Abstract
Although electroacupuncture (EA) is widely used to treat pain, its mechanisms have not been completely understood. The present study investigated the descending inhibitory system involvement in EA action. Inflammatory pain was induced by injecting complete Freund’s adjuvant subcutaneously into one hind paw of rats with dorsolateral funiculus lesions and sham-operated rats. EA treatment, 10 Hz at 3 mA, was given twice for 20 min each, once immediately post- and again 2 h post-Freund’s adjuvant at GB 30, at the junction of the lateral 1/3 and medial 2/3 of the distance between the greater trochanter and sacral hiatus. For sham EA control, acupuncture needles were inserted bilaterally into GB 30 without electrical or manual stimulation. Paw withdrawal latency to a noxious thermal stimulus was measured at baseline and 20 min after EA treatment. Compared to sham EA, EA significantly (P<0.05, n=9) increased withdrawal latency of the inflamed hind paws in the sham-operated rats but not in those with dorsolateral funiculus lesions, indicating that lesioning blocked EA-produced anti-hyperalgesia. EA, compared to sham EA, also significantly inhibited Fos expression in laminae I-II of the spinal cord in the sham-operated rats (58.4 ± 6.5 vs 35.2 ± 5.4 per section) but not in those with dorsolateral funiculus lesions. Further, EA activated serotonin- and catecholamine-containing neurons in the nucleus raphe magnus and locus coeruleus that project to the spinal cord. The results demonstrate that EA inhibits transmission of noxious messages and hyperalgesia by activating supraspinal neurons that project to the spinal cord.
Keywords: Hyperalgesia, Acupuncture, Spinal cord, Nucleus raphe magnus, Locus coeruleus, Dorsolateral funiculus, Rats
1. Introduction
Most previous acupuncture analgesia studies have employed uninjured animal models and transient noxious stimulation (Ulett et al., 1998), which are unlike the chronic pain conditions seen in clinics. Recent chronic pain acupuncture/ electroacupuncture (EA) studies, including our own (Lao et al., 2004), have shown that EA produces anti-hyperalgesia in ankle sprain and inflammatory pain animal models (Koo et al., 2002; Zhang et al., 2002). Although our study showed that EA significantly inhibited peripheral inflammation-induced hyperalgesia and spinal Fos expression, the mechanisms of these effects were not understood.
It is known that brain stem descending pathways play an important role in the control of pain transmission. Hyperalgesia in animal models of inflammatory pain is closely linked to activities of descending modulatory circuits (Millan, 2002). Carrageenan-induced hyperalgesia is significantly greater in rats with lesions of the dorsolateral funiculus than in sham-operated rats (Ren and Dubner, 1996). Previous studies demonstrate that electrical activation of dorsolateral funiculus inhibits inflammation-induced Fos expression in the spinal cord (Zhang et al., 1994). These data suggest that the descending inhibitory system suppresses hyperalgesia and spinal Fos expression during peripheral inflammation. Whether the effect of EA involves descending pathways has not been investigated. In the present study, we tested our hypothesis that EA engages the descending inhibitory system originating from the nucleus raphe magnus and locus coeruleus in complete Freund’s adjuvant-induced inflammatory pain rat model and showed that dorsolateral funiculus lesion abolished EA’s inhibitory effects.
2. Results
2.1 Reconstruction of lesions
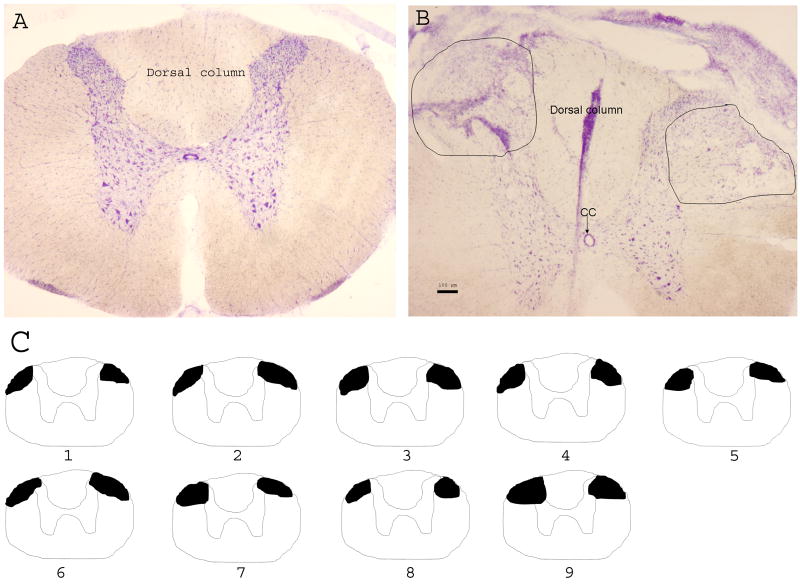
To verify the extent of the dorsolateral funiculus lesion, 30 μm sections of the thoracic spinal cord were cut and stained with Cresyl violet. The extent of lesion was reconstructed, showing that the damage mainly was to the dorsolateral funiculus and the adjacent dorsal horn (Fig. 1).
Fig. 1.
A-B: Photographs showing the extent of the dorsolateral funiculus lesion in the lower thoracic spinal cord. A. The intact spinal section. B. The section with lesion. Scale bar is 100 μm; CC: central canal. C: Lower thoracic spinal cord drawing to show the extent of the dorsolateral funiculus lesion (black areas) in the rats.
2.2 Effect of dorsolateral funiculus lesion on EA-produced inhibition of hyperalgesia
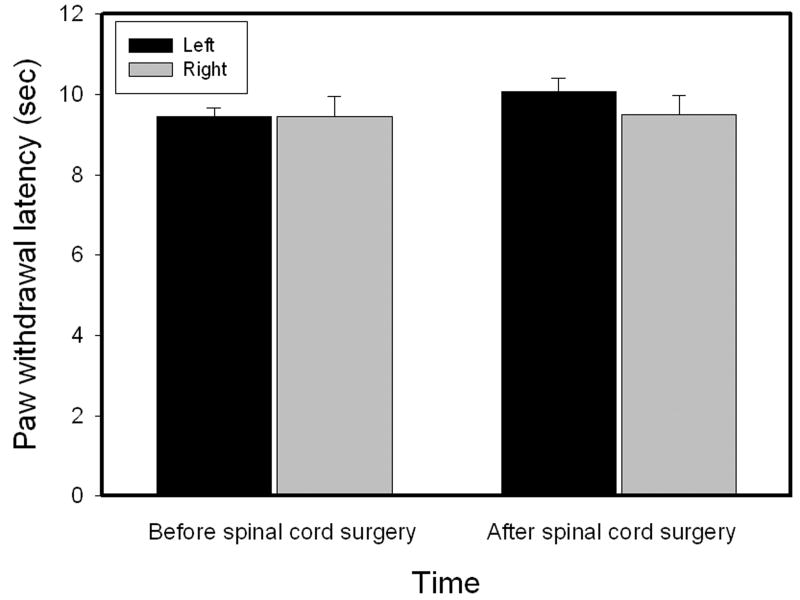
Before dorsolateral funiculus lesioning, hind paw withdrawal latencies to noxious heat stimuli were similar in the left (9.44 ± 0.21 sec) and right (9.44 ± 0.50 sec) hind paws. After lesioning, they were also similar in left (10.06 ± 0.35 sec) and right (9.51 ± 0.46 sec) hind paws (Fig. 2). There were no significant paw withdrawal latency differences before and after lesioning, and paw withdrawal latencies were also unchanged in sham-operated rats (data not shown).
Fig. 2.
Paw withdrawal latencies measured before and after dorsolateral funiculus lesioning (n=9). Dorsolateral funiculus lesions did not produce a significant effect on paw withdrawal latencies.
As shown in Fig. 3, before Freund’s adjuvant injection, paw withdrawal latencies were similar in left and right hind paws in rats with dorsolateral funiculus lesions and sham-operated rats. Following an injection of 0.08 ml Freund’s adjuvant in sham-operated rats, withdrawal latencies of the injected paws of sham EA-treated rats became significantly shorter (5.23 ± 0.38 sec) than those of the contralateral hind paws (10.09 ± 0.63 sec), which were unchanged from baseline. The EA treatment, 10 Hz at bilateral GB 30, significantly (P<0.05) increased withdrawal latency of the ipsilateral hind paws (6.78 ± 0.36 sec) compared to sham EA (5.23 ± 0.38 sec). See Fig. 3A. This indicates that EA significantly inhibited Freund’s adjuvant-induced hyperalgesia in sham-operated rats. EA did not increase withdrawal latency of the contralateral hind paws. Following Freund’s adjuvant injection in rats with dorsolateral funiculus lesions, paw withdrawal latency of the injected paws of sham EA-treated rats also became significantly shorter (4.38 ± 0.35 sec) than those of the contralateral hind paws (10.52 ± 0.53 sec). However, EA treatment did not significantly increase withdrawal latency of the injected paws (4.48 ± 0.30 sec) compared to sham EA (4.38 ± 0.35 sec) in lesioned rats (Fig. 3B). This demonstrates that EA did not inhibit Freund’s adjuvant-induced hyperalgesia in rats with dorsolateral funiculus lesioning. It should be noted that hyperalgesia was greater in rats with dorsolateral funiculus lesioning than in sham-operated rats, although the difference is not statistically significant (p=0.08).
Fig. 3.
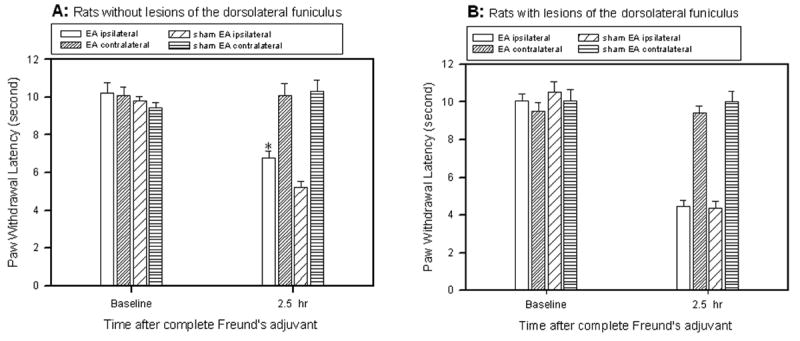
Effects of dorsolateral funiculus lesioning on EA-produced inhibition of inflammatory hyperalgesia (n=9). A: EA treatment significantly inhibited the decrease of ipsilateral paw withdrawal latencies in sham-operated rats compared to sham EA (* P<0.05). B: EA treatment, compared to sham EA, showed no significant effect on paw withdrawal latencies in rats with dorsolateral funiculus lesioning. Note that hyperalgesia was greater in sham EA-treated rats with dorsolateral funiculus lesioning than in sham EA-treated rats with no dorsolateral funiculus lesioning, although the difference is not statistically significant.
2.3 Effect of dorsolateral funiculus lesion on EA inhibition of Spinal Fos Expression
Fos-immunoreactive nuclear profiles were mainly localized in ipsilateral laminae I–II (58.4 ± 6.5 per section) and V-VI (25.2 ± 3.5 per section), especially in the medial two-thirds of the superficial dorsal horn after inflammation (Fig. 4A). EA significantly inhibited spinal cord Fos expression in ipsilateral laminae I–II (35.2 ± 5.4 per section) (Fig. 4B). By contrast, in rats with dorsolateral funiculus lesioning, the number of Fos-labeled cells was similar in EA- and sham EA-treated rats in laminae I-II (87.0 ± 8.6 vs 79.2 ± 9.7 per section) and V-VI (55.3 ± 7.8 vs 60.3 ± 9.1) (Fig. 4C-D). The data demonstrate that EA did not inhibit Freund’s adjuvant-induced Fos expression in the spinal cord in rats with dorsolateral funiculus lesions, suggesting that the lesion blocked EA inhibition of Fos expression at the spinal level. It was also noted that Fos expression in laminae I-II and V-VI was significantly (P<0.05) greater in rats with dorsolateral funiculus lesioning than in sham-operated rats.
Fig. 4.
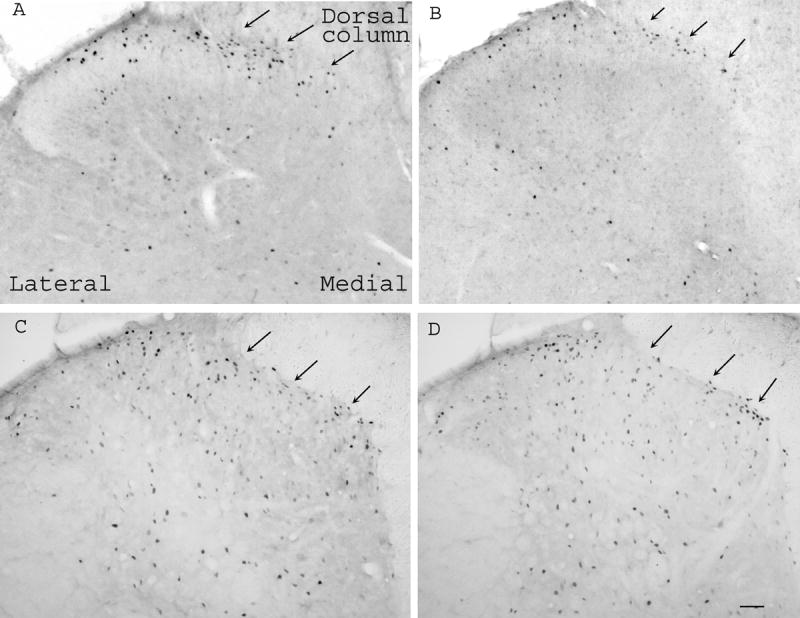
Microphotographs showing Fos expression in the spinal cord in rats with sham operation (A, B) or dorsolateral funiculus lesion (C, D). Note that EA (B) inhibited Fos expression compared to sham EA (A) in sham-operated rats, and that EA (D) did not show significant inhibition of Fos expression compared to sham EA (C) in rats with dorsolateral funiculus lesioning. Arrows point to medial superficial laminae. Scale bars are 50 μm.
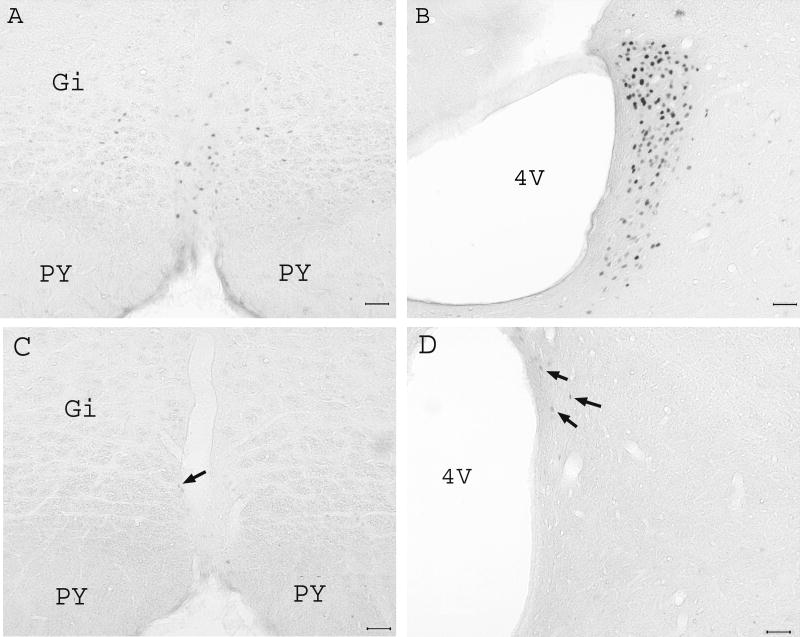
2.4 Fos expression in the nucleus raphe magnus and locus coeruleus
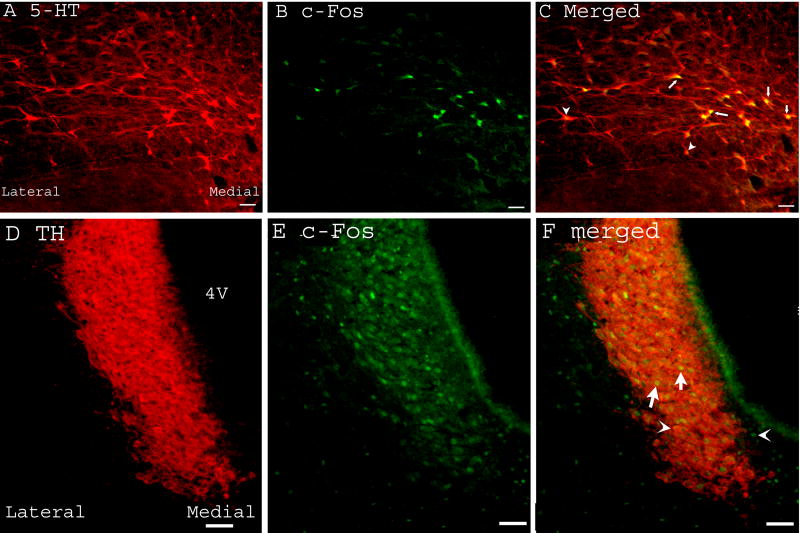
EA induced abundant Fos expression in the nucleus raphe magnus (36.5 ± 5/ per 50 μm section) and bilateral locus coeruleus (110.4 ± 9/ per 50 μm section, Fig. 5A-B). Untreated naive rats expressed little Fos in these two nuclei (Fig. 5C-D). Double immunoflurence staining demonstrated that Fos was completely co-localized with serotonin and tyrosine hydroxylase, an indicator of catecholamine, in the nucleus raphe magnus and locus coeruleus, respectively (Fig. 6). This indicates that EA activated seretonergic and catecholaminergic neurons in these nuclei. Double staining of Fos and the retrograde tracer Fluorogold, which was injected into the lumbar spinal cord, demonstrated that most EA-activated neurons in the nucleus raphe magnus and some in the locus coeruleus projected to the lumbar spinal cord (Fig. 7). The reddish brown reaction product for Fluorogold was localized in cytoplasm and dendrites while the dark reaction product associated with Fos was present in the nucleus, reliably distinguishing the single-labeled Fluorogold and Fos from the double-labeled Fluorogold / Fos neurons.
Fig. 5.
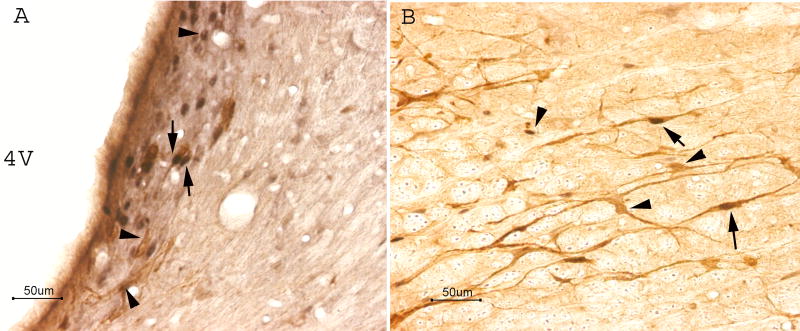
Effects of EA on brainstem Fos expression in uninjured rats (n=3). Note that EA induced abundant Fos expression in the nucleus raphe magnus (A) and locus coeruleus (B). Naive rats with no EA treatment showed little Fos expression (arrows) in the nucleus raphe magnus (C) and locus coeruleus (D). Scale bars are 50 μm; PY: pyramidal tract; Gi: Gigantocellular reticular nucleus; 4V: the fourth ventricle.
Fig. 6.
Micrographs showing co-localization of Fos and serotonin or tyrosine hydroxylase in the nucleus raphe magnus (A-C) and locus coeruleus (D-F) respectively. A-C: Sections containing nucleus raphe magnus were double-labeled with anti-serotonin (red) and anti-Fos (green). A. Serotonin-immunoreactive neurons; B. Fos-immunoreactive neurons; C. Merged graphs of A and B. Arrows indicate double-labeled serotonin/Fos neurons (yellow). Arrow heads point to single-labeled neurons. D-F: Sections containing locus coeruleus were double-labeled with anti-tyrosine hydroxylase (red) and anti-Fos (green). D. tyrosine hydroxylase-immunoreactive neurons; E. Fos-immunoreactive neurons; F. Merged graphs of A and B. Arrows indicate double-labeled tyrosine hydroxylase/Fos neurons (yellow). Arrow heads point to single-labeled neurons. 4V: the fourth ventricle. Bars = 50 μm.
Fig. 7.
Representative photograph showing the colocalization of fluorogold and Fos in the nucleus raphe magnus (A) and locus coeruleus (B). Note that the immunostaining product for fluorogold is localized in cytoplasm and dendrites, while that associated with Fos is present in the nucleus. Arrows indicate doubled-labeled Fluorogold/Fos neurons. Bars = 50 μm.
3. Discussion
The results of the present study suggest that EA activates the dorsolateral funiculus to inhibit Freund’s adjuvant-induced hyperalgesia and transmission of noxious inputs at the spinal level, as shown by the fact that dorsolateral funiculus lesioning prevented EA-produced inhibition of hyperalgesia and Fos expression. Baseline paw withdrawal latencies were unchanged before and after the lesioning, which rules out the possibility that the rats became unresponsive after surgery, which might block EA action. It should be noted that dorsolateral funiculus lesioning caused more hyperalgesia than did sham surgery 2.5 hours after the Freund’s adjuvant injection, although this result is not statistically significant. The result somewhat parallels a previous report that carrageenan induced greater hyperalgesia in rats with dorsolateral funiculus lesioning than in sham-operated rats (Ren and Dubner, 1996). EA had no significant effect on withdrawal latency of the contralateral hind paw although it significantly inhibited the ipsilateral paw withdrawal latency. The data indicate that EA at 10Hz, 3 mA, which is much lower than the EA intensity used in previous studies (Romita et al., 1997), had little anti-nociceptive effect in normal rats but had a significant anti-hyperalgesic effect in rats with hind paw inflammation. Dose-response curves for intrathecally administered mu- and/or delta-opioid agonists, determined by hind paw withdrawal latency to a noxious thermal stimulus, were shifted to the left for carrageenan-inflamed hind paws compared to contralateral non-inflamed paws (Hylden et al., 1991). The data demonstrated that the spinal cord ipsilateral to the inflamed paw was hyper-excitable and hyper-responsive compared to the contralateral spinal cord. This may explain why the relatively low EA current intensity (i.e. 3 mA) used the present study was sufficient to induce a therapeutic effect ipsilaterally but not contralaterally. It was also found that Fos expression was significantly greater in rats with dorsolateral funiculus lesions than in sham-operated rats. This is consistent with our previous report that electrical stimulation of the dorsolateral funiculus inhibited Fos expression in the spinal cord (Zhang et al., 1994), thus confirming that the activities of the descending dorsolateral funiculus inhibit spinal Fos expression.
It is known that the major descending pathways in the dorsolateral funiculus of the spinal cord originate from supraspinal nuclei (e.g. the nucleus raphe magnus and locus coeruleus) (Millan, 2002; Watkins et al., 1980; Watkins et al., 1981). Previous studies have demonstrated that lesions of rostral medulla serotoninergic neurons caused by 5,7-DHT, a selective neurotoxin for serotoninergic neurons, induced a significant increase in Fos expression in the spinal dorsal horn compared to vehicle-injected controls (Wei et al., 1999). Intrathecal pretreatment with cyproheptadine (a 5-HT-receptor antagonist) increased Fos expression induced by formalin injection into an animal’s hind paw (Liu et al., 1997). Intrathecal pretreatment with high-efficacy 5-hydroxytryptamine1A (5-HT1A) receptor agonist, F 13640, significantly reduced formalin-induced Fos expression in the spinal cord (Buritova et al., 2005). Behaviorally, intrathecal 5-HT significantly inhibited both phases of biting/licking behavior in the formalin test (Bonnefont et al., 2005). Anatomical data demonstrated that spinal Fos-immunoreactive neurons received input from serotonin-immunoreactive axonal varicosities (Pretel et al., 1991). Our data demonstrate that EA-activated neurons in the nucleus raphe magnus contain serotonin and that EA-activated neurons project to the spinal cord. Collectively, these data suggest that EA activates the nucleus raphe magnus, which sends descending fibers to the spinal cord to inhibit hyperalgesia and noxious stimuli-induced Fos expression by releasing 5-HT at the spinal level (Liu et al., 1997; Wei et al., 1999).
Similar data also suggest that descending fibers in the dorsolateral funiculus that originate in the locus coeruleus inhibit noxious stimuli-induced Fos expression in the spinal cord by releasing norepinephrine (NE) at the spinal level (Liu et al., 1997; Wei et al., 1999). Our results demonstrate that EA activates catecholaminergic neurons in the locus coeruleus and that these activated neurons project to the spinal cord. Taken together, these data indicate that EA causes catecholaminergic locus coeruleus-spinal cord projection neurons to release NE that inhibits hyperalgesia and Fos expression. Moreover, previous study showed that descending norepinephrinergic terminals activate the spinal opioid system to inhibit Fos expression in the spinal cord (Liu et al., 1999). Our previous data demonstrate that EA inhibition of hyperalgesia is involved in the release of mu and delta receptor agonists (Zhang et al., 2004), and it has been shown that intrathecal injection of a mu receptor agonist inhibits Fos expression in the spinal cord (Hammond et al., 1998). Therefore it is plausible that EA may activate norepinephrinergic neurons in the locus coeruleus that descend to the spinal cord to activate the opioid system and inhibit Fos expression and hyperalgesia. Whether the nucleus raphe magnus and locus coeruleus are involved in EA action may be shown in subsequent experiments that inhibit cellular activity in these areas during EA.
In conclusion, the present study demonstrates that EA activates seretonergic and catecholaminergic neurons in the nucleus raphe magnus and locus coeruleus that project to the spinal cord through the dorsolateral funiculus to inhibit transmission of noxious messages and hyperalgesia. This data sheds light on the mechanisms of EA and may have potential implications for the clinical treatment of chronic pain.
4. Experimental Procedure
4.1 Experimental Design
Four experiments were conducted. In experiment 1, dorsolateral funiculus-lesioned and sham-operated rats were injected with Freund’s adjuvant and randomly divided into the following groups (n=9 per group): 1) dorsolateral funiculus lesion plus EA; 2) dorsolateral funiculus lesion plus sham EA; 3) sham operation plus EA; 4) sham operation plus sham EA. EA was applied at 10 Hz, 3 mA, 0.1 ms pulse width for two 20-minute periods, once at the beginning and once at the end of a 2 hour period starting immediately after a Freund’s adjuvant injection. Paw withdrawal latency to a noxious thermal stimulus was tested at baseline and 20 minutes after the EA treatment to determine thermal hyperalgesia. In experiment 2, rats were grouped into the same four groups as in experiment 1 (n=6 per group), and their spinal cords were removed after EA treatment to measure Fos protein immunoreactivity, a marker of neuronal activation. Rats used in the immunohistochemical study were not tested for paw withdrawal latency in order to avoid possible radiant stimuli-induced effects on Fos expression. In experiment 3, EA was given to a group of rats (n=3) to determine whether EA activates neurons in the supraspinal brain stem, the nucleus raphe magnus and locus coeruleus, and whether EA-induced Fos is co-localized with serotonin in the nucleus raphe magnus and with tyrosine hydroxylase in the locus coeruleus. In experiment 4, EA was given to a group of rats (n=3) in which the retrograde tracer Fluorogold (4% in H2O) was injected into the lumbar spinal cord. The brain stem was removed after EA treatment to determine whether EA-induced Fos is expressed in Fluorogold-labeled neurons in the nucleus raphe magnus and locus coeruleus.
4.2 Dorsolateral funiculus lesion
Male Sprague–Dawley rats weighing 200-220g (Harlan) were kept under controlled conditions (22°C ± 0.5°C, relative humidity 40-60%, 7 am to 7 pm alternate light-dark cycles, food and water ad libitum). Under anesthesia with pentobarbital sodium (50 mg/kg, i.p.), dorsolateral funiculus lesion was performed on the spine at T10–12. The skin at T10–12 was incised, and a laminectomy was performed under a surgical microscope. The dura mater was cut open with iris scissors, and a 0.1 ml 2% lidocaine solution was applied to the dorsal surface of the cord. Five minutes later, bilateral dorsolateral funiculus lesion was performed by cutting a portion of the dorsolateral quadrant of the spinal cord with a sharp knife. The wound was closed with sutures. For sham rats, the dura mater was opened, but the spinal cord was not lesioned. Post-operatively, rats were carefully monitored and rats with motor impairment were excluded from the study. The extent of the spinal lesions was verified in all animals at the conclusion of the experiments.
4.3 Induction of Hyperalgesia
Seven days after dorsolateral funiculus lesioning, complete Freund’s adjuvant (Sigma, St Louis, MO; suspended in an 1:1 oil/saline emulsion, 0.08 ml, 0.04 mg Mycobacterium tuberculosis) was injected subcutaneously into the plantar surface of one hind paw of each rat using a 25-gauge hypodermic needle (Iadarola et al., 1988; Lao et al., 2001; Lao et al., 2004). The inflammation, manifesting as redness, edema, and hyper-responsiveness to noxious stimuli, was limited to the injected paw, appeared shortly after the injection, and lasted for about two weeks. Hyperalgesia was determined by a decrease in paw withdrawal latency to a noxious thermal stimulus.
4.4 Acupuncture Treatment
To obtain maximum anti-hyperalgesia, two 20-minute EA treatments were given, a prophylactic treatment immediately after the Freund’s adjuvant injection and a second treatment at the end of a 2-hour period starting immediately after a Freund’s adjuvant injection (Lao et al., 2004). Previously determined EA parameters of 10 Hz at 3 mA and 0.1 ms pulse width, which showed significant anti-hyperalgesic effects on the rat inflammation model (Lao et al., 2001; Lao et al., 2004; Zhang et al., 2004), were chosen for the present study. EA was applied bilaterally to the equivalent of the human acupoint GB 30 (Cheng, 1999) on the rat’s hind limbs, the negative pole being ipsilateral to the Freund’s adjuvant injection and the positive pole being contralateral.
GB 30 was chosen based on traditional Chinese medicine (TCM) meridian theory (O’connor and Bensky, 1981) and its successful use in previous studies (Lao et al., 2001; Lao et al., 2004; Xu et al., 1993). In humans, GB 30 is located at the junction of the lateral one-third and medial two-thirds of the distance between the greater trochanter and the hiatus of the sacrum; underneath are the sciatic nerve, inferior gluteal nerve, and gluteal muscles (Cheng, 1999). Comparable landmarks were used to locate GB 30 in the rats. Our previous study (Lao et al., 2004) demonstrated that EA at acupoint GB 30 produced significant anti-hyperalgesia, while EA at the point comparable to the human acupoint TE 5 on the foreleg and at sham points, including one at the opposite aspect of GB 30 and one on the abdomen, did not.
EA treatment was given as reported previously (Lao et al., 2004). Briefly, an acupuncture needle was inserted into GB 30 on each flank of the animal. The needles were connected to an electrical stimulator (A300 Pulsemaster, World Precision Instruments). While frequency was held constant, intensity was adjusted slowly over a period of two minutes to the designated level of 3 mA, which is the maximum intensity a conscious animal can tolerate. Mild muscle twitching was observed. During EA treatment, each rat was placed under an inverted clear plastic chamber (approximately 5”× 8”× 11”) but was neither restrained nor given any anesthetic. The animals remained awake and still, and gave no observable signs of distress during treatment (Lao et al., 2004).
For the sham EA control, acupuncture needles were inserted bilaterally into GB 30 without electrical or manual stimulation. This procedure showed little anti-hyperalgesia in our previous study (Lao et al., 2004) and seems to be an appropriate control for non-specific needling effects.
4.5 Behavioral Testing
Rats were tested for hind paw thermal hyperalgesia by a method developed previously (Hargreaves et al., 1988; Lao et al., 2004). Briefly, rats were placed under a clear plastic chamber on the glass surface of the Paw Thermal Stimulator System (UCSD, San Diego) and allowed to acclimatize for 30 minutes. A radiant heat stimulus, directed onto the plantar surface of each hind paw, was applied from underneath the glass floor with a high intensity projector lamp bulb (CXL/CXR, 8 V, 50 W), and paw withdrawal latency to the nearest 0.1 second was automatically determined. A 20-second cut-off was used to prevent tissue damage.
Mean paw withdrawal latencies were established by averaging the latency of four tests with a five-minute interval between each test. Paw withdrawal latency measurements were made pre- dorsolateral funiculus lesion, post-lesioning (pre-Freund’s adjuvant), and 2.5 hours post-Freund’s adjuvant. The investigator who performed the behavioral tests was blind to the treatment assignments.
4.6 Immunohistochemistry and immunofluorescence
Rats were deeply anesthetized with sodium pentobarbital (80 mg/kg, i.p.) and perfused transcardially with 100 ml of saline followed by 500 ml of 4% paraformaldehyde in 0.1 mol/L phosphate buffer at pH 7.4. The lumbar (L4-5) spinal cord was removed, immersed in the same fixative for 2 hours and transferred to a solution of 30% sucrose in a phosphate buffer for overnight cryoprotection. Thirty micrometer-thick sections were cut with a cryostat at −20°C. Free-floating tissue sections were rinsed in phosphate-buffered saline (PBS) with 0.75% Triton X-100 and 1% H2O2 for one hour and then in PBS with 3% normal goat serum (NGS) for 30 minutes. The sections were incubated overnight in primary antiserum against Fos (Oncogene, 1:20,000) and incubated for 1 hour in biotinylated goat anti-rabbit IgG (Vector, 1:400) and then ABC (Vector, 1:200) solution, respectively. Then, the sections were visualized with the nickel-diaminobenzidine method (DAB substrate kit, Vector labs). Tissue sections were washed twice for 20 minutes in PBS between the antibody incubations. Finally, the sections were mounted on gelatin-coated slides, air-dried, dehydrated in graded alcohol, cleared in xylene, and coverslipped with DPX (Electron Microscopy Sciences). Control sections without primary antiserum showed no immunoreactive staining. The Fos-immunoreactive neurons were counted on ten randomly selected sections of laminae I-II and V-VI of each rat; the totals were averaged separately for each animal and then averaged for the group. Similarly, brain stem sections were immunostained for Fos, and five sections were selected to count the Fos-immunoreactive neurons of the RVM and locus coeruleus.
For double immunofluorescence labeling, spinal cord sections were blocked in PBS with 10% normal donkey serum for 60 minutes, incubated overnight at room temperature with a mixture of rabbit polyclonal antibody against Fos (Oncogene, 1:1000) and mouse monoclonal antibodies against TH (Chemicon, 1:500), or goat polyclonal antibody against 5-HT (ImmunoStar, 1:250). After three 10-minute washings in PBS, sections were incubated in a mixture of CY2-conjugated donkey anti-rabbit (1:100, Jackson ImmunoResearch Laboratories) and CY3-congugated donkey anti-mouse (1:400) or CY3-congugated donkey anti-goat (1:500) for 1 hour at room temperature. Control sections were similarly processed, except that the primary antisera were omitted. The stained sections were mounted on gelatin-coated slides, coverslipped with aqueous mounting medium (Biomeda Corp., CA) and examined under a Nikon fluorescence microscope. Control sections without primary antiserum showed no immunoreactive staining.
For Fos and Fluorogold double labeling, Fluorogold (4%, 0.2 μl) was injected into the lumbar spinal cord 4 days before EA treatment after a laminectomy under anesthesia with pentobarbital sodium (50 mg/kg, i.p.). After EA treatment, the rats were perfused as above. The spinal cord sections were double stained for Fos and Fluorogold with the avidin-biotin-peroxidase (ABC) method. The sections were treated with 2% hydrogen peroxide for 30 minutes to remove the endogenous peroxidase and then incubated in 5% normal goat serum with 0.3% Triton X-100 in PBS for 1 hour. They were incubated overnight in primary antiserum against Fos (Oncogene, 1:15,000) and incubated for 1 hour in biotinylated goat anti-rabbit IgG (Vector, 1:200) and ABC (Vector, 1:100) solution, respectively. Finally they were developed with the nickel-diaminobenzidine method (DAB substrate kit, Vector labs), which results in a black stain. The primary and secondary antibodies were diluted in 5% normal goat serum with 0.3% Triton X-100 in PBS; the ABC was diluted in PBS alone. Tissue sections were washed 3 times, 10 minutes each, in PBS between antibody incubations. All incubations were carried out at room temperature. Fluorogold immunostaining was identical to the above methods, with the exception that the primary antibody was rabbit anti-Fluorogold (Chemicon, 1:5000) and the peroxidase was reacted in diaminobenzidine solution (DAB substrate kit, Vector labs) to yield a brown stain.
4.7 Data Analysis
Paw withdrawal latency data were presented as mean ± SE and analyzed using analysis of variance (ANOVA) with repeated measures followed by Dunnett’s post-hoc comparisons (GraphPad InStat). The immunohistochemical data were analyzed with one-way between-subject ANOVA followed by the Dunnett’s post hoc test. P<0.05 was considered significant in all cases.
Acknowledgments
We would like to thank Dr. Lyn Lowry for her editorial support. This work was funded by NIH grant P01 AT002605.
Abbreviations
- EA
Electroacupuncture
- NE
Norepinephrine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bonnefont J, et al. Spinal 5-HT1A receptors differentially influence nociceptive processing according to the nature of the noxious stimulus in rats: effect of WAY-100635 on the antinociceptive activities of paracetamol, venlafaxine and 5-HT. Pain. 2005;114:482–490. doi: 10.1016/j.pain.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Buritova J, et al. Effects of the high-efficacy 5-HT1A receptor agonist, F 13640 in the formalin pain model: A c-Fos study. European Journal of Pharmacology. 2005;514:121–130. doi: 10.1016/j.ejphar.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Cheng X, editor. Chinese Acupuncture and Moxibustion. Foreign Languages Press; Beijing: 1999. [Google Scholar]
- Hammond DL, et al. Differential effects of intrathecally administered delta and mu opioid receptor agonists on formalin-evoked nociception and on the expression of Fos-like immunoreactivity in the spinal cord of the rat. Journal of Pharmacology & Experimental Therapeutics. 1998;284:378–87. [PubMed] [Google Scholar]
- Hargreaves K, et al. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hylden J, et al. Spinal opioid analgesic effects are enhanced in a model of unilateral inflammation/hyperalgesia: possible involvement of noradrenergic mechanisms. European Journal of Pharmacology. 1991;194:135–143. doi: 10.1016/0014-2999(91)90097-a. [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, et al. Enhancement of dynorphin gene expression in spinal cord following experimental inflammation: stimulus specificity, behavioral parameters and opioid receptor binding. Pain. 1988;35:313–26. doi: 10.1016/0304-3959(88)90141-8. [DOI] [PubMed] [Google Scholar]
- Koo ST, et al. Acupuncture analgesia in a new rat model of ankle sprain pain. Pain. 2002;99:423–431. doi: 10.1016/S0304-3959(02)00164-1. [DOI] [PubMed] [Google Scholar]
- Lao L, et al. Electroacupuncture attenuates behavioral hyperalgesia and selectively reduces spinal Fos protein expression in rats with persistent inflammation. Journal of Pain. 2001;2:111–117. doi: 10.1054/jpai.2001.19575. [DOI] [PubMed] [Google Scholar]
- Lao L, et al. A parametric study of electroacupuncture on persistent hyperalgesia and Fos protein expression in rats. Brain Research. 2004;1020:18–29. doi: 10.1016/j.brainres.2004.01.092. [DOI] [PubMed] [Google Scholar]
- Liu R-J, et al. Interrelations of opioids with monoamines in descending inhibition of nociceptive transmission at the spinal level: an immunocytochemical study. Brain Research. 1999;830:183–190. doi: 10.1016/s0006-8993(99)01293-7. [DOI] [PubMed] [Google Scholar]
- Liu RJ, et al. Effects of intrathecal monoamine antagonists on the nociceptive c-fos expression in a lesioned rat spinal cord. International Journal of Neuroscience. 1997;91:169. doi: 10.3109/00207459708986374. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Progress in Neurobiology. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- O’connor J, Bensky D. Acupuncture: A Comprehensive Text. Eastland Press; Chicago: 1981. [Google Scholar]
- Pretel S, et al. Enkephalin, substance P, and serotonin axonal input to c-fos-like immunoreactive neurons of the rat spinal cord. Peptides. 1991;12:1243–50. doi: 10.1016/0196-9781(91)90202-z. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. Enhanced descending modulation of nociception in rats with persistent hindpaw inflammation. Journal of Neurophysiology. 1996;76:3025–3037. doi: 10.1152/jn.1996.76.5.3025. [DOI] [PubMed] [Google Scholar]
- Romita VV, et al. Parametric Studies on Electroacupuncture-Like Stimulation in a Rat Model: Effects of Intensity, Frequency, and Duration of Stimulation on Evoked Antinociception. Brain Research Bulletin. 1997;42:289–296. doi: 10.1016/s0361-9230(96)00264-x. [DOI] [PubMed] [Google Scholar]
- Ulett GA, et al. Electroacupuncture: mechanisms and clinical application. Biological Psychiatry. 1998;44:129–138. doi: 10.1016/s0006-3223(97)00394-6. [DOI] [PubMed] [Google Scholar]
- Watkins LR, et al. The somatotopic organization of the nucleus raphe magnus and surrounding brain stem structures as revealed by HRP slow-release gels. Brain Research. 1980;181:1–15. doi: 10.1016/0006-8993(80)91255-x. [DOI] [PubMed] [Google Scholar]
- Watkins LR, et al. Identification and somatotopic organization of nuclei projecting via the dorsolateral funiculus in rats: a retrograde tracing study using HRP slow-release gels. Brain Research. 1981;223:237–55. doi: 10.1016/0006-8993(81)91139-2. [DOI] [PubMed] [Google Scholar]
- Wei F, et al. Laminar-selective noradrenergic and serotoninergic modulation includes spinoparabrachial cells after inflammation. Neuroreport. 1999;10:1757–61. doi: 10.1097/00001756-199906030-00024. [DOI] [PubMed] [Google Scholar]
- Xu R, et al. Influence of capsaicin treating sciatic nerve on the pain threshold and the effect of acupuncture analgesia of rats. Acupuncture Research. 1993;18:280–4. [PubMed] [Google Scholar]
- Zhang R-X, et al. Involvement of opioid receptors in electroacupuncture-produced anti-hyperalgesia in rats with peripheral inflammation. Brain Research. 2004;1020:12–17. doi: 10.1016/j.brainres.2004.05.067. [DOI] [PubMed] [Google Scholar]
- Zhang RX, et al. Effects of descending inhibitory systems on the c-Fos expression in the rat spinal cord during formalin-induced noxious stimulation. Neuroscience. 1994;58:299–304. doi: 10.1016/0306-4522(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Zhang Y-Q, et al. Excitatory amino acid receptor antagonists and electroacupuncture synergetically inhibit carrageenan-induced behavioral hyperalgesia and spinal fos expression in rats. Pain. 2002;99:525–535. doi: 10.1016/S0304-3959(02)00268-3. [DOI] [PubMed] [Google Scholar]