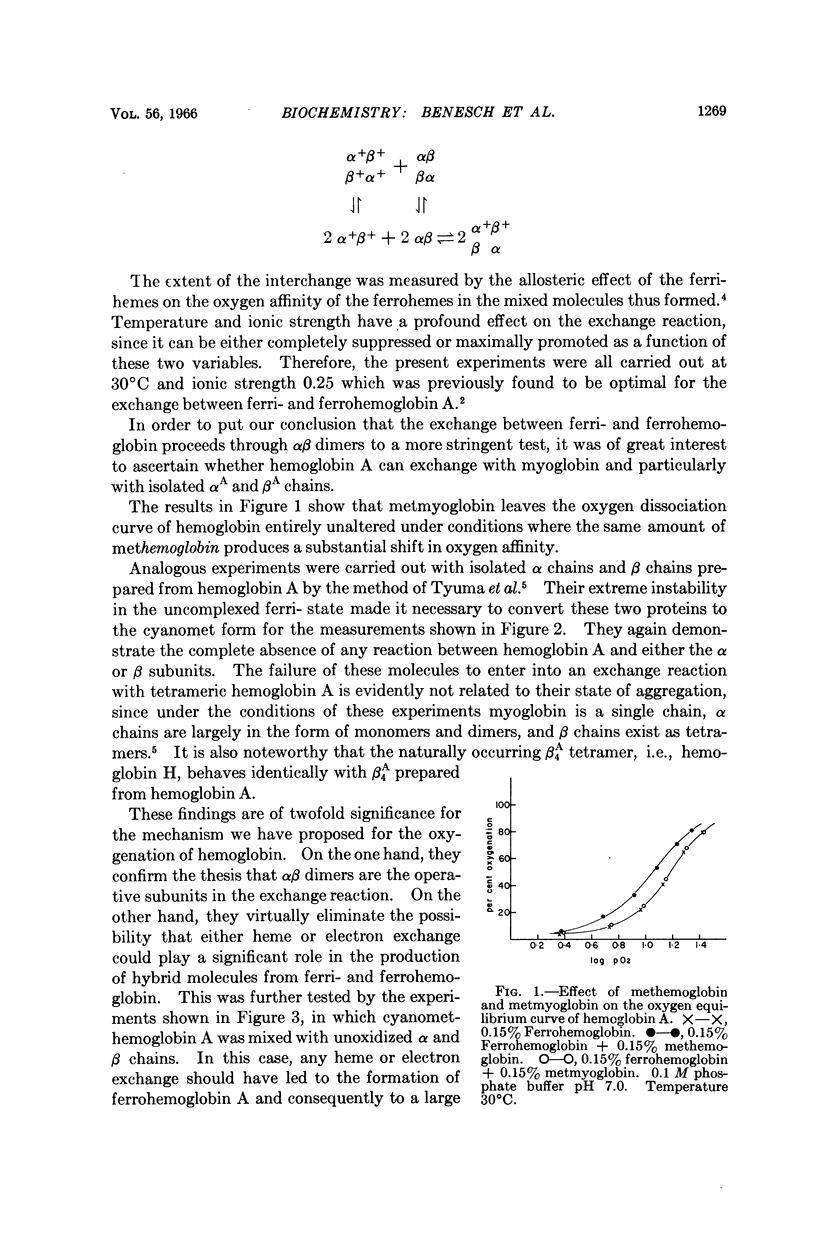
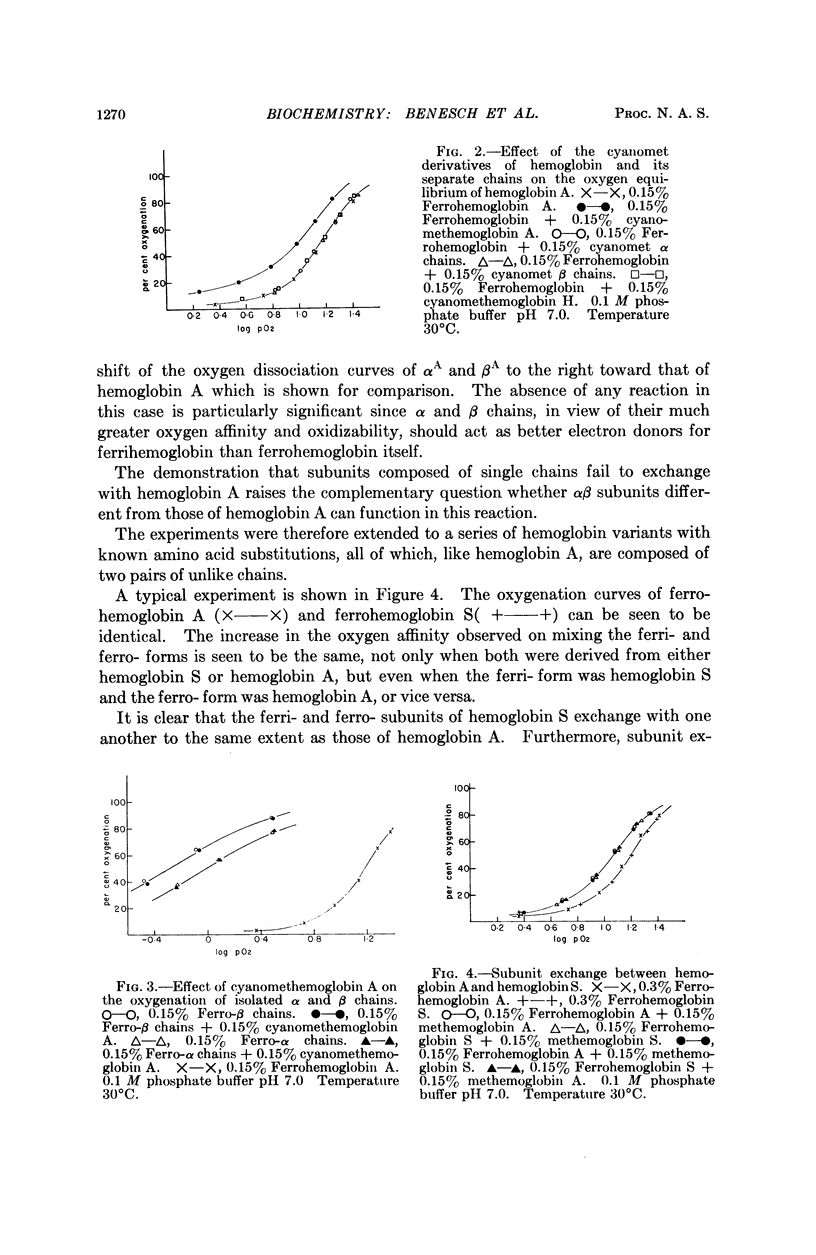
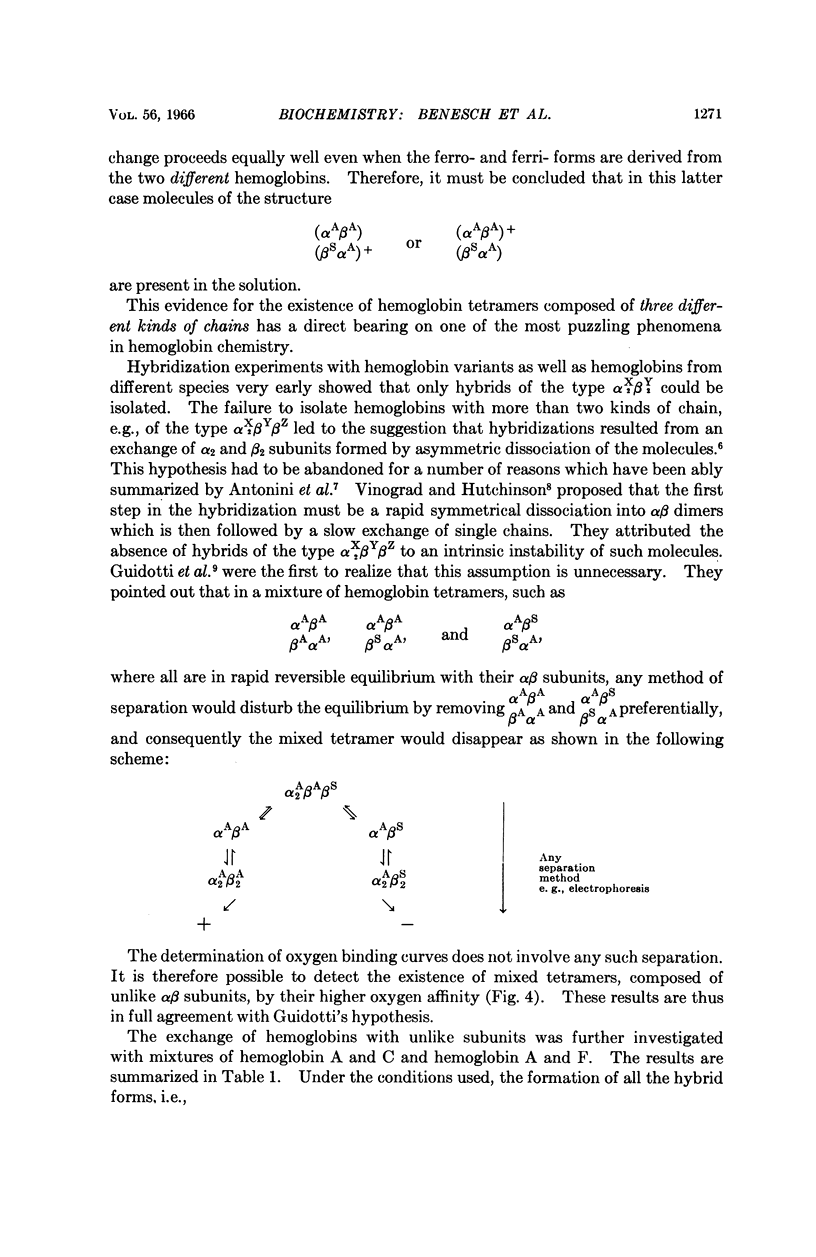
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTONINI E., WYMAN J., BUCCI E., FRONTICELLI C., ROSSI-FANELLI A. The dissociation and recombination of subunits of human, horse, and canine hemoglobin. J Mol Biol. 1962 May;4:368–375. doi: 10.1016/s0022-2836(62)80017-5. [DOI] [PubMed] [Google Scholar]
- BENESCH R., MACDUFF G., BENESCH R. E. DETERMINATION OF OXYGEN EQUILIBRIA WITH A VERSATILE NEW TONOMETER. Anal Biochem. 1965 Apr;11:81–87. doi: 10.1016/0003-2697(65)90045-x. [DOI] [PubMed] [Google Scholar]
- Benesch R. E., Benesch R., Macduff G. Subunit exchange and ligand binding: a new hypothesis for the mechanism of oxygenation of hemoglobin. Proc Natl Acad Sci U S A. 1965 Aug;54(2):535–542. doi: 10.1073/pnas.54.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENOKI Y., TYUMA I. FURTHER STUDIES ON HEMOGLOBIN-OXYGEN EQUILIBRIUM. Jpn J Physiol. 1964 Jun 15;14:280–298. doi: 10.2170/jjphysiol.14.280. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H. The photochemical formation of a quickly reacting form of haemoglobin. Biochem J. 1959 Feb;71(2):293–303. doi: 10.1042/bj0710293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUIDOTTI G., KONIGSBERG W., CRAIG L. C. ON THE DISSOCIATION OF NORMAL ADULT HUMAN HEMOGLOBIN. Proc Natl Acad Sci U S A. 1963 Oct;50:774–782. doi: 10.1073/pnas.50.4.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUISMAN T. H., JONXIS J. H. The detection and estimation of fetal hemoglobin by means of the alkali denaturation test. Blood. 1956 Nov;11(11):1009–1018. [PubMed] [Google Scholar]
- ITANO H. A., ROBINSON E. Electrophoretic separation of intermediate compounds in two reactions of ferrihemoglobin. Biochim Biophys Acta. 1958 Sep;29(3):545–555. doi: 10.1016/0006-3002(58)90011-8. [DOI] [PubMed] [Google Scholar]
- Itano H. A., Singer S. J. ON DISSOCIATION AND RECOMBINATION OF HUMAN ADULT HEMOGLOBINS A, S, AND C. Proc Natl Acad Sci U S A. 1958 Jun;44(6):522–529. doi: 10.1073/pnas.44.6.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- MUIRHEAD H., PERUTZ M. F. STRUCTURE OF HAEMOGLOBIN. A THREE-DIMENSIONAL FOURIER SYNTHESIS OF REDUCED HUMAN HAEMOGLOBIN AT 5-5 A RESOLUTION. Nature. 1963 Aug 17;199:633–638. doi: 10.1038/199633a0. [DOI] [PubMed] [Google Scholar]
- ROSSI-FANELLI A., ANTONINI E., CAPUTO A. Studies on the relations between molecular and functional properties of hemoglobin. II. The effect of salts on the oxygen equilibrium of human hemoglobin. J Biol Chem. 1961 Feb;236:397–400. [PubMed] [Google Scholar]
- Sund H., Weber K. The quaternary structure of proteins. Angew Chem Int Ed Engl. 1966 Feb;5(2):231–245. doi: 10.1002/anie.196602311. [DOI] [PubMed] [Google Scholar]
- VINOGRAD J., HUTCHINSON W. D. Carbon-14 labelled hybrids of haemoglobin. Nature. 1960 Jul 16;187:216–218. doi: 10.1038/187216a0. [DOI] [PubMed] [Google Scholar]



