Abstract
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system (CNS). In the recent years, accumulating evidence has supported an immunosuppressive role for regulatory T cells (Tregs). Most studies in the context of autoimmunity have focused on the defects of the CD4+CD25high Tregs. However, we recently demonstrated an altered function of Tr1 Treg cells in MS, characterized by a lack of IL-10 secretion. Therefore, several major regulatory T cell defects are involved in human autoimmune disease. Hence, the induction of Tregs or the stimulation of Treg activity may be beneficial for the treatment of such diseases.
Keywords: Multiple sclerosis, Tr1 regulatory T cells, IL-10, CD46
1- Introduction
Despite advances in the understanding of the mechanisms regulating T cell activation, T cell-mediated autoimmune diseases are still not well understood. Among them, multiple sclerosis (MS) is a complex genetic disease with inflammation in the central nervous system (CNS) white matter mediated by activated autoreactive lymphocytes (Feldmann and Steinman, 2005; Hafler and De Jager, 2005; Hafler et al., 2005; Hohlfeld and Wekerle, 2004). Once in the CNS, these autoreactive T cells target the myelin basic protein on the myelin sheath, and recruit more inflammatory immune cells to the site of attack (Bruck, 2005; Liu et al., 2006; McQualter and Bernard, 2007). The pathology of the inflammatory reaction is consistent with a T-cell mediated immune response, leading to tissue damage through activated macrophages and microglia. This repeated inflammation and subsequent demyelination will then instigate nerve impulses to be slowed or stopped, causing the symptoms of MS. Therefore, the understanding of the factors controlling T cell activation, inflammation and migration within the brain is of crucial importance (Adorini, 2004; Hohlfeld and Wekerle, 2004). Worldwide, MS may affect 2.5 million individuals including 400,000 subjects in the US, and 80,000 individuals in the UK and it is the most common disease affecting young adults. Hence, new approaches need to be developed in treating this disease.
In the recent years, the characterization of regulatory T cells and of their role in controlling the immune response has been highlighted. Indeed, the loss of Treg function seems to be a critical factor in the pathogenesis of human autoimmune diseases (Kretschmer et al., 2006; Paust and Cantor, 2005; Wraith et al., 2004). Several classes of Tregs have now been identified, including the naturally occurring CD4+CD25high Tregs, as well as induced Tregs such as Tr1 and Th3 cells. Most studies of these cells in the context of autoimmunity have focused on the defects of the CD4+CD25high Tregs. However, we also recently demonstrated an altered function of Tr1 regulatory T cells in MS, characterized by a lack of IL-10 secretion. Therefore, several major regulatory T cell defects that encompass the various sorts of Tregs are involved in human autoimmune diseases. This suggests that therapies aiming at enhancing or inducing Treg responses might be beneficial for such diseases.
2- Regulatory T cells and Multiple Sclerosis (MS)
In the past years, a resurgence of interest in regulatory T cells (Tregs) has emerged. Such T cells have been shown to regulate the immune response by turning off the signals initiated during the immune response. A variety of lymphocyte populations with suppressive capabilities have been reported in both animals and humans. Shimon Sakaguchi first described Tregs as the major contributors in controlling autoreactive T cells and maintaining a state of peripheral tolerance to a range of self-antigens (Sakaguchi et al., 1985; Sakaguchi et al., 1995). An early observation on suppressive activity that was lessened in patients with MS was published in 1986 (Antel et al., 1986). The absence or depletion of Tregs cells leads to autoimmune destruction of a wide range of target organs (Fontenot et al., 2003; Hori et al., 2003; Khattri et al., 2003).
2a- CD4+CD25high regulatory T cells
Characterization
CD4+CD25high regulatory T cells contribute to the maintenance of peripheral tolerance by active suppression and require cell contact in vitro to exert their negative regulation. These cells were initially characterized in mice by expression of CD25 on CD4+ T cells (Sakaguchi et al., 1995), and the expression of FoxP3 transcription factor is crucial to their development and function (Hori et al., 2003; Khattri et al., 2003). Several groups demonstrated that Tregs also exist in humans and that they are very similar in phenotype and function to their murine counterparts (Baecher-Allan et al., 2001; Dieckmann et al., 2001; Jonuleit et al., 2001; Stephens et al., 2001; Taams et al., 2001; Taylor et al., 2001). However, while CD25 is a useful marker to identify murine Tregs, only high CD25 expression should be considered as Tregs in human, as intermediate CD25 expressing T cells do not exhibit suppressive activity (Baecher-Allan et al., 2001; Baecher-Allan et al., 2003; Roncador et al., 2005). They also expressed FoxP3, although again activated human T cells also express low amounts of FoxP3 albeit in lower amounts than Tregs (Roncador et al., 2005; Walker et al., 2003). They are anergic when stimulated by T cell receptor (TCR) cross-linking in vitro, and suppress T cell activation in a non-HLA-restricted, contact-dependent manner. IL-2 signaling is required for maintaining the homeostasis of Treg cells in vivo (Fontenot et al., 2005; Maloy and Powrie, 2005). Additional phenotypic characterizations include CD62L expression that is downregulated on effector T cells, no expression of CCR7 (Hoffmann et al., 2004; Noma et al., 2005), the exclusion of cells expressing the early activation marker CD69 (Gray et al., 2003; McNeill et al., 2007), and high-level expression of glucocorticoid-induced TNFR family-related gene/protein (GITR) (Ono et al., 2006). Finally, the expression of E3 ubiquitin ligase, GRAIL, is upregulated in CD4+CD25+ Tregs, and its forced expression induces a regulatory phenotype (Mackenzie et al., 2007).
CD4+CD25+ T cells in MS
A role of these CD4+CD25high regulatory T cells has first been shown in regulating autoimmune diseases in animal models, including EAE (Kohm et al., 2002; Nishibori et al., 2004). In MS patients, the levels of circulating CD4+CD25+ T cells and CD4+CD25high Treg cells are not altered (Putheti et al., 2004). However, we and others have reported a decrease of CD4+CD25high regulatory T cell function in patients with MS (Haas et al., 2005; Huan et al., 2005; Viglietta et al., 2004). Indeed, a significant decrease in the suppressive activity of CD4+CD25high regulatory T cells from peripheral blood of patients with MS as compared with healthy donors was observed (Viglietta et al., 2004). Interestingly, only patients in the relapsing-remitting phase exhibit impaired Treg function, characterized by a reduction in proliferation and interferon-gamma production of CD4+CD25− responder T cells (Venken et al., 2006). Secondary progressive patients have normal CD4+CD25+ Tregs. Furthermore, consistently with their suppressive capacity, CD4+CD25+ Tregs from secondary progressive MS patients have normal levels of FoxP3 expression while FoxP3 expression was decreased in relapsing remitting MS patients.
2b. Tr1 regulatory T cells and IL-10 production
Characterization and induction
Two other populations of regulatory T cells have been described. Type 1 regulatory (Tr1) T cells mainly exert their suppressive activity through the secretion of IL-10 (Roncarolo et al., 2001), a potent immunosuppressive cytokine (Moore et al., 2001), while Th3 cells suppress cell activation through TGFβ release (Bach, 2001; Chen et al., 1994). Both in vitro and in vivo studies with recombinant IL-10 and neutralizing antibodies revealed pleiotropic activities of IL-10 on B, T, and mast cells (Moore et al., 2001). The anti-inflammatory role of IL-10 was demonstrated by the development of inflammatory responses in IL-10 deficient (IL-10−/−) mice (Berg et al., 1995; Kuhn et al., 1993). Indeed, IL-10-deficient mice spontaneously develop inflammatory bowel disease (Kuhn et al., 1993) due to a defect in Tr1 cells that attenuates sensitivity to intestinal flora (Asseman et al., 1999). Hence, IL-10 and IL-10 secreting cells appear to play a role in peripheral tolerance and in protection against autoimmunity.
Tr1 cells were first identified by Roncarolo and colleagues (Groux et al., 1997). Unlike CD4+CD25+ Tregs, no cell surface marker uniquely identifies Tr1 cells. They are not characterized by CD25 expression, although they may express low levels following activation. They don’t express FoxP3 (Vieira et al., 2004) and are functionally defined by their secretion of large amounts of IL-10, modest amounts of IFN-γ, and no IL-2 or IL-4 (Bacchetta et al., 1994; Groux et al., 1997). In vitro induction of Tr1 might be achieved by stimulation of naïve human CD4+ T cells with anti-CD3 mAb in the presence of exogenous IL-10 and IFNα (Levings et al., 2001). Similarly, the combination of the two immunosuppressive drugs, vitamin D3 and dexamethasone, induces human and mouse naive CD4+ T cells to differentiate in vitro into regulatory T cells secreting large amounts of IL-10 (Barrat et al., 2002; Cantorna et al., 1996). Finally, CD46 activation of T cells in the presence of IL-2 leads to Tr1 differentiation characterized by a massive secretion of IL-10 and bystander CD4+ T cell suppression (Kemper et al., 2003), and will be further discussed in the following section. On the other hand, activation of OX40L pathway will inhibit Tr1 differentiation (Ito et al., 2006).
Tr1, IL-10 and MS
Numerous data have revealed the importance of IL-10 in regulating EAE (Anderson et al., 2004; Bettelli et al., 1998; Burkhart et al., 1999; Cua et al., 1999; Rott et al., 1994; Zhang et al., 2004). The neutralization of endogenous IL-10 increased the severity and incidence of SEB- or TNF-induced EAE relapse (Crisi et al., 1995) and the severity of the disease is more severe in IL-10 deficient mice than in wild-type (Bettelli et al., 1998; Samoilova et al., 1998). Mice transgenic for human IL-10 expressed under the control of the MHC class-II promoter were completely protected from induced EAE (Cua et al., 1999). In humans, while a preferential up-modulation of TNFα and lymphotoxin α is observed in active MS, an increased IL-10 production is associated with stable disease (Navikas et al., 1995), and by IFNβ treatment (Chabot and Yong, 2000). Alternatively, a decreased production of IL-10 associated with a significant increased production of IL-12p40 is detected in patients with secondary progressive MS (Balashov et al., 2000; Soldan et al., 2004; van Boxel-Dezaire et al., 1999). Dendritic cells from patients with MS produce more IL-23 than healthy controls, which affects IL-10 production (Vaknin-Dembinsky et al., 2006). Low amounts of IL-10 production are associated with higher disability and MRI lesion load in secondary progressive multiple sclerosis (Petereit et al., 2003). Patients with MS have also diminished frequencies of IL-10 secreting innate TCR-reactive T cells (Vandenbark et al., 2001). Altogether, these data suggest a likely defect in T cell activation leading to generation of regulatory T cells and regulatory cytokines such as IL-10 in MS (Beebe et al., 2002). As mentioned above, vitamin D3 induces Tr1 cells secreting IL-10, and inhibits Th1-mediated autoimmune diseases including EAE (Cantorna et al., 1996). A definitive proof of the role of IL-10 in controlling EAE was recently shown by Spach and colleagues (Spach et al., 2006). The authors demonstrated that the strong inhibition of myelin oligodendrocyte peptide (MOG(35–55))-induced EAE development by vitamin D3 and 1,25-(OH)(2)D(3) was dependent on the functional expression of both IL-10 and IL-10R (Spach et al., 2006). These data also suggest that 1,25-(OH)(2)D(3) may be enhancing an anti-inflammatory loop involving IL-10 secreting Tr1 cells This is further supported by a study in severe asthma patients that describes the in vitro inhibitory potential of human Tr1 cells induced by vitamin D3 and dexamethasone, to inhibit cytokine production by allergen-specific Th2 cells (Xystrakis et al., 2006). Dexamethasone is ineffective in the induction of IL-10 in CD4+ T cells from glucocorticoid resistant asthma patients as compared with their glucocorticoid-sensitive counterparts. The authors now show that the addition of vitamin D3 with dexamethasone could potentially increase the therapeutic response to glucocorticoids in glucocorticoid resistant asthma patients, via the induction of IL-10 producing cells. Hence, a definitive role of IL-10 and IL-10 secreting cells has been demonstrated in human pathologies.
3- CD46, T cell activation and IL-10 production
3a. CD46
CD46 (previously called Membrane Cofactor Protein, MCP) is a ubiquitously expressed protein, first identified as a member of the regulators of complement activation family (Seya et al., 1999). It is a type I membrane protein which is a regulatory part of the complement system. It has cofactor activity for inactivation of complement components C3b and C4b by serum factor I, which protects the host cell from autolysis by complement (Kemper and Atkinson, 2007). In addition, CD46 can act as a receptor for many pathogens (Cattaneo, 2004; Riley-Vargas et al., 2004), including the Edmonston strain of measles virus, human herpesvirus-6, adenoviruses A and B, type IV pili of Neisseria gonorrhoeae and Neisseria meningitidis as well as group A streptococcus, and has been called a “pathogens’ magnet” (Cattaneo, 2004). The basic structure of CD46 is composed of four “short consensus repeats” and a region rich in serine, threonine and proline (STP region) followed by a transmembrane segment, an intracytoplasmic anchor of 12 amino acids and a short cytoplasmic tail. So far, eighteen isoforms are produced due to the alternative splicing of various exons (Dhiman et al., 2004). In particular, four major isoforms are produced (BC1, BC2, C1, and C2), depending on the alternative splicing of an exon in the STP region (B) and of the exon 13 that results in two distinct intracytoplasmic tails of 16 (Cyt-1) or 23 (Cyt-2) amino acids (Russell et al., 1992). These isoforms are usually co-expressed in any given tissue, except for brain and kidney where a preferential expression of Cyt-2 is observed (Johnstone et al., 1993).
3b. CD46 and the CNS
The blood-brain barrier (BBB) is composed of tight junctions, which prevent the entry of large proteins into the CNS, and crossing this barrier is precisely regulated and crucial for the immune surveillance of the brain. Interestingly, CD46 is highly expressed at the BBB (Shusta et al., 2002). This has been shown by a subtractive expression cloning methodology, identifying proteins with enriched expression at the BBB in comparison to liver and kidney tissues. Johansson et al. analyzed the infection of human CD46-expressing transgenic mice by Neisseria meningitides, the causative agent of meningococcal meningitis (Johansson et al., 2003), which binds to CD46 (Kallstrom et al., 2001). They show that transgenic mice expressing human CD46 were susceptible to meningococcal disease, because bacteria crossed their BBB. Therefore, CD46 mediates access to the meninges by promoting passage of the BBB. As Cyt-2 is predominantly expressed in the human brain, it might enhance inflammatory responses, and explain the lethal effect of Neisserial infection in CD46 transgenic mice. Therefore, one can hypothesize that CD46 plays a role in the activation and/or migration of T cells in the brain of patients with MS.
3c. CD46 is a costimulatory molecule for human T cells
T cell activation occurs upon TCR engagement. However, efficient T cell activation needs a concomitant stimulation with a costimulatory molecule. The major costimulatory molecule described so far is CD28, a member of the B7 family (Sharpe and Freeman, 2002). However, CD3/CD46 costimulation promotes T cell proliferation with a potency comparable to CD28 (Astier et al., 2000). Enhanced proliferation was accompanied by drastic morphological changes of primary human T cells and actin relocalization (Zaffran et al., 2001), along with activation of Vav, critical for TCR activation and T cell activation-induced actin cytoskeleton rearrangements, as well as Rac activation, a GTPase of the Rho family. Such findings were reinforced by a recent report showing that CD46 modifies T cell and NK cell polarization (Oliaro et al., 2006). Of note, the functional orthologue of CD46 (that is not expressed in rodents) in rat or in mice (Crry) is also a costimulatory molecule for murine T cells (Fernandez-Centeno et al., 2000; Jimenez-Perianez et al., 2005), suggesting a new biological function for these complement regulatory molecules (Morgan et al., 2005).
3d. CD46 is an inductor of human Tr1 cells
The role of CD46 in human T cell activation has been strongly supported by the fact that CD46/CD3 costimulation of human primary T cells in the presence of IL-2 induced a T regulatory (Tr1) phenotype, characterized by a massive production of IL-10 and granzyme B, and the ability to suppress the proliferation of bystander CD4+ T cells (Grossman et al., 2004; Kemper et al., 2003). Low strength of TCR stimulation leads to a lack of sustained proliferation of CD3/CD46-generated Tr1-like cells that is due, at least partially, to a G0/G1 blockage in their cell cycle progression, with the inability to degrade p27/kip1, and to an increased sensitivity to cell death (Meiffren et al., 2006). However, depending on the costimulatory signals, CD46 activated T cells can also differentiate towards a Th1 response with increased IL-10, IL-2 and IFNγ secretion, but decreased IL-5 production (Sanchez et al., 2004). As CD46 acts as a receptor for many pathogens, Kemper’s group has investigated if such pathogens could directly induce Tr1-like cells through their interaction with CD46 (Price et al., 2005). They showed that interaction of the streptococcal ligand for CD46 indeed led to Tr1 differentiation. These data highlight the importance of CD46 in the regulation of the immune response through the induction of Tr1 cells and IL-10 production.
4- Tr1 induction is dysregulated in patients with MS
The importance of regulatory T cells in the pathology of autoimmune diseases has been demonstrated by various groups, who demonstrated a defect in the CD4+CD25high regulatory T cells in patients with MS (Balandina et al., 2005; Haas et al., 2005; Huan et al., 2005; Viglietta et al., 2004) as well as in other human autoimmune diseases (Bluestone and Tang, 2005; Christen and von Herrath, 2004; Feldmann and Steinman, 2005). Considering the central role of Tr1 cells and IL-10 in regulating immune responses, we postulated that patients with MS would have multiple defects in immunoregulatory T cells, including Tr1 cells. As mentioned above, CD46-activated T cells acquire a Tr1 phenotype. We therefore determined whether CD46 activation was impaired in patients with MS. A striking difference was observed between healthy donors and patients (Astier et al., 2006). While no significant difference was observed in the proliferation of the cells, little to no IL-10 was secreted by CD46-activated T cells from patients with MS as compared to healthy donors. The lack of IL-10 production was specific to CD46 as it was not affected upon CD28 stimulation. Increasing strength of stimulation by stronger TCR stimulation or enhanced IL-2 concentrations did not restore IL-10 production. The deficit in IL-10 secretion was also specific to this cytokine as the concentrations of IFNγ secreted by CD46-activated T cells were not affected. These data demonstrate that human autoimmune diseases can be associated with multiple defects in regulatory T cell populations.
As mentioned above, an increased IL-10 production is often associated with remissions (Clerici et al., 2001; Correale et al., 1995; Navikas et al., 1995), and induced by IFNβ treatment (Chabot and Yong, 2000; Ozenci et al., 1999). However, when IL-10 secretion by T cells from untreated and IFNβ treated patients was examined, no difference was observed between these two groups of patients. This suggests that while IFNβ has a therapeutic effect, it does not appear to target Tr1 cells, but likely acts on the other cells producing Il-10 such as Th2 cells, B cells or monocytes. It would be interesting to determine what affects IL-10 production by Tr1 cells. Nevertheless, our results suggest that pharmacologic interventions that induce IL-10 secretion by CD4 cells are viable interventions in patients with MS.
5. Divergent roles of the two intracytoplasmic isoforms in a murine model of inflammation
The two intracellular tails of CD46 produced by alternative splicing, Cyt1 and Cyt2, are co-expressed in human cells, although the proportion of Cyt1 to Cyt2 isoforms can slightly vary (Russell et al., 1992), with a predominant expression of Cyt2 in the brain. As suggested by Russell, it is possible that a selective recruitment of each isoform to determined specific signaling complexes might result in a different signaling outcome, and therefore in a different biological response (Russell, 2004). The specific role of each cytoplasmic isoform has been evaluated using a model of transgenic mice expressing either one of the intracytoplasmic isoforms. Mice do not express CD46, except in testes. Furthermore, there is no homology between the sequences of the mouse and human cytoplasmic domains. The two cytoplasmic tails exhibited antagonist effect on T cell-dependent contact hypersensitivity reaction. Cyt1 inhibited the inflammatory reaction, whereas Cyt2 augmented the inflammation (Marie et al., 2002). The two isoforms exerted opposite effects on CD4+ T cell proliferation, as Cyt1 expression enhanced proliferation while Cyt2 inhibited it. Of note, the morphological changes observed in human T cells after CD46 stimulation were only reproduced when CD46-Cyt1 was expressed. In contrast, only the Cyt2 isoform promoted CD8+ T cell cytotoxicity. Finally Cyt1 engagement was shown to inhibit IL-2 secretion, while the Cyt2 isoform inhibited IL-10 secretion (Marie et al., 2002). Thus, CD46 differentially regulates T cell–mediated inflammatory responses and contact hypersensitivity reactions depending on its cytoplasmic tail. This suggests that depending on which cytoplasmic tail is expressed or activated, CD46 stimulated T cells might acquire or not a regulatory phenotype.
6. Altered cytoplasmic isoforms expression in T cells from patients with MS
As discussed above, the analysis of CD46 transgenic mice showed that the two distinct cytoplasmic isoforms of CD46 have distinct functions in terms of T cell activation and cytokine production, and differentially control inflammation. As these two isoforms are co-expressed in any given tissue, except for brain and kidney where a preferential expression of Cyt2 is observed (Johnstone et al., 1993), their role in IL-10 secretion hadn’t been elucidated in humans. Expression of both CD46 cytoplasmic isoforms was studied in healthy donors and patients with MS (Astier et al., 2006). Their relative expression was determined by qRT-PCR using primers specific for each cytoplasmic tail. When patients with MS were compared to healthy donors, no difference was observed in freshly isolated T cells. However, upon activation, an increase in Cyt2 isoform was detected in patients with MS, but not in healthy donors. Hence, the reduced secretion of IL-10 by Tr1 cells from patients with MS was associated with an increased expression of the Cyt2 isoform of CD46. Of note, these data correlated with the results found in the mouse, where Cyt1 inhibited inflammation while Cyt2 augmented it (Marie et al., 2002). These data suggest that CD46 Cyt2 might be the most important isoform in the regulation of inflammation in human, although more data should be collected, and it should be assessed in different autoimmune diseases.
7. CD46 and HHV6 in MS
As mentioned above CD46 is also the receptor for HHV6 (Santoro et al., 1999). This is of much interest in the case of MS, as links between viral infection such as HHV6 infection and development of MS have been demonstrated. HHV-6 is present in active MS plaques (Challoner et al., 1995), and the patients have increased IgM response towards HHV6 antigens during the RR phase of the disease (Soldan et al., 1997). Moreover, a recent study followed the HHV6 viral load and clinical data in a one-year follow-up of a cohort of 63 patients and healthy donors (Alvarez-Lafuente et al., 2006). They show that RR patients in relapse have active HHV6 replication and increased EDSS, suggesting that exacerbations are associated with active HHV6 replication. Hence, one may hypothesize that the increasing viral load will activate the T cell population in the brain through CD46. As CD46 is deficient in patients with MS, this will lead to further damage and inflammation.
8- Future pathways
MS is a complex disease with genetic predisposition and environmental influences, as well as immunological defects. It has proven heritability, and the association of selective allelic variants likely leads to a higher risk of developing disease (Hafler and De Jager, 2005; Hafler et al., 2005). Ultimately, several immunologic alterations will lead to the profound loss of tolerance associated with CNS white matter inflammation. Hence, future investigations can examine defects in IL-10 secretion and whole genome association scans to determine whether it is related to genetic or environmental influences. Similarly, the use of whole genome RNAi libraries will be of use to determine new genes involved in the regulation of IL-10 (Astier et al, manuscript in preparation). Future studies will then focus on the role of the newly discovered genes in IL-10 production in patients with MS. Ultimately, the precise dissection of the cascade leading to IL-10 production and Tr1 differentiation will be understood. It will open new means to manipulate the immune system in humans, with an impact in autoimmune diseases such as MS in which a deficit in IL-10 production likely participates in the neuroinflammation observed in these patients.
Conclusion
CD46 has been only recently identified as a regulator of T cell activation. However, our recent data as well as others’ have demonstrated its crucial role in the fine regulation of the immune response. According to the results found in the CD46 transgenic mouse model, CD46 cytoplasmic isoforms could induce either an anti-inflammatory response through Tr1 differentiation or a pro-inflammatory response. This is further supported by our findings in MS. CD46 is dysregulated in patients with impaired IL-10 production, and an increased Cyt2 isoform expression, as summarized in Figure 1. Hence, the interference with the signal transduction cascade initiated by CD46 on human T cells may be targets of novel strategies to treat autoimmune diseases, such as MS.
Figure 1. Altered Tr1 differentiation in patients with MS.
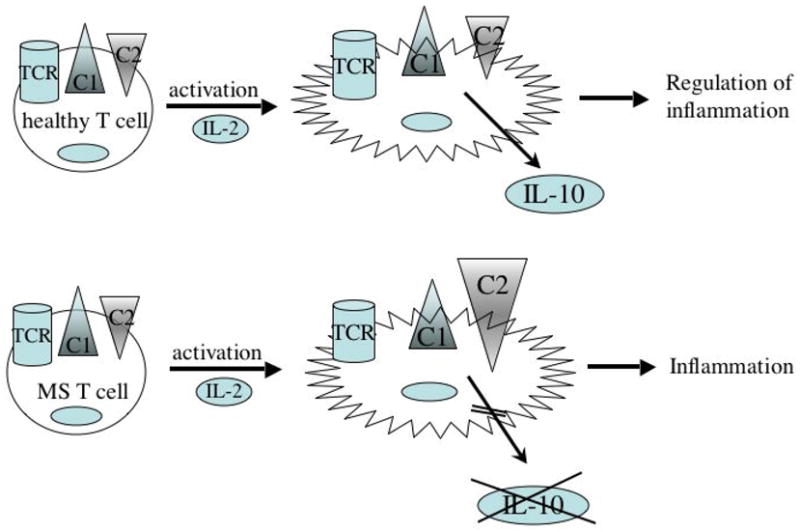
CD46 activation of human T cells induces Tr1 differentiation and IL-10 secretion with an equivalent level of both CD46 cytoplasmic isoforms Cyt1 (C1) and Cyt2 (C2). This pathway is dysfunctional in patients with MS, as upon CD46 stimulation, T cells do not produce IL-10. This is also associated with an increased expression of CD46 –Cyt2 (C2) isoform. This likely contributes to the neuro-inflammation observed in these patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adorini L. Immunotherapeutic approaches in multiple sclerosis. J Neurol Sci. 2004;223:13–24. doi: 10.1016/j.jns.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Alvarez-Lafuente R, Garcia-Montojo M, De Las Heras V, Bartolome M, Arroyo R. Clinical parameters and HHV-6 active replication in relapsing-remitting multiple sclerosis patients. J Clin Virol. 2006;37(Suppl 1):S24–26. doi: 10.1016/S1386-6532(06)70007-5. [DOI] [PubMed] [Google Scholar]
- Anderson AC, Reddy J, Nazareno R, Sobel RA, Nicholson LB, Kuchroo VK. IL-10 plays an important role in the homeostatic regulation of the autoreactive repertoire in naive mice. J Immunol. 2004;173:828–834. doi: 10.4049/jimmunol.173.2.828. [DOI] [PubMed] [Google Scholar]
- Antel J, Bania M, Noronha A, Neely S. Defective suppressor cell function mediated by T8+ cell lines from patients with progressive multiple sclerosis. J Immunol. 1986;137:3436–3439. [PubMed] [Google Scholar]
- Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astier A, Trescol-Biemont MC, Azocar O, Lamouille B, Rabourdin-Combe C. Cutting edge: CD46, a new costimulatory molecule for T cells, that induces p120CBL and LAT phosphorylation. J Immunol. 2000;164:6091–6095. doi: 10.4049/jimmunol.164.12.6091. [DOI] [PubMed] [Google Scholar]
- Astier AL, Meiffren G, Freeman S, Hafler DA. Alterations in CD46-mediated Tr1 regulatory T cells in patients with multiple sclerosis. J Clin Invest. 2006;116:3252–3257. doi: 10.1172/JCI29251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchetta R, Bigler M, Touraine JL, Parkman R, Tovo PA, Abrams J, de Waal Malefyt R, de Vries JE, Roncarolo MG. High levels of interleukin 10 production in vivo are associated with tolerance in SCID patients transplanted with HLA mismatched hematopoietic stem cells. J Exp Med. 1994;179:493–502. doi: 10.1084/jem.179.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach JF. Non-Th2 regulatory T-cell control of Th1 autoimmunity. Scand J Immunol. 2001;54:21–29. doi: 10.1046/j.1365-3083.2001.00945.x. [DOI] [PubMed] [Google Scholar]
- Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25+ regulatory cells from human peripheral blood express very high levels of CD25 ex vivo. Novartis Found Symp. 2003;252:67–88. 88–91, 106–114. doi: 10.1002/0470871628.ch6. [DOI] [PubMed] [Google Scholar]
- Balandina A, Lecart S, Dartevelle P, Saoudi A, Berrih-Aknin S. Functional defect of regulatory CD4(+)CD25+ T cells in the thymus of patients with autoimmune myasthenia gravis. Blood. 2005;105:735–741. doi: 10.1182/blood-2003-11-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balashov KE, Comabella M, Ohashi T, Khoury SJ, Weiner HL. Defective regulation of IFNgamma and IL-12 by endogenous IL-10 in progressive MS. Neurology. 2000;55:192–198. doi: 10.1212/wnl.55.2.192. [DOI] [PubMed] [Google Scholar]
- Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O’Garra A. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe AM, Cua DJ, de Waal Malefyt R. The role of interleukin-10 in autoimmune disease: systemic lupus erythematosus (SLE) and multiple sclerosis (MS) Cytokine Growth Factor Rev. 2002;13:403–412. doi: 10.1016/s1359-6101(02)00025-4. [DOI] [PubMed] [Google Scholar]
- Berg DJ, Leach MW, Kuhn R, Rajewsky K, Muller W, Davidson NJ, Rennick D. Interleukin 10 but not interleukin 4 is a natural suppressant of cutaneous inflammatory responses. J Exp Med. 1995;182:99–108. doi: 10.1084/jem.182.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Das MP, Howard ED, Weiner HL, Sobel RA, Kuchroo VK. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J Immunol. 1998;161:3299–3306. [PubMed] [Google Scholar]
- Bluestone JA, Tang Q. How do CD4+CD25+ regulatory T cells control autoimmunity? Curr Opin Immunol. 2005;17:638–642. doi: 10.1016/j.coi.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Bruck W. Clinical implications of neuropathological findings in multiple sclerosis. J Neurol. 2005;252(Suppl 3):iii10–iii14. doi: 10.1007/s00415-005-2011-5. [DOI] [PubMed] [Google Scholar]
- Burkhart C, Liu GY, Anderton SM, Metzler B, Wraith DC. Peptide-induced T cell regulation of experimental autoimmune encephalomyelitis: a role for IL-10. Int Immunol. 1999;11:1625–1634. doi: 10.1093/intimm/11.10.1625. [DOI] [PubMed] [Google Scholar]
- Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci U S A. 1996;93:7861–7864. doi: 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R. Four viruses, two bacteria, and one receptor: membrane cofactor protein (CD46) as pathogens’ magnet. J Virol. 2004;78:4385–4388. doi: 10.1128/JVI.78.9.4385-4388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabot S, Yong VW. Interferon beta-1b increases interleukin-10 in a model of T cell-microglia interaction: relevance to MS. Neurology. 2000;55:1497–1505. doi: 10.1212/wnl.55.10.1497. [DOI] [PubMed] [Google Scholar]
- Challoner PB, Smith KT, Parker JD, MacLeod DL, Coulter SN, Rose TM, Schultz ER, Bennett JL, Garber RL, Chang M, et al. Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc Natl Acad Sci U S A. 1995;92:7440–7444. doi: 10.1073/pnas.92.16.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- Christen U, von Herrath MG. Initiation of autoimmunity. Curr Opin Immunol. 2004;16:759–767. doi: 10.1016/j.coi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Clerici M, Saresella M, Trabattoni D, Speciale L, Fossati S, Ruzzante S, Cavaretta R, Filippi M, Caputo D, Ferrante P. Single-cell analysis of cytokine production shows different immune profiles in multiple sclerosis patients with active or quiescent disease. J Neuroimmunol. 2001;121:88–101. doi: 10.1016/s0165-5728(01)00431-3. [DOI] [PubMed] [Google Scholar]
- Correale J, Gilmore W, McMillan M, Li S, McCarthy K, Le T, Weiner LP. Patterns of cytokine secretion by autoreactive proteolipid protein-specific T cell clones during the course of multiple sclerosis. J Immunol. 1995;154:2959–2968. [PubMed] [Google Scholar]
- Crisi GM, Santambrogio L, Hochwald GM, Smith SR, Carlino JA, Thorbecke GJ. Staphylococcal enterotoxin B and tumor-necrosis factor-alpha-induced relapses of experimental allergic encephalomyelitis: protection by transforming growth factor-beta and interleukin-10. Eur J Immunol. 1995;25:3035–3040. doi: 10.1002/eji.1830251108. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Groux H, Hinton DR, Stohlman SA, Coffman RL. Transgenic interleukin 10 prevents induction of experimental autoimmune encephalomyelitis. J Exp Med. 1999;189:1005–1010. doi: 10.1084/jem.189.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhiman N, Jacobson RM, Poland GA. Measles virus receptors: SLAM and CD46. Rev Med Virol. 2004;14:217–229. doi: 10.1002/rmv.430. [DOI] [PubMed] [Google Scholar]
- Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–1310. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M, Steinman L. Design of effective immunotherapy for human autoimmunity. Nature. 2005;435:612–619. doi: 10.1038/nature03727. [DOI] [PubMed] [Google Scholar]
- Fernandez-Centeno E, de Ojeda G, Rojo JM, Portoles P. Crry/p65, a membrane complement regulatory protein, has costimulatory properties on mouse T cells. J Immunol. 2000;164:4533–4542. doi: 10.4049/jimmunol.164.9.4533. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- Gray CP, Arosio P, Hersey P. Association of increased levels of heavy-chain ferritin with increased CD4+ CD25+ regulatory T-cell levels in patients with melanoma. Clin Cancer Res. 2003;9:2551–2559. [PubMed] [Google Scholar]
- Grossman WJ, Verbsky JW, Tollefsen BL, Kemper C, Atkinson JP, Ley TJ. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood. 2004;104:2840–2848. doi: 10.1182/blood-2004-03-0859. [DOI] [PubMed] [Google Scholar]
- Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- Haas J, Hug A, Viehover A, Fritzsching B, Falk CS, Filser A, Vetter T, Milkova L, Korporal M, Fritz B, Storch-Hagenlocher B, Krammer PH, Suri-Payer E, Wildemann B. Reduced suppressive effect of CD4+CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur J Immunol. 2005;35:3343–3352. doi: 10.1002/eji.200526065. [DOI] [PubMed] [Google Scholar]
- Hafler DA, De Jager PL. Applying a new generation of genetic maps to understand human inflammatory disease. Nat Rev Immunol. 2005;5:83–91. doi: 10.1038/nri1532. [DOI] [PubMed] [Google Scholar]
- Hafler DA, Slavik JM, Anderson DE, O’Connor KC, De Jager P, Baecher-Allan C. Multiple sclerosis. Immunol Rev. 2005;204:208–231. doi: 10.1111/j.0105-2896.2005.00240.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann P, Eder R, Kunz-Schughart LA, Andreesen R, Edinger M. Large-scale in vitro expansion of polyclonal human CD4(+)CD25high regulatory T cells. Blood. 2004;104:895–903. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R, Wekerle H. Autoimmune concepts of multiple sclerosis as a basis for selective immunotherapy: from pipe dreams to (therapeutic) pipelines. Proc Natl Acad Sci U S A. 2004;101(Suppl 2):14599–14606. doi: 10.1073/pnas.0404874101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Huan J, Culbertson N, Spencer L, Bartholomew R, Burrows GG, Chou YK, Bourdette D, Ziegler SF, Offner H, Vandenbark AA. Decreased FOXP3 levels in multiple sclerosis patients. J Neurosci Res. 2005;81:45–52. doi: 10.1002/jnr.20522. [DOI] [PubMed] [Google Scholar]
- Ito T, Wang YH, Duramad O, Hanabuchi S, Perng OA, Gilliet M, Qin FX, Liu YJ. OX40 ligand shuts down IL-10-producing regulatory T cells. Proc Natl Acad Sci U S A. 2006;103:13138–13143. doi: 10.1073/pnas.0603107103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Perianez A, Ojeda G, Criado G, Sanchez A, Pini E, Madrenas J, Rojo JM, Portoles P. Complement regulatory protein Crry/p65-mediated signaling in T lymphocytes: role of its cytoplasmic domain and partitioning into lipid rafts. J Leukoc Biol. 2005;78:1386–1396. doi: 10.1189/jlb.1104642. [DOI] [PubMed] [Google Scholar]
- Johansson L, Rytkonen A, Bergman P, Albiger B, Kallstrom H, Hokfelt T, Agerberth B, Cattaneo R, Jonsson AB. CD46 in meningococcal disease. Science. 2003;301:373–375. doi: 10.1126/science.1086476. [DOI] [PubMed] [Google Scholar]
- Johnstone RW, Russell SM, Loveland BE, McKenzie IF. Polymorphic expression of CD46 protein isoforms due to tissue-specific RNA splicing. Mol Immunol. 1993;30:1231–1241. doi: 10.1016/0161-5890(93)90038-d. [DOI] [PubMed] [Google Scholar]
- Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–1294. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallstrom H, Blackmer Gill D, Albiger B, Liszewski MK, Atkinson JP, Jonsson AB. Attachment of Neisseria gonorrhoeae to the cellular pilus receptor CD46: identification of domains important for bacterial adherence. Cell Microbiol. 2001;3:133–143. doi: 10.1046/j.1462-5822.2001.00095.x. [DOI] [PubMed] [Google Scholar]
- Kemper C, Atkinson JP. T-cell regulation: with complements from innate immunity. Nat Rev Immunol. 2007;7:9–18. doi: 10.1038/nri1994. [DOI] [PubMed] [Google Scholar]
- Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–392. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- Kretschmer K, Apostolou I, Jaeckel E, Khazaie K, von Boehmer H. Making regulatory T cells with defined antigen specificity: role in autoimmunity and cancer. Immunol Rev. 2006;212:163–169. doi: 10.1111/j.0105-2896.2006.00411.x. [DOI] [PubMed] [Google Scholar]
- Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Levings MK, Sangregorio R, Galbiati F, Squadrone S, de Waal Malefyt R, Roncarolo MG. IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J Immunol. 2001;166:5530–5539. doi: 10.4049/jimmunol.166.9.5530. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hao W, Letiembre M, Walter S, Kulanga M, Neumann H, Fassbender K. Suppression of microglial inflammatory activity by myelin phagocytosis: role of p47-PHOX-mediated generation of reactive oxygen species. J Neurosci. 2006;26:12904–12913. doi: 10.1523/JNEUROSCI.2531-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie DA, Schartner J, Lin J, Timmel A, Jennens-Clough M, Fathman CG, Seroogy CM. GRAIL is upregulated in CD4+CD25+ T regulatory cells and is sufficient for conversion of T cells to a regulatory phenotype. J Biol Chem. 2007 doi: 10.1074/jbc.M604192200. [DOI] [PubMed] [Google Scholar]
- Maloy KJ, Powrie F. Fueling regulation: IL-2 keeps CD4+ Treg cells fit. Nat Immunol. 2005;6:1071–1072. doi: 10.1038/ni1105-1071. [DOI] [PubMed] [Google Scholar]
- Marie JC, Astier AL, Rivailler P, Rabourdin-Combe C, Wild TF, Horvat B. Linking innate and acquired immunity: divergent role of CD46 cytoplasmic domains in T cell induced inflammation. Nat Immunol. 2002;3:659–666. doi: 10.1038/ni810. [DOI] [PubMed] [Google Scholar]
- McNeill A, Spittle E, Backstrom BT. Partial depletion of CD69low-expressing natural regulatory T cells with the anti-CD25 monoclonal antibody PC61. Scand J Immunol. 2007;65:63–69. doi: 10.1111/j.1365-3083.2006.01870.x. [DOI] [PubMed] [Google Scholar]
- McQualter JL, Bernard CC. Multiple sclerosis: a battle between destruction and repair. J Neurochem. 2007;100:295–306. doi: 10.1111/j.1471-4159.2006.04232.x. [DOI] [PubMed] [Google Scholar]
- Meiffren G, Flacher M, Azocar O, Rabourdin-Combe C, Faure M. Cutting edge: abortive proliferation of CD46-induced Tr1-like cells due to a defective Akt/Survivin signaling pathway. J Immunol. 2006;177:4957–4961. doi: 10.4049/jimmunol.177.8.4957. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Morgan BP, Marchbank KJ, Longhi MP, Harris CL, Gallimore AM. Complement: central to innate immunity and bridging to adaptive responses. Immunol Lett. 2005;97:171–179. doi: 10.1016/j.imlet.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Navikas V, Link J, Palasik W, Soderstrom M, Fredrikson S, Olsson T, Link H. Increased mRNA expression of IL-10 in mononuclear cells in multiple sclerosis and optic neuritis. Scand J Immunol. 1995;41:171–178. doi: 10.1111/j.1365-3083.1995.tb03550.x. [DOI] [PubMed] [Google Scholar]
- Nishibori T, Tanabe Y, Su L, David M. Impaired development of CD4+ CD25+ regulatory T cells in the absence of STAT1: increased susceptibility to autoimmune disease. J Exp Med. 2004;199:25–34. doi: 10.1084/jem.20020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma K, Yamaguchi Y, Okita R, Matsuura K, Toge T. The spleen plays an immunosuppressive role in patients with gastric cancer: involvement of CD62L+ cells and TGF-beta. Anticancer Res. 2005;25:643–649. [PubMed] [Google Scholar]
- Oliaro J, Pasam A, Waterhouse NJ, Browne KA, Ludford-Menting MJ, Trapani JA, Russell SM. Ligation of the cell surface receptor, CD46, alters T cell polarity and response to antigen presentation. Proc Natl Acad Sci U S A. 2006;103:18685–18690. doi: 10.1073/pnas.0602458103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Shimizu J, Miyachi Y, Sakaguchi S. Control of autoimmune myocarditis and multiorgan inflammation by glucocorticoid-induced TNF receptor family-related protein(high), Foxp3-expressing CD25+ and CD25- regulatory T cells. J Immunol. 2006;176:4748–4756. doi: 10.4049/jimmunol.176.8.4748. [DOI] [PubMed] [Google Scholar]
- Ozenci V, Kouwenhoven M, Huang YM, Xiao B, Kivisakk P, Fredrikson S, Link H. Multiple sclerosis: levels of interleukin-10-secreting blood mononuclear cells are low in untreated patients but augmented during interferon-beta-1b treatment. Scand J Immunol. 1999;49:554–561. doi: 10.1046/j.1365-3083.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- Paust S, Cantor H. Regulatory T cells and autoimmune disease. Immunol Rev. 2005;204:195–207. doi: 10.1111/j.0105-2896.2005.00247.x. [DOI] [PubMed] [Google Scholar]
- Petereit HF, Pukrop R, Fazekas F, Bamborschke SU, Ropele S, Kolmel HW, Merkelbach S, Japp G, Jongen PJ, Hartung HP, Hommes OR. Low interleukin-10 production is associated with higher disability and MRI lesion load in secondary progressive multiple sclerosis. J Neurol Sci. 2003;206:209–214. doi: 10.1016/s0022-510x(02)00420-3. [DOI] [PubMed] [Google Scholar]
- Price JD, Schaumburg J, Sandin C, Atkinson JP, Lindahl G, Kemper C. Induction of a regulatory phenotype in human CD4+ T cells by streptococcal M protein. J Immunol. 2005;175:677–684. doi: 10.4049/jimmunol.175.2.677. [DOI] [PubMed] [Google Scholar]
- Putheti P, Pettersson A, Soderstrom M, Link H, Huang YM. Circulating CD4+CD25+ T regulatory cells are not altered in multiple sclerosis and unaffected by disease-modulating drugs. J Clin Immunol. 2004;24:155–161. doi: 10.1023/B:JOCI.0000019780.93817.82. [DOI] [PubMed] [Google Scholar]
- Riley-Vargas RC, Gill DB, Kemper C, Liszewski MK, Atkinson JP. CD46: expanding beyond complement regulation. Trends Immunol. 2004;25:496–503. doi: 10.1016/j.it.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Roncador G, Brown PJ, Maestre L, Hue S, Martinez-Torrecuadrada JL, Ling KL, Pratap S, Toms C, Fox BC, Cerundolo V, Powrie F, Banham AH. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur J Immunol. 2005;35:1681–1691. doi: 10.1002/eji.200526189. [DOI] [PubMed] [Google Scholar]
- Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- Rott O, Fleischer B, Cash E. Interleukin-10 prevents experimental allergic encephalomyelitis in rats. Eur J Immunol. 1994;24:1434–1440. doi: 10.1002/eji.1830240629. [DOI] [PubMed] [Google Scholar]
- Russell S. CD46: a complement regulator and pathogen receptor that mediates links between innate and acquired immune function. Tissue Antigens. 2004;64:111–118. doi: 10.1111/j.1399-0039.2004.00277.x. [DOI] [PubMed] [Google Scholar]
- Russell SM, Loveland BE, Johnstone RW, Thorley BR, McKenzie IF. Functional characterisation of alternatively spliced CD46 cytoplasmic tails. Transplant Proc. 1992;24:2329–2330. [PubMed] [Google Scholar]
- Sakaguchi S, Fukuma K, Kuribayashi K, Masuda T. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J Exp Med. 1985;161:72–87. doi: 10.1084/jem.161.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Samoilova EB, Horton JL, Chen Y. Acceleration of experimental autoimmune encephalomyelitis in interleukin-10-deficient mice: roles of interleukin-10 in disease progression and recovery. Cell Immunol. 1998;188:118–124. doi: 10.1006/cimm.1998.1365. [DOI] [PubMed] [Google Scholar]
- Sanchez A, Feito MJ, Rojo JM. CD46-mediated costimulation induces a Th1-biased response and enhances early TCR/CD3 signaling in human CD4+ T lymphocytes. Eur J Immunol. 2004;34:2439–2448. doi: 10.1002/eji.200324259. [DOI] [PubMed] [Google Scholar]
- Santoro F, Kennedy PE, Locatelli G, Malnati MS, Berger EA, Lusso P. CD46 is a cellular receptor for human herpesvirus 6. Cell. 1999;99:817–827. doi: 10.1016/s0092-8674(00)81678-5. [DOI] [PubMed] [Google Scholar]
- Seya T, Hirano A, Matsumoto M, Nomura M, Ueda S. Human membrane cofactor protein (MCP, CD46): multiple isoforms and functions. Int J Biochem Cell Biol. 1999;31:1255–1260. doi: 10.1016/s1357-2725(99)00092-8. [DOI] [PubMed] [Google Scholar]
- Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- Shusta EV, Zhu C, Boado RJ, Pardridge WM. Subtractive expression cloning reveals high expression of CD46 at the blood-brain barrier. J Neuropathol Exp Neurol. 2002;61:597–604. doi: 10.1093/jnen/61.7.597. [DOI] [PubMed] [Google Scholar]
- Soldan SS, Alvarez Retuerto AI, Sicotte NL, Voskuhl RR. Dysregulation of IL-10 and IL-12p40 in secondary progressive multiple sclerosis. J Neuroimmunol. 2004;146:209–215. doi: 10.1016/j.jneuroim.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Soldan SS, Berti R, Salem N, Secchiero P, Flamand L, Calabresi PA, Brennan MB, Maloni HW, McFarland HF, Lin HC, Patnaik M, Jacobson S. Association of human herpes virus 6 (HHV-6) with multiple sclerosis: increased IgM response to HHV-6 early antigen and detection of serum HHV-6 DNA. Nat Med. 1997;3:1394–1397. doi: 10.1038/nm1297-1394. [DOI] [PubMed] [Google Scholar]
- Spach KM, Nashold FE, Dittel BN, Hayes CE. IL-10 signaling is essential for 1,25-dihydroxyvitamin D3-mediated inhibition of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:6030–6037. doi: 10.4049/jimmunol.177.9.6030. [DOI] [PubMed] [Google Scholar]
- Stephens LA, Mottet C, Mason D, Powrie F. Human CD4(+)CD25(+) thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur J Immunol. 2001;31:1247–1254. doi: 10.1002/1521-4141(200104)31:4<1247::aid-immu1247>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Taams LS, Smith J, Rustin MH, Salmon M, Poulter LW, Akbar AN. Human anergic/suppressive CD4(+)CD25(+) T cells: a highly differentiated and apoptosis-prone population. Eur J Immunol. 2001;31:1122–1131. doi: 10.1002/1521-4141(200104)31:4<1122::aid-immu1122>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Taylor PA, Noelle RJ, Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J Exp Med. 2001;193:1311–1318. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaknin-Dembinsky A, Balashov K, Weiner HL. IL-23 is increased in dendritic cells in multiple sclerosis and down-regulation of IL-23 by antisense oligos increases dendritic cell IL-10 production. J Immunol. 2006;176:7768–7774. doi: 10.4049/jimmunol.176.12.7768. [DOI] [PubMed] [Google Scholar]
- van Boxel-Dezaire AH, Hoff SC, van Oosten BW, Verweij CL, Drager AM, Ader HJ, van Houwelingen JC, Barkhof F, Polman CH, Nagelkerken L. Decreased interleukin-10 and increased interleukin-12p40 mRNA are associated with disease activity and characterize different disease stages in multiple sclerosis. Ann Neurol. 1999;45:695–703. doi: 10.1002/1531-8249(199906)45:6<695::aid-ana3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Vandenbark AA, Finn T, Barnes D, Culbertson N, Chou YK, Hicks K, Bakke A, Mass M, Whitham R, Offner H, Bourdette D. Diminished frequency of interleukin-10-secreting, T-cell receptor peptide-reactive T cells in multiple sclerosis patients might allow expansion of activated memory T cells bearing the cognate BV gene. J Neurosci Res. 2001;66:171–176. doi: 10.1002/jnr.1209. [DOI] [PubMed] [Google Scholar]
- Venken K, Hellings N, Hensen K, Rummens JL, Medaer R, D’Hooghe MB, Dubois B, Raus J, Stinissen P. Secondary progressive in contrast to relapsing-remitting multiple sclerosis patients show a normal CD4+CD25+ regulatory T-cell function and FOXP3 expression. J Neurosci Res. 2006;83:1432–1446. doi: 10.1002/jnr.20852. [DOI] [PubMed] [Google Scholar]
- Vieira PL, Christensen JR, Minaee S, O’Neill EJ, Barrat FJ, Boonstra A, Barthlott T, Stockinger B, Wraith DC, O’Garra A. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5986–5993. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, Ziegler SF. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25- T cells. J Clin Invest. 2003;112:1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wraith DC, Nicolson KS, Whitley NT. Regulatory CD4+ T cells and the control of autoimmune disease. Curr Opin Immunol. 2004;16:695–701. doi: 10.1016/j.coi.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Xystrakis E, Kusumakar S, Boswell S, Peek E, Urry Z, Richards DF, Adikibi T, Pridgeon C, Dallman M, Loke TK, Robinson DS, Barrat FJ, O’Garra A, Lavender P, Lee TH, Corrigan C, Hawrylowicz CM. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest. 2006;116:146–155. doi: 10.1172/JCI21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaffran Y, Destaing O, Roux A, Ory S, Nheu T, Jurdic P, Rabourdin-Combe C, Astier AL. CD46/CD3 costimulation induces morphological changes of human T cells and activation of Vav, Rac, and extracellular signal-regulated kinase mitogen-activated protein kinase. J Immunol. 2001;167:6780–6785. doi: 10.4049/jimmunol.167.12.6780. [DOI] [PubMed] [Google Scholar]
- Zhang X, Koldzic DN, Izikson L, Reddy J, Nazareno RF, Sakaguchi S, Kuchroo VK, Weiner HL. IL-10 is involved in the suppression of experimental autoimmune encephalomyelitis by CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:249–256. doi: 10.1093/intimm/dxh029. [DOI] [PubMed] [Google Scholar]


