .10) is also presented to show trends that may help to better illustrate overall relationships.
Results
Table 1 presents the individual patient characteristics of age, BMI, ethnicity, breast cancer stage, cancer type, tumor size, presence of positive nodes, estrogen receptor and progesterone receptor expression, Her-2 expression, chemotherapy regimen, and type of surgery. The mean age of the sample was 50.04 years (SD ± 10.01). Compared to patients who completed the larger study on chemotherapy and disturbed sleep and fatigue, the patients who completed this sub-study on inflammatory markers did not significantly differ on any of the characteristics that appear in Table 1.
Table 1.
Patient, Disease and Treatment Characteristics
| N | 35 |
| Age (years) | 50.04 (SD = 10.1; range = 34 to 79) |
| BMI | 27.65 (5.64) |
| Ethnicity | 26 - Caucasian
2 - African-American 3 - Hispanic 3 - Asian 1 - Native American |
| Stage | 11 - Stage I
9 - Stage II 1 - Stage III 4 - Stage III-A |
| Type of cancer | 3 - Infiltrating Lobular Carcinoma
30 - Infiltrating Ductal Carcinoma 2 - Infiltrating Mixed Carcinoma |
| Mean tumor Size (cm.) | 2.72 (1.74) |
| Positive Nodes | 19 - No
16 - Yes |
| Estrogen
Receptor Expression |
14 - No
21 - Yes |
| Progesterone
Receptor Expression |
17 - No
18 - Yes |
| Her-2 Expression | 27 - No
8 - Yes |
| Chemotherapy regimen | 19- Adriamycin and Cyclophosphamide alone
7 - Adriamycin and Cyclophosphamide with Taxanes 9 - Epirubicin and Cytoxan with 5Fluorouracil |
| Surgery | 18 - Lumpectomy
17 - Mastectomy |
Inflammatory Markers Pre-Chemotherapy
Circulating levels of each inflammatory marker at Pre-C1 are presented in Table 2. Prior to chemotherapy, IL-6 levels were higher in individuals with negative versus positive estrogen receptor expression (4.7 pg/ml, SD = 4.5 versus 1.4 pg/ml, SD = .811, respectively) (F= 7.01, p = 0.012) and in individuals with negative versus positive progesterone receptor expression (4.34 pg/ml, SD = 4.4 versus 1.34 pg/ml, SD = .721, respectively) (F= 5.66, p = 0.023). The only significant correlation Pre-C1, was between CRP levels and BMI (r = .412, p = 0.016).
Table 2.
Levels of Inflammatory Markers Prior to and During Chemotherapy
| (mean ± SD) | Pre-Cycle 1 | Pre-Cycle 4 | F; P values |
|---|---|---|---|
| CRP (mg/L) | 2.93 (2.65) | 3.42 (3.76) | 0.381; 0.54 |
| TNF-α (pg/ml) | 1.16 (1.48) | 1.19 (0.73) | 0.136; 0.83 |
| IL1-RA (pg/ml) | 1337 (1465) | 1357 (1181) | 0.05; 0.95 |
| VEGF (pg/ml) | 77.8 (96.1) | 119.2 (91.4) | 6.65; 0.01 |
| sICAM-1 (ng/ml) | 297.07 (78.3) | 334.02 (78.3) | 11.31; 0.002 |
| IL-6 (pg/ml) | 2.39 (2.21) | 3.096 (3.23) | 1.43; 0.242 |
| sP-selectin (ng/ml) | 90.5 (47.2) | 116.12 (58.2) | 5.65; 0.021 |
| vWf (%) | 94.32 (64.7) | 149.11 (167.4) | 5.72; 0.023 |
Inflammatory Markers During Chemotherapy
The MANOVA was significant at F=3.55, p=0.019. Individual repeated measures ANOVA’s showed that chemotherapy led to significant elevations in circulating levels of VEGF (p≤0.01), sICAM-1(p≤0.01), sP-selectin (p≤0.02) and vWf (p≤0.05). CRP, TNF- α, IL1-RA, and IL-6 levels did not significantly change in response to chemotherapy (Table 2).
The results of the multiple regression analyses are presented in Table 3. Levels of inflammatory markers at Pre-C4 were predicted by the Pre-C1 levels in all cases except sP-selectin and IL1-RA. CRP levels at the start of cycle 4 were predicted by higher pre-chemotherapy levels of CRP (model R2 = .177, F= 5.81, p≤0.05). Similarly, levels of TNF-α at the start of cycle 4 were predicted by higher pre-chemotherapy levels of TNF-α (model R2 = .257, F=8.63, p≤0.01).
Table 3.
Multiple Regression Predictors of Inflammation In Response to Chemotherapy
| Inflammatory Marker | Individual predictor variables (β coefficients; p values) | Model R2; adjusted R2; p value |
|---|---|---|
| CRP | Pre-chemotherapy CRP (.526; 0.023) | .177; .147; .023 |
| TNF-α | Pre-chemotherapy TNF-α (.322; 0.007) | .257; .227; 0.007 |
| IL1-RA | Pre-chemotherapy IL1-RA (−.159; .379); ER+ status (−.330; .069) | .133; .069; 0.146 |
| VEGF | Pre-chemotherapy VEGF (.374; .009); tumor size (−.104; 0.028) | .342; .009; .004 |
| sICAM-1 | Pre-chemotherapy sICAM-1 (.620; 0.001) | .396; .369; 0.001 |
| IL-6 | Pre-chemotherapy IL-6 (.588; 0.009); tumor size (.078; .041) | .311; .262;0.005 |
| sP-selectin | Pre-chemotherapy sP-selectin (.096; 0.52); tumor size (−.052; .008); chemotherapy (.069; 0.014) | .436; .349; 0.0048 |
| vWf | Pre-chemotherapy vWf (.706; 0.001) | .335; .332; 0.001 |
Levels of VEGF at the start of cycle 4 were predicted by higher pre-chemotherapy levels of VEGF, as well as smaller tumor size (model R2 = .342, F=7.01, p≤0.01). Cycle 4 sICAM-1 levels were predicted by sICAM-1 levels at cycle 1 (model R2 = . 396, F = 13.77, p≤0.01).
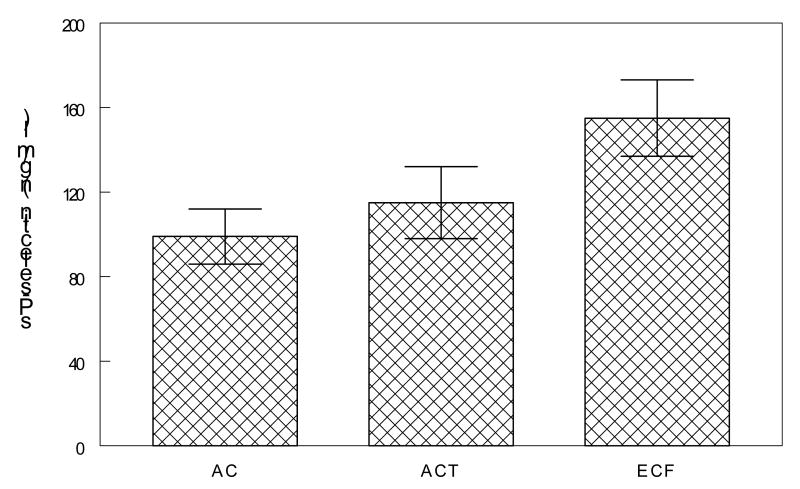
IL-6 levels at cycle 4 were predicted by higher pre-chemotherapy IL-6 levels as well as larger tumor size (model R2 = .311, F=6.31, p≤0.01). Levels of cycle 4 sP-selectin were predicted by smaller tumor size and type of chemotherapy treatment (model R2 = .436, F=5.02, p≤0.01). Post hoc analysis of the chemotherapy treatment effect showed that cycle 4 sP-selectin levels were higher (p <0.05) in individuals who received epirubicin and cytoxan with 5fluorouracil (154 ng/ml, SD=56) versus adriamycin and cyclophosphamide alone (115 ng/ml, SD=44 ) and adriamycin and cyclophosphamide with taxanes (99 ng/ml, SD=60) (Figure 1).
Figure 1.
In a multiple regression analysis, levels of pre-cycle 4 sP-selectin were predicted by type of chemotherapy regimen such that levels were higher in patients who received epirubicin and cytoxan with 5fluorouracil (ECF) versus adriamycin and cyclophosphamide alone (AC) and adriamycin and cyclophosphamide with taxanes (ACT) (p <0.05).
Circulating levels of vWf at cycle 4 were predicted by higher pre-chemotherapy levels of vWf (model R2 = .355, F= 8.57, p<0.01). The regression model for cycle 4 IL1-RA was not statistically significant (model R2 = .133, F=2.06, p = 0.146).
Discussion
Among the eight inflammatory markers examined, four were found to be elevated in response to chemotherapy. Consistent with prior studies (Ueno et al., 2006), levels of VEGF, sICAM-1, sP-selectin and vWf were significantly higher in circulation following three cycles of chemotherapy. Studies suggest that the elevated levels of sICAM-1 in breast cancer reflect the acute phase response while elevated vWf levels reflect endothelial activation and damage, respectively (Blann et al., 2002). sP-selectin’s elevation is believed to reflect platelet but not endothelial activation (Fox et al., 1995). It is not clear whether elevations of sICAM-1, sP-selectin, and vWF observed following chemotherapy reflect the same endothelial and platelet sources as seen in breast cancer prior to treatment. Our findings of elevated markers of endothelial and platelet activation may provide an explanation for observations of increased risk for thrombosis in patients undergoing chemotherapy (Yeh et al., 2004; Youssef and Links, 2005).
It is not yet clear how long after chemotherapy these elevated levels might persist. Caine et al. reported that compared to pre-treatment, radiotherapy and/or chemotherapy for breast cancer led to a significant reduction in circulating VEGF, IL6 and sP-selectin levels at 3 months and at 12 months following treatment (Caine et al., 2006). In the current study VEGF and sP-selectin levels, but not IL-6 levels, were elevated during chemotherapy. Unfortunately, we do not have long term follow-up data on our patients to address the duration of these elevations.
We found no significant differences in pre- versus post-chemotherapy levels of TNF-α, IL1-RA, IL-6 or CRP. These findings are not wholly consistent with prior reports. Previous studies found that TNF-α increased and IL-6 decreased in 30 advanced breast cancer patients in response to chemotherapy, although these effects were observed following a longer treatment regimen of 6 full cycles of chemotherapy (with taxanes) (Tsavaris et al., 2002). It could be that for some inflammatory factors longer duration of treatment is needed to induce activation. Pusztai et al. (Pusztai et al., 2004) reported that 70 stage I-III breast cancer patients with adjuvant or neoadjuvant every 3-week paclitaxel chemotherapy led to significant increases in circulating levels of IL-6, but not TNF-α. In contrast, 5-fluoruracil, adriamycin and cyclophosphamide chemotherapy at 21-day intervals for six full cycles in 23 stage I-III breast cancer patients had no significant effect on serum levels of IL-6, IL-10, or TNF-α (Mendonca et al., 2006).
A primary aim of this study was to identify factors that might be related to levels of inflammatory markers following chemotherapy. Six of eight markers examined post-chemotherapy in this study were significantly related to their respective levels prior to the start of chemotherapy, implying that degree of inflammation present in a patient following chemotherapy is, in part, determined by the degree of inflammation that existed before beginning chemotherapy. This held true for markers that did (VEGF, sICAM-1, vWf ) and did not (TNF- α, IL-6, CRP) change significantly in response to chemotherapy. In addition, among the several demographic and disease related variables that we examined, estrogen receptor status, tumor size and chemotherapy regimen were also related to one or more inflammatory marker following chemotherapy. Berberoglu reported that neoadjuvant chemotherapy in non-inflammatory stage III-B breast cancer led to a reduction of circulating TNF-α levels that was greater in patients with partial and complete responses, suggesting that degree of clinical response was related to post-chemotherapy TNF-α levels (Berberoglu et al., 2004). Consistent with our findings, circulating levels of inflammatory markers have been found to be related to the expression of estrogen and/or progesterone receptors on tumor cells. Fuksiewicz et al (Fuksiewicz et al., 2006) reported in older breast cancer patients significantly higher concentrations of the proinflammatory cytokine IL-8 in those tumors lacking progesterone receptors and higher IL-1RA concentrations in those lacking estrogen receptors. Prior to chemotherapy, we found that IL-6 levels were higher in patients with negative estrogen and progesterone receptor expressing tumors. Consistent with Fuksiewicz et al’s observation, following chemotherapy we found that IL1-RA levels were (marginally) higher in patients with negative estrogen receptor expressing tumors. We also found that tumor size was positively related to post-chemotherapy IL-6 levels but negatively related to post-chemotherapy VEGF and sP-selectin levels. There are reports of a positive not negative association between tumor size and plasma VEGF levels in breast cancer (Nishimura et al., 2003; Wu et al., 2002). IL-6 expression is not typically associated with tumor size but with estrogen and progesterone receptor expression (Fontanini et al., 1999), as was observed in this study prior to chemotherapy. These particular aspects of our findings suggest that endothelial versus immune cell responses to chemotherapy are differentially related to initial severity of disease. An effect was also observed for chemotherapy regimen such that post-chemotherapy sP-selectin levels were highest in women who had received epirubicin, cytoxan, 5fluorouracil treatment. We are not aware of any literature suggesting that epirubicin, cytoxan, 5fluorouracil treatment differentially activates platelets compared to adriamycin (Cottu et al., 2001; Ottosson et al., 1999).
There are several potential limitations of this study. Breast cancer patients were non-metastatic and our findings might not generalize to more severe disease. As the minimum number of cycles of chemotherapy at the time of our study was four 3-week cycles, our study was limited to women who were receiving this regimen. Our results therefore may not be generalizeable to women in two-week cycles or those getting more than four cycles of treatment. In addition, we obtained a single blood sample at each study timepoint which is not ideal for factors such as IL-6 which display diurnal variability (Kanabrocki et al., 1999; Muc-Wierzgon et al., 1996). We attempted to address this limitation by sampling blood within a fixed time each testing day but in the context of the clinic setting we could only limit our blood sampling times to within a range of approximately 4 hours. It is possible therefore that our biomarker levels were not fully representative of endogenous levels. Finally, our study was restricted to examining markers of endothelial and platelet activation. Markers of T and B cell activation and/or destruction merit study in the context of examining the inflammatory effects of chemotherapy for breast cancer.
In summary, the results of this study indicate that three cycles of anthracycline-based chemotherapy for breast cancer lead to elevations in inflammatory markers associated with endothelial and platelet activation. Circulating levels of inflammatory markers during chemotherapy are most significantly related to levels that existed prior to chemotherapy, but also potentially to disease characteristics of estrogen receptor status and tumor size and also to type of chemotherapy treatment.
Acknowledgments
Our thanks to Sherella Johnson for coordinating the study and for help with data collection.
Supported by NCI CA85264, NCI CA112035, CBCRP 11IB-0034, NIH M01 RR00827, NHLBI HL-57265, UCSD General Clinical Research Center MO1-RR00827, and the Research Service of the Veterans Affairs San Diego Healthcare System.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albuquerque KV, Price MR, Badley RA, Jonrup I, Pearson D, Blamey RW, Robertson JF. Pre-treatment serum levels of tumour markers in metastatic breast cancer: a prospective assessment of their role in predicting response to therapy and survival. Eur J Surg Oncol. 1995;21:504–509. doi: 10.1016/s0748-7983(95)96935-7. [DOI] [PubMed] [Google Scholar]
- Bachelot T, Ray-Coquard I, Menetrier-Caux C, Rastkha M, Duc A, Blay JY. Prognostic value of serum levels of interleukin 6 and of serum and plasma levels of vascular endothelial growth factor in hormone-refractory metastatic breast cancer patients. British journal of cancer. 2003;88:1721–1726. doi: 10.1038/sj.bjc.6600956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berberoglu U, Yildirim E, Celen O. Serum levels of tumor necrosis factor alpha correlate with response to neoadjuvant chemotherapy in locally advanced breast cancer. The International journal of biological markers. 2004;19:130–134. doi: 10.1177/172460080401900207. [DOI] [PubMed] [Google Scholar]
- Blann AD, Byrne GJ, Baildam AD. Increased soluble intercellular adhesion molecule-1, breast cancer and the acute phase response. Blood Coagul Fibrinolysis. 2002;13:165–168. doi: 10.1097/00001721-200203000-00012. [DOI] [PubMed] [Google Scholar]
- Blann AD, Gurney D, Wadley M, Bareford D, Stonelake P, Lip GY. Increased soluble P-selectin in patients with haematological and breast cancer: a comparison with fibrinogen, plasminogen activator inhibitor and von Willebrand factor. Blood Coagul Fibrinolysis. 2001;12:43–50. doi: 10.1097/00001721-200101000-00007. [DOI] [PubMed] [Google Scholar]
- Boudreau N, Myers C. Breast cancer-induced angiogenesis: multiple mechanisms and the role of the microenvironment. Breast Cancer Res. 2003;5:140–146. doi: 10.1186/bcr589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozcuk H, Uslu G, Samur M, Yildiz M, Ozben T, Ozdogan M, Artac M, Altunbas H, Akan I, Savas B. Tumour necrosis factor-alpha, interleukin-6, and fasting serum insulin correlate with clinical outcome in metastatic breast cancer patients treated with chemotherapy. Cytokine. 2004;27:58–65. doi: 10.1016/j.cyto.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Caine GJ, Stonelake PS, Lip GY, Blann AD. Changes in plasma vascular endothelial growth factor, angiopoietins, and their receptors following surgery for breast cancer. Cancer Lett. 2006 doi: 10.1016/j.canlet.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Cho DH, Song HK, Kang HS, Yoon SR, Lee HG, Pyun KH, Lee WJ, Kim YB, Choi I. Ligation of ICAM-1 molecules inhibits target cell-induced granule exocytosis of IL-12-activated natural killer cells. Cellular immunology. 2000;199:1–7. doi: 10.1006/cimm.1999.1592. [DOI] [PubMed] [Google Scholar]
- Cottu PH, Extra JM, Espie M, Marolleau JP, de Roquancourt A, Makke J, Miclea JM, Laurence V, Mayeur D, Lerebours F, Cuvier C, Marty M. High-dose sequential epirubicin and cyclophosphamide with peripheral blood stem cell support for advanced breast cancer: results of a phase II study. British journal of cancer. 2001;85:1240–1246. doi: 10.1054/bjoc.2001.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanini G, Campani D, Roncella M, Cecchetti D, Calvo S, Toniolo A, Basolo F. Expression of interleukin 6 (IL-6) correlates with oestrogen receptor in human breast carcinoma. British journal of cancer. 1999;80:579–584. doi: 10.1038/sj.bjc.6690394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SB, Turner GD, Gatter KC, Harris AL. The increased expression of adhesion molecules ICAM-3, E- and P-selectins on breast cancer endothelium. The Journal of pathology. 1995;177:369–376. doi: 10.1002/path.1711770407. [DOI] [PubMed] [Google Scholar]
- Fuksiewicz M, Kaminska J, Kotowicz B, Kowalska M, Rubach M, Pienkowski T. Serum cytokine levels and the expression of estrogen and progesterone receptors in breast cancer patients. Clin Chem Lab Med. 2006;44:1092–1097. doi: 10.1515/CCLM.2006.192. [DOI] [PubMed] [Google Scholar]
- Kaihara A, Iwagaki H, Gouchi A, Hizuta A, Isozaki H, Takakura N, Tanaka N. Soluble intercellular adhesion molecule-1 and natural killer cell activity in gastric cancer patients. Research communications in molecular pathology and pharmacology. 1998;100:283–300. [PubMed] [Google Scholar]
- Kanabrocki EL, Sothern RB, Messmore HL, Roitman-Johnson B, McCormick JB, Dawson S, Bremner FW, Third JL, Nemchausky BA, Shirazi P, Scheving LE. Circadian interrelationships among levels of plasma fibrinogen, blood platelets, and serum interleukin-6. Clin Appl Thromb Hemost. 1999;5:37–42. doi: 10.1177/107602969900500108. [DOI] [PubMed] [Google Scholar]
- Klein B, Levin I, Kfir B, Mishaeli M, Shapira J, Klein T. The significance of soluble interleukin-2, soluble interleukin-2 receptors, soluble ICAM-1 and beta 2-microglobulin in breast cancer patients. Tumour Biol. 1995;16:290–296. [PubMed] [Google Scholar]
- Knupfer H, Preiss R. Significance of interleukin-6 (IL-6) in breast cancer (review) Breast cancer research and treatment. 2006 doi: 10.1007/s10549-006-9328-3. [DOI] [PubMed] [Google Scholar]
- Kummel S, Eggemann H, Luftner D, Thomas A, Jeschke S, Zerfel N, Heilmann V, Emons G, Zeiser T, Ulm K, Kobl M, Korlach S, Schmid P, Sehouli J, Elling D, Blohmer JU. Changes in the circulating plasma levels of VEGF and VEGF-D after adjuvant chemotherapy in patients with breast cancer and 1 to 3 positive lymph nodes. Anticancer research. 2006;26:1719–1726. [PubMed] [Google Scholar]
- Lithgow D, Covington C. Chronic inflammation and breast pathology: a theoretical model. Biological research for nursing. 2005;7:118–129. doi: 10.1177/1099800405280823. [DOI] [PubMed] [Google Scholar]
- Mendonca MA, Cunha FQ, Murta EF, Tavares-Murta BM. Failure of neutrophil chemotactic function in breast cancer patients treated with chemotherapy. Cancer chemotherapy and pharmacology. 2006;57:663–670. doi: 10.1007/s00280-005-0086-4. [DOI] [PubMed] [Google Scholar]
- Merendino RA, Gangemi S, Ruello A, Bene A, Losi E, Lonbardo G, Purello-Dambrosio F. Serum levels of interleukin-18 and sICAM-1 in patients affected by breast cancer: preliminary considerations. The International journal of biological markers. 2001;16:126–129. doi: 10.1177/172460080101600207. [DOI] [PubMed] [Google Scholar]
- Mettler L, Salmassi A, Heyer M, Schmutzier A, Schollmeyer T, Jonat W. Perioperative levels of interleukin-1beta and interleukin-6 in women with breast cancer. Clinical and experimental obstetrics & gynecology. 2004;31:20–22. [PubMed] [Google Scholar]
- Miller RG. Simultaneous Statistical Inference. Springer-Verlag, Springer Series in Statistics; 1981. [Google Scholar]
- Mills PJ, Parker B, Jones V, Adler KA, Perez CJ, Johnson S, Cohen-Zion M, Marler M, Sadler GR, Dimsdale JE, Ancoli-Israel S. The effects of standard anthracycline-based chemotherapy on soluble ICAM-1 and vascular endothelial growth factor levels in breast cancer. Clin Cancer Res. 2004;10:4998–5003. doi: 10.1158/1078-0432.CCR-0734-04. [DOI] [PubMed] [Google Scholar]
- Muc-Wierzgon M, Madej K, Baranowski M, Kokot T. Fluctuation of endogenous TNF alpha concentration in plasma in advanced cancer patients. Journal of biological regulators and homeostatic agents. 1996;10:25–26. [PubMed] [Google Scholar]
- Nicolini A, Carpi A, Rossi G. Cytokines in breast cancer. Cytokine Growth Factor Rev. 2006 doi: 10.1016/j.cytogfr.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Nishimura R, Nagao K, Miyayama H, Matsuda M, Baba K, Yamashita H, Fukuda M. Higher plasma vascular endothelial growth factor levels correlate with menopause, overexpression of p53, and recurrence of breast cancer. Breast cancer (Tokyo, Japan) 2003;10:120–128. doi: 10.1007/BF02967636. [DOI] [PubMed] [Google Scholar]
- O’Hanlon DM, Fitzsimons H, Lynch J, Tormey S, Malone C, Given HF. Soluble adhesion molecules (E-selectin, ICAM-1 and VCAM-1) in breast carcinoma. Eur J Cancer. 2002;38:2252–2257. doi: 10.1016/s0959-8049(02)00218-6. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Hirakawa K, Nakata B, Fujihara T, Sawada T, Kato Y, Yoshikawa K, Sowa M. Expression of intercellular adhesion molecule-1 in invasive breast cancer reflects low growth potential, negative lymph node involvement, and good prognosis. Clin Cancer Res. 1998;4:31–36. [PubMed] [Google Scholar]
- Ottosson S, Magnusson K, Hultborn R. Acute hematologic feasibility of G-CSF supported dose-escalated FEC therapy as adjuvant treatment after breast cancer surgery. Anticancer research. 1999;19:4429–4434. [PubMed] [Google Scholar]
- Pusztai L, Mendoza TR, Reuben JM, Martinez MM, Willey JS, Lara J, Syed A, Fritsche HA, Bruera E, Booser D, Valero V, Arun B, Ibrahim N, Rivera E, Royce M, Cleeland CS, Hortobagyi GN. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine. 2004;25:94–102. doi: 10.1016/j.cyto.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Roberts WL, Moulton L, Law TC, Farrow G, Cooper-Anderson M, Savory J, Rifai N. Evaluation of nine automated high-sensitivity C-reactive protein methods: implications for clinical and epidemiological applications. Part 2. Clinical chemistry. 2001;47:418–425. [PubMed] [Google Scholar]
- Shirai A, Furukawa M, Yoshizaki T. Expression of intercellular adhesion molecule (ICAM)-1 in adenoid cystic carcinoma of the head and neck. The Laryngoscope. 2003;113:1955–1960. doi: 10.1097/00005537-200311000-00019. [DOI] [PubMed] [Google Scholar]
- Singer CF, Kronsteiner N, Hudelist G, Marton E, Walter I, Kubista M, Czerwenka K, Schreiber M, Seifert M, Kubista E. Interleukin 1 system and sex steroid receptor expression in human breast cancer: interleukin 1alpha protein secretion is correlated with malignant phenotype. Clin Cancer Res. 2003;9:4877–4883. [PubMed] [Google Scholar]
- Tsavaris N, Kosmas C, Vadiaka M, Kanelopoulos P, Boulamatsis D. Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes. British journal of cancer. 2002;87:21–27. doi: 10.1038/sj.bjc.6600347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno T, Chow LW, Toi M. Increases in circulating VEGF levels during COX-2 inhibitor treatment in breast cancer patients. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2006;60:277–279. doi: 10.1016/j.biopha.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Vasse M, Thibout D, Paysant J, Legrand E, Soria C, Crepin M. Decrease of breast cancer cell invasiveness by sodium phenylacetate (NaPa) is associated with an increased expression of adhesive molecules. British journal of cancer. 2001;84:802–807. doi: 10.1054/bjoc.2000.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kanel R, Dimsdale JE, Ziegler MG, Mills PJ, Patterson TL, Lee SK, Grant I. Effect of acute psychological stress on the hypercoagulable state in subjects (spousal caregivers of patients with Alzheimer’s disease) with coronary or cerebrovascular disease and/or systemic hypertension. The American journal of cardiology. 2001;87:1405–1408. doi: 10.1016/s0002-9149(01)01564-8. [DOI] [PubMed] [Google Scholar]
- Wu Y, Saldana L, Chillar R, Vadgama JV. Plasma vascular endothelial growth factor is useful in assessing progression of breast cancer post surgery and during adjuvant treatment. International journal of oncology. 2002;20:509–516. [PubMed] [Google Scholar]
- Yamazaki Y, Morita T. Molecular and functional diversity of vascular endothelial growth factors. Mol Divers. 2006 doi: 10.1007/s11030-006-9027-3. [DOI] [PubMed] [Google Scholar]
- Yeh ET, Tong AT, Lenihan DJ, Yusuf SW, Swafford J, Champion C, Durand JB, Gibbs H, Zafarmand AA, Ewer MS. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation. 2004;109:3122–3131. doi: 10.1161/01.CIR.0000133187.74800.B9. [DOI] [PubMed] [Google Scholar]
- Youssef G, Links M. The prevention and management of cardiovascular complications of chemotherapy in patients with cancer. Am J Cardiovasc Drugs. 2005;5:233–243. doi: 10.2165/00129784-200505040-00003. [DOI] [PubMed] [Google Scholar]
- Zhang GJ, Adachi I. Serum levels of soluble intercellular adhesion molecule-1 and E-selectin in metastatic breast carcinoma: correlations with clinicopathological features and prognosis. International journal of oncology. 1999;14:71–77. [PubMed] [Google Scholar]