Abstract
HLA-G is a non-classical HLA-class Ib molecule with multiple immunoregulatory properties. Its main function in physiological conditions is to abrogate maternal NK cell activity against foetal tissue and to establish immune tolerance at maternal-foetal interface. HLA-G is expressed not only as a membrane bound molecule on the surface of cells, but also as a soluble moiety in body fluids. The major isoforms of HLA-G present in serum are soluble HLA-G1 and HLA-G5 which are generated by shedding or proteolytic cleavage of the membrane bound isoform and by secretion of a soluble isoform, respectively.
Here we review the data about soluble HLA-G (sHLA-G) serum levels in different pathological conditions, including immune-mediated disorders, transplantation, and malignancies. In particular, we focus on sHLA-G expression and function in human neuroblastoma, a pediatric tumor, with special emphasis on a novel potential immuno escape mechanism utilized by NB to instruct monocytes to produce and release sHLA-G. Finally, the potential clinical relevance of sHLA-G serum levels is discussed.
Keywords: HLA-G, immune escape, immune-mediated diseases, transplantation, tumors
Introduction
Malignant transformation of cells may be associated with de novo expression of HLA-G, although the frequency of this phenotypic change varies markedly among different types of tumors [1-6]. This topic has been discussed in detail in recently published reviews, to which we refer the interested reader [1, 3]. Although the available information has to be interpreted with caution because of the limited number of lesions investigated in some types of tumors and of the conflicting results obtained by different investigators, the following points are noteworthy, i) glioblastoma (GB); multiple myeloma (MM) HLA-G has the highest expression in cutaneous lymphoma, clear cell renal carcinoma and ovarian carcinoma in which it has been detected in about 40% of the lesions analyzed; ii) it has an intermediate expression in breast and lung carcinoma as well as in cutaneous melanoma, in which it has been detected in less than 30% of the lesions tested; and iii) it has not been detected in the uveal melanoma and laryngeal carcinoma lesions tested. The mechanism(s) underlying the differential expression of HLA-G within a tumor type and among different tumors remain(s) to be determined.
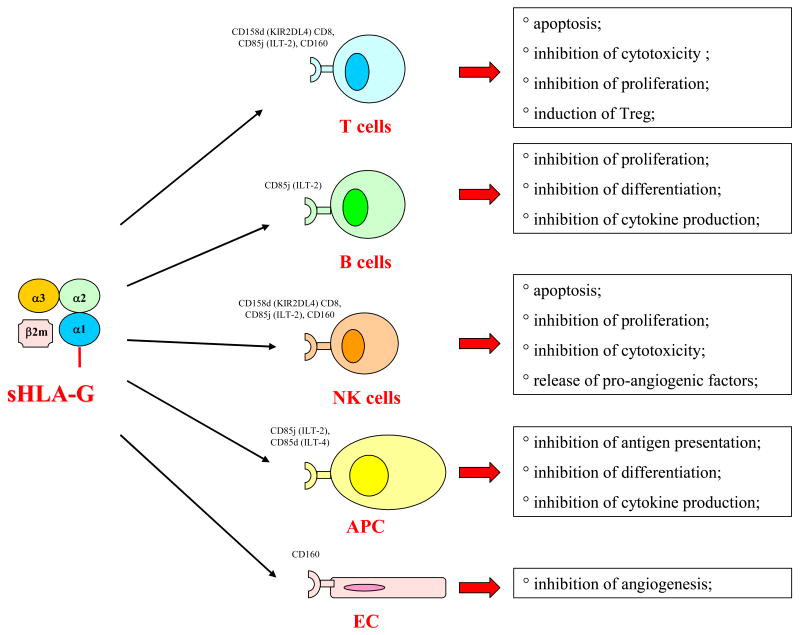
The immunosuppressive properties of HLA-G, in conjunction with the emphasis in recent years on the escape mechanisms utilized by tumor cells to avoid immune recognition and destruction (7), have provided the impetus to investigate whether and how HLA-G expression in tumor cells impacts on their interactions with the host immune system. Several HLA-G mediated escape mechanisms have been described. As summarized in Fig.1, they include i) inhibition of cytotoxic activity of CTL and NK cells [8, 9], ii) inhibition of CD4+ T cell proliferation and cytokine release [10, 11], iii) inhibition of cell cycle progression in human alloreactive T cells [12]; iv) generation of a new type of regulatory CD4+ or CD8+ T cells through transfer of membrane HLA-G from antigen presenting cells to activated T cells (“trogocytosis”) [13]; v) induction of tolerogenic dendritic cells (DC) associated with inhibition of their differentiation [14], and vi) induction of a Th2-like cytokine profile at tumor site through stimulation of IL-3, IL-4 and IL-10 secretion [15].
Figure 1.
Immunoregulatory activities mediated by sHLA-G. Target cells and receptors involved are also indicated.
Whether these escape mechanisms play a role in the clinical course of the disease and/or whether they represent useful prognostic biomarkers and/or molecular targets for therapeutic intervention is not known. The evidence available in the literature is compatible with at least some of these possibilities. Thus HLA-G expression on the surface of tumor cells in B cell-chronic lymphocytic leukemia (B-CLL) has been shown to have on more than 23% of malignant cells. Furthermore, suppression of the humoral and cellular immune response, as measured by IgG serum level, total T cell number, and CD4+ T cell number, was worse in B-CLL patients who had surface HLA-G expression on more than 23% of malignant cells than in those who had a lower percentage of HLA-G positive malignant cells [16].
Along this line, HLA-G expression was detected in a proportion of both primary and metastatic ovarian carcinoma lesions. The presence of a high proportion of HLA-G positive tumor cells in effusions obtained before chemotherapy administration correlated with better response to chemotherapy and overall survival. This finding suggests that HLA-G expression by ovarian carcinoma cells in effusions represents a possible marker of tumor susceptibility to chemotherapy [2].
Finally, in a recent study on neuroblastoma (NB) that is discussed in detail below, we have shown that serum levels of soluble HLA-G (sHLA-G) were significantly higher in patients who developed a local or disseminated relapse than in those who remained in remission over a 3-6 year follow-up. There was also a trend, that however did not reach statistical significance, to higher serum sHLA-G levels in patients with poor clinical outcome than in those who were in complete remission [17]. These findings suggest that sHLA-G levels may predict NB relapse and have therefore prognostic value. However, multicentric studies with larger cohorts of patients are needed to obtain conclusive evidence in support of this possibility.
Like other types of histocompatibility antigens (18, 19), HLA-G are expressed not only on the surface membrane of cells, but also in body fluids. In the present paper we will review the molecular and functional characteristics of sHLA-G, their expression in physiological and pathological conditions and their potential clinical relevance.
Molecular and functional characteristics of sHLA-G
Seven HLA-G isoforms are generated by alternative splicing of the primary HLA-G transcript. Four of them, HLA-G1, -G2, -G3 and -G4, are bound to the cell surface, while the remaining three, HLA-G5, -G6 and –G7 are soluble. The latter three are the counterparts of HLA-G1, -G2 and –G3, respectively. HLA-G1 is the only isoform derived from the translation of the total HLA-G transcript. The other membrane bound isoforms lack one or two globular domains. The structure of the soluble isoforms resembles that of the corresponding membrane bound isoforms in the extracellular part, but differs at the C-terminus. The extracellular domain and the intracytoplasmic tail which are present in the membrane bound isoforms are replaced in the secreted isoforms by a short hydrophilic tail (20-23). These differences provide a marker to distinguish shed or proteolytically cleaved HLA-G isoforms from secreted HLA-G isoforms. The major isoforms are HLA-G1 and HLA-G5, that share immunosuppressive activities mediated by their binding to the receptors CD85j (ILT-2), CD85d (ILT-4), CD158d (KIR2DL4) and CD160 (BY55). These receptors have a differential cellular distribution, since CD85j (ILT-2) is expressed by B, NK and T cells, CD160 (BY55) is expressed by endothelial, NK and T cells, and CD85d (ILT-4) is expressed only by macrophages and CD158d (KIR2DL4) only by NK cells.
sHLA-G derives from the secretion of soluble isoforms, especially HLA-G5, as well as from the shedding of proteolitycally cleaved surface isoforms, like HLA-G1. In physiological conditions, monocytes/macrophages together with myeloid and plasmacytoid dendritic cells are the major producers of sHLA-G [24]. Like their surface membrane bound counterparts, sHLA-G have immunosuppressive properties. The mechanisms underlying these functional properties are similar to those summarized in the Introduction of this paper, but with some distinct characteristics. Thus, sHLA-G5 inhibits T cell proliferation induced by allogeneic dendritic cells without affecting their differentiation and maturation [25]. Furthermore, naïve T cells incubated with sHLA-G5 for 18 hours become anergic to subsequent antigenic stimulation and acquire regulatory properties, as demonstrated by their ability to inhibit proliferation of other T cells in an antigen non-specific fashion [26].
Like classical sHLA class I antigens (27,28), sHLA-G have been shown by Fournel et al (29) and by one of us (30) to induce apoptosis of activated CD8+ T cells and CD8+ NK cells, although this result has not been confirmed by Hunt (31) who however used recombinant HLA-G in her experiments. Binding to CD8 leads to Fas ligand (L) upregulation, soluble FasL secretion and activated CD8+ cell apoptosis by Fas/sFasL interaction [30]. However there is conflicting information in the literature about he potency of sHLA-G and classical sHLA class I antigens in triggering activated CD8+ cell apoptosis. The latter antigens have been reported to be less potent than the former ones by Fournel et al [29], while opposite results have been obtained by one of us (30). Whether these discrepancies reflect the different source of antigens used, the different methods of purification and/or other technical issues remains to be determined. In any case, it is likely that in physiological conditions sHLA-G molecules do not play a major role, since their level in serum is about one order of magnitude below that required to induce CD8+ T cell and CD8+ NK cell apoptosis in vitro (30). Thus, the potential role of HLA-G in the regulation of the immune response would be restricted to pathological conditions associated with a marked increase in the level of sHLA-G in serum or in a given anatomic site. It also remains to be determined whether sHLA-G cooperates with classical sHLA class I antigens in inducing apoptosis and whether this effect is additive or synergistic.
Like classical sHLA class I antigens (18,19), sHLA-G1 inhibits the cytotoxic activity of HLA class I antigen restricted, antigen-specific CTL [9, 30, 32] sHLA-G5 also inhibits CD4 and CD8 T cell alloproliferation by blocking cell cycle progression. However sHLA-G5 does not induce apoptosis of alloreactive T cells [12].
Rajagopalan et al. [33] have recently showed that sHLA-G, following binding to the CD158d receptor on the surface of human resting NK cells, is endocytosed and triggers the expression of a set of chemokines and cytokines driving a proinflammatory/proangiogenic response. These findings suggest that NK cells can exert beneficial effects at sites of HLA-G expression, such as stimulation of vascularization in the maternal decidua during pregnancy. Furthermore, a novel effect of sHLA-G on angiogenesis has been recently described. Specifically, sHLA-G1 has been shown to inhibit in vitro and in vivo angiogenesis by inducing endothelial cell apoptosis upon binding to the CD160/BY55 receptor [34, 35]. Since the latter receptor was detected also in the vasculature of a murine tumor, it is tempting to speculate that CD160 may represent an attractive therapeutic target to inhibit tumor-associated neoangiogenesis.
Expression of sHLA-G in physiological conditions
sHLA-G has been detected in serum from healthy individuals utilizing variations of a double determinant immunoassay as a test system. This assay has been recently standardized in a workshop in order to minimize the interference of interlaboratory variability in the comparison of results obtained by different investigators [36]. Two ELISAs were validated: one detects sHLA-G1 in combination with sHLA-G5, while the other one quantifies only sHLA-G5 molecules. The mean plasma levels of sHLA-G1 + G5 in healthy donors ranged from 27.7 to 95.9 ng/ml, while those of sHLA-G5 from 14.6 to 85.8 ng/ml. In some of these samples the totality of sHLA-G was represented by sHLA-G5, whereas in others only sHLA-G5 and both sHLA-G1 + G5 were detected. In this regard it is noteworthy that amniotic fluid contains sHLA-G1 molecules only, while ascites contains sHLA-G5 molecules only [37]. There was also evidence for a third form of naturally expressed sHLA-G in which the intron-4 encoded sequence is accessible to the binding of the mAb 5A6G7. The resemblance of the structure of this HLA-G isoform to that of classical HLA class I molecules accounts for its reactivity with mAb W6/32, which recognizes a determinant expressed on β2 microglobulin-associated HLA class I heavy chains. On the other hand, the epitope recognized by the mAb MEM-G/9 appears to be hidden, since the latter mAb does not react with the novel sHLA-G structure [38].
sHLA-G plasma levels, which are markedly lower than those of classical HLA class I antigens (30), are influenced by several variables. Among them is the gender of the donors, since the level of sHLA-G is higher in women than in men [39]. Furthermore, as previously observed for classical HLA class I antigens (18,19), another important variable is represented by the HLA-G polymorphism. Thus, healthy individuals carrying the HLA-G*01013 allele or the “null” allele HLA-G*0105N have significantly lower sHLA-G levels than subjects carrying the more frequent HLA-G*01011 and HLA-G*01012 alleles. In addition, subjects with the latter alleles have significantly lower sHLA-G levels than individuals with the HLA-G*01041 allele. Polymorphisms in the 3′UTR and the 5′URR of the HLA-G gene may further influence the sHLA-G level [40].
There is no correlation between sHLA-G1/HLA-G5 and IL-10 concentrations in serum irrespective of genotypes [40]. In contrast, in LPS-activated PBMC cultures from healthy individuals with the +14/+14 bp HLA-G insertion polymorphism, an autocrine loop involving HLA-G5/sHLA-G1 and IL-10 may result in high IL-10 production (41).
There is suggestive evidence that sHLA-G levels are increased in serum and amniotic fluid during pregnancy. However these results were obtained using different assays with different antibodies prior to the standardization workshop discussed above; therefore the conclusions must be interpreted with caution, since one cannot exclude that the reported differences reflect different sensitivity of the assays used by different investigators. At any rate Hunt et al(42) have shown that serum levels of sHLA-G are significantly higher in pregnant than in non pregnant women. Furthermore sHLA-G1 levels are higher in amniotic fluid than in cord serum and in maternal serum and decrease significantly toward term; the levels of sHLA-G1 in maternal serum show a trend to decrease during the third trimester, but the difference from the second trimester is not statistcally significant [37]. The decline in the level of sHLA-G1 in amniotic fluid may stimulate a maternal immune response against the fetus and contribute to the initiation of parturition. In this regard, sHLA-G was detected in eight cell-stage embryo culture supernatants obtained by in vitro fertilization (IVF), and positive embryo implantation occurred only in women with sHLA-G1/ -G5 molecules in embryo culture supernatants [42-44].
Expression of sHLA-G in pathological conditions
The immunoregulatory characteristics of HLA-G have prompted a number of studies aimed at analyzing the expression of sHLA-G in sera from patients affected by a variety of disorders. These studies have mostly focused on disorders of the immune system, on transplantation and on malignant diseases.
i) Immune-mediated disorders
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system of autoimmune origin [45]; based upon the clinical course, MS is classified in three distinct subtypes, i.e. relapsing remitting (RR), secondary progressive (SP), and primary progressive (PP). Both HLA-G and the CD85d (ILT-2) receptor were detected by immunohistochemistry in acute and chronic plaques, perilesional areas and normal white matter of MS patients [46]. sHLA-G and soluble HLA class I (sHLA-I) were tested in serum and cerebrospinal fluid (CSF) from MS patients and patients with non inflammatory neurological disorders (NIND). Intrathecal production of sHLA-G and sHLA-I was significantly more frequent in MS than in NIND patients [46]. However, sHLA-I levels in CSF were significantly higher in MS patients with clinically and MRI active disease, whereas sHLA-G levels in CSF were significantly more elevated in MS patients with clinically and MRI stable disease. Furthermore, while sHLA-I serum levels were low in clinically active MS patients, sHLA-G serum levels were decreased in clinically stable MS patients. Thus, sHLA-I and sHLA-G1 display opposite trends in relation to disease activity in MS patients [47]. In another study, the same group of investigators correlated sHLA-G levels in the CSF from MS patients with the clinical course of the disease and the results of MRI imaging in patients with RR, SP and PP MS. sHLA-G levels in CSF were significantly increased in clinically stable and MRI inactive individuals, indicating that sHLA-G mediated immunosuppression may be involved in disease stabilization. Furthermore sHLA-G and IL10 levels in the CSF from patients with RR MS were correlated with each other and were found to be increased in MS patients without lesional activity on MRI scans, suggesting the involvement of both molecules in disease remission [47].
sHLA-G serum levels were significantly decreased in a cohort of patients with rheumatoid arthritis (RA), a chronic inflammatory disorder of joints with an immune pathogenesis. Furthermore, sHLA-G levels in RA patients positively correlated with parameters of disease activity and presence of HLADRB1 associated epitopes. The low levels of sHLA-G detected in RA patients suggest that T and NK cell activation are not adequately downregulated by sHLA-G molecules [49].
In asthmatic children, the overall levels of serum sHLA-G were not different from those detected in controls. However, when the analysis was restricted to atopic asthmatics, sHLA-G levels were significantly higher in patients than in healthy controls (50).
HLA-G expression was detected also in intestinal biopsies and sera from patients with celiac disease, an autoimmune disorder characterized by an immune response to ingested gluten. This finding may suggest that HLA-G expression reflects an attempt to restore tolerance to gluten and counterbalance inflammation [51].
ii) Transplantation
A major risk of transplantation is rejection of the transplanted organ by the host immune system. In principle, sHLA-G may help reverse rejection by blocking CTL and NK cell mediated mechanisms.
Studies carried out in transplant recipients have made the following observations: i) in renal allograft recipients, the presence of serum sHLA-G is positively correlated with functioning transplants [52], ii) heart transplanted patients displaying a significant increase in serum sHLA-G in the first month after transplantation have a lower incidence of severe rejection episodes than patients with low levels of the molecule [53], and iii) in liver transplanted patients, high serum levels of sHLA-G showed a positive correlation with normal liver function tests, whereas a fall in sHLA-G levels was rapidly followed by deterioration of liver functional parameters [25,54].
Taken together, these results suggest that patients showing increased serum levels of sHLA-G shortly after transplantation have lower incidence of rejection episodes likely due to the immunosuppressive effects of sHLA-G [55]. Additional studies are needed to assess whether sHLA-G levels may have a prognostic value in solid organ transplantation.
iii) Tumors
sHLA-G have been detected in the plasma (56) of patients with various types of malignant diseases. They include glioblastoma multiforme, breast and ovarian cancer [57], lymphoblastic and monocytic acute leukemia [58,59], malignant melanoma (60) and multiple myeloma [61].
Glioblastoma (GB) is a highly malignant tumor of the central nervous system that is thought to originate from astrocytes or astrocytic precursor cells; in spite of aggressive treatment, GB patients have a median survival time of 12 months. In GB patients, sHLA-G serum levels did not differ from those found in healthy controls, but patients with high sHLA-G levels had a significantly shorter survival than those with low sHLA-G levels [56].
Seventy four percent of patients suffering from acute myeloid leukemia (AML), especially of the FABM4 and FABM5 subtypes, showed markedly increasedsHLA-G serum levels. This figure reached 89% in acute lymphoblastic leukemia (ALL) patients, with a higher frequency of upregulated sHLA-G serum concentrations in T than in B-ALL; specifically, high serum levels of sHLA-G were found in 16 out of 17 patients with T-ALL vs 711 patients with B-. Correlations between up-regulation of serum sHLA-G and clinical course of the disease in acute leukemia patients were limited to absence of anterior myelodysplasia and high level of leukocytosis [59].
In melanoma, serum sHLA-G levels were significantly higher in patients than in healthy controls. Although sHLA-G up-regulation was positively correlated with advanced stage of the disease and with tumor load, no correlation with patient prognosis was identified [60]. Treatment with IFN-α was associated with increased serum levels of sHLA-G, irrespective of disease stage and tumor load. On the other hand the increased sHLA-G serum level was found to be related to IFN-α induced up-regulation of surface HLA-G on peripheral blood monocytes [60].
Malignant ascites from patients with breast or ovarian cancer have significantly higher levels of sHLA-G than ascites of non-neoplastic origin, suggesting that sHLA-G level may represent a useful adjunct to cytology in the differential diagnosis of malignant vs benign ascites [57].
sHLA-I and sHLA-G serum levels have been investigated in patients with multiple myeloma (MM), an aggressive plasma cell tumor characterized by secretion of high level monoclonal immunoglobulin in serum. Both sHLA-I and sHLA-G levels were significantly higher in sera from patients with MM than in those from healthy controls. sHLA-I level was predictive of short survival, while sHLA-G level was of no prognostic value [61].
HLA-G and neuroblastoma
Until recently, no information was available about HLA-G expression in pediatric tumors and their role in the course of the disease. In the frame of a program aimed at identifying the immune escape mechanisms that contribute to NB growth and spreading [62-65] we have recently investigated whether membrane-bound and soluble HLA-G play any role in the biology of NB cells and in the clinical course of this disease [17]
NB, that represents the most frequent extra-cranial solid tumor and the first cause of lethality in pre-school age children, originates from the sympathetic nervous system and is characterized by heterogeneous pathological and clinical presentation [66]. A short description of these latter features is provided to facilitate the interpretation of our findings. NB presenting as disseminated disease after the first year of life is one of the most aggressive solid tumors of childhood [66]. This tumor, classified as stage 4 according to the International Neuroblastoma Staging System (INSS) classification, occurs predominantly in children aged 4 to 6 years and accounts for about 50% of all NB cases. Stage 4 NB usually originates from the adrenal gland and spreads virtually to every organ; the most common metastatic sites are cortex of long bones, skull, lymph nodes and bone marrow. The majority of patients with stage 4 NB have grim prognosis, with 70-75% of them dying in 5 years from diagnosis. Malignant neuroblasts have a special propensity to localize to the bone marrow [66].
Localized NB at diagnosis includes stages 1, 2A-B, and 3. Patients with stage 1 disease have a completely resectable tumor, whereas stage 2A-B and 3 patients have a localized tumor that can be completely or partially excised, and may or may not show ipsilateral lymph node involvement. Stage 1 and 2 patients have an overall survival (OS) of approximately 95% at 5 years from diagnosis, while OS of stage 3 patients approximates 75% [66].
A third type of NB, the so-called stage 4s, includes patients with less than one year of age who present with metastatic lesions at onset, localized in skin, liver and/or bone marrow. In spite of the disseminated disease, these patients have an OS that approximates 75% at five years. In most of these patients, the tumor regresses spontaneously without any treatment or following supportive treatment only. Regression is probably related to delayed neuroblast cell differentiation and/or late activation of programmed cell death [66].
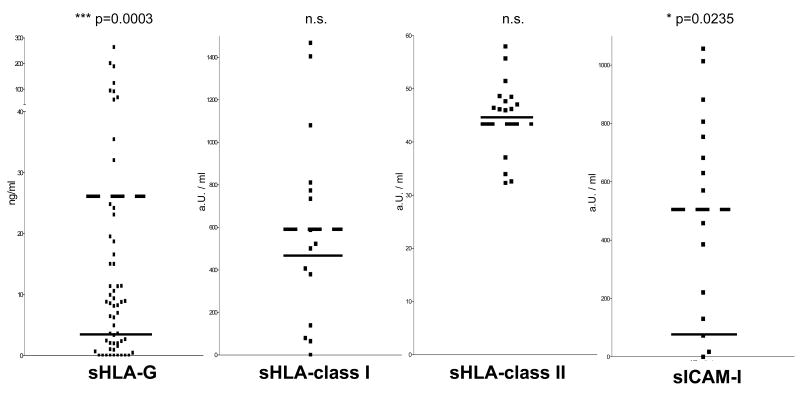
We investigated serum levels of sHLA-G in fifty three untreated NB patients, 25 of whom had localized disease, 20 metastatic disease at diagnosis (stage 4) and 8 stage 4s disease. Altogether, sHLA-G serum levels were significantly higher in NB patients than in age-matched healthy controls. However no difference was detected among patient subgroups. The pattern of expression of serum HLA-G is similar to that of the intercellular adhesion molecule sICAM-1. As shown in Fig. 2, sICAM-1 was significantly increased in serum from the latter patients, and there was a clear trend, that however did not reach statistical significance, towards a parallel increase in serum sHLA-G. Lack of statistical correlation between sICAM-1 and sHLA-G in these patients is likely due to the limited number of patients tested for both parameters.
Figure 2.
Serum levels of sHLA-G, sHLA-class I, sHLA-class II and sICAM-1 in NB patients. Black and broken lines indicate the mean of serum levels obtained for each molecule in healthy subjects and in NB patients, respectively. Results are expressed as ng/ml for sHLA-G and as arbritrary Units (aU) for the remaining molecules. Only sHLA-G and sICAM-1 are significantly increased in NB patients' sera.
Whether the increased serum levels of sHLA-G and sICAM-1 play a role in tumor growth and/or in the interactions of NB cells with the host immune system, as well as whether they represent useful tumor markers, warrants further investigation. To the best of our knowledge, NB represents the first example of malignant disease with a combined increase of serum levels of sHLA-G and sICAM-1. Such increase appears to be selective, since it was detected in NB patients with normal serum levels of classical sHLA-I and soluble HLA class II (sHLA-II) antigens (Fig. 2). The latter results also suggest that sHLA-G and classical sHLA-I and sHLA-II are controlled by different regulatory mechanisms, at least in this malignancy. The available experimental evidence argues against the association between presence of sHLA-G in serum and cell surface expression of HLA-G by NB cells is conflicting [17]. First, the level of sHLA-G was low in the supernatants of five NB cell lines without detectable cell surface expression of HLA-G as well as in those of six NB cell lines with low to moderate cell surface expression of HLA-G. Second, HLA-G was present in sera from NB patients, although its expression could not be detected by immunohistochemical analyses of primary tumors. However the latter results have to be testes to be interpreted with caution, since, in recent studies, flow cytometric analysis of metastatic NB cells isolated from the bone marrow of two stage 4 patients and stained with HLA-G-specific mAb has detected HLA-G expression on neuroblasts gated on the ground of GD2 expression. The results are shown in Fig. 3, panel A. Several mechanisms may account for the different results derived from the analysis of neuroblasts present in primary and metastatic NB lesions. First, the metastatic NB cells and the primary NB lesions we analyzed were derived from different patients. Therefore we cannot exclude that the different results we obtained reflect individual variability in the regulation of HLA-G expression by NB cells. Second, different assay systems with different sensitivity, i.e. flow cytometry and immunohistochemistry, were used to analyze neuroblasts isolated from metastatic lesions and those present in primary NB lesions, respectively. Immunohistochemistry is less sensitive than flow cytometry, raising the possibility that the different sensitivity of the assays contributed to the different results obtained. Lastly, the mechanism we favour is represented by the different epigenetic control of HLA-G gene expression in primary and metastatic lesions because of the diverse environmental conditions. In this regard, previous studies carried out with various human tumor cell lines have shown that both CpG methylation and histone deacetylation play a role in transcriptional silencing of the HLA-G gene, although to a different extent in the individual cell lines analyzed [67]. Experiments performed with histone deacetylase (HDAC) and DNA methyl transferase (DNMT) inhibitors have demonstrated that demethylation with 5-aza-2′-deoxycytidine (5-AC) is more effective at activating HLA-G mRNA and protein expression than incubation of cell lines with HDAC inhibitors, such as trychostatin A (TSA) [67]. Along this line our own studies still in progress with five NB cell lines displaying low to absent surface HLA-G expression have shown that their incubation with 5-AC or with TSA upregulated HLA-G expression, as assessed by flow cytometric analysis of cells stained with HLA-G-specific mAb. These experiments also showed that the five NB cell lines analyzed have a differential sensitivity to 5-AC and HDAC inhibitors. The effects of 5-AC and TSA on the representative GI-LI-N NB cell line are shown in Figure 3, panels B and C, respectively. If epigenetic mechanisms do indeed contribute to the control of HLA-G expression by NB cells, one can envision differential HLA-G expression in tumor lesions located in different anatomic sites, changes in HLA-G expression by NB cells during the course of the disease because of changes in the microenvironment, and differences among patients. If this scenario is correct, such variables should be taken into account when analyzing the influence of HLA-G cell surface expression on the serum level of sHLA-G.
Figure 3.
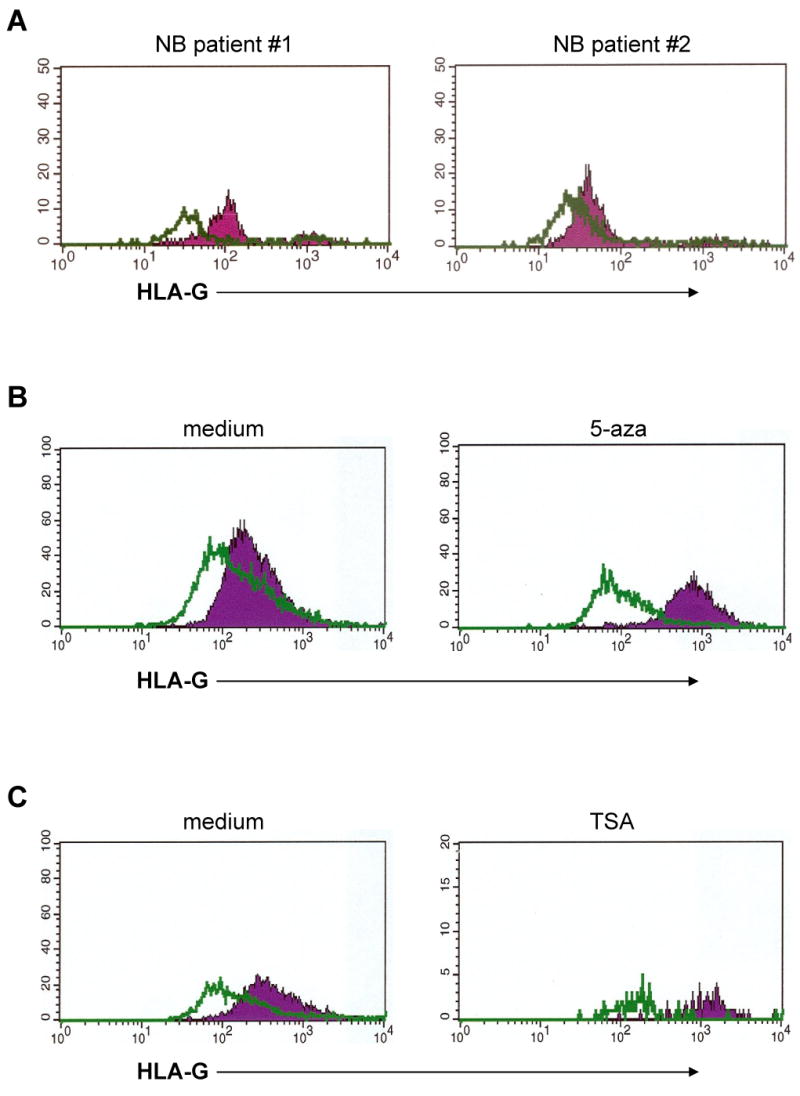
Potential role of epigenetic mechanisms in the differential HLA-G expression by freshly isolated NB cells and by NB cells in long term culture. Panel A. GD2+ cells were purified from BM of two NB patients (right and left panel,), stained with HLA-G1-specific mAb (filled profile) or with an irrelevant isotype-matched mAb (empty profiles) and analyzed with a flow cytometer. Panel B. Cultured NB GI-LI-N cells were cultured for 72 h at 37° C in medium supplemented with 5 μM 5-Aza (right panel) or in medium alone (left panel). Cells were then stained with HLA-G-specific mAb (filled profiles) or with an irrelevant isotype matched mAb (empty profiles) and analyzed with a flow cytometer. Panel C. Cultured NB GI-LI-N cells were cultured for 72 h at 37° C in medium supplemented with trichostatin A (100 ng/ml) (right panel) or in medium alone (left panel). Cells were then stained with HLA-G-specific mAb (filled profiles) or with an irrelevant isotype matched mAb (empty profiles) and analyzed with a flow cytometer.
The available evidence strongly suggests that sHLA-G present in NB patients' sera is derived from monocytes, that represent together with mature myeloid and plasmacytoid dendritic cells the major source of sHLA-G in physiological conditions. Indeed, monocytes from NB patients spontaneously release significantly higher levels of sHLA-G than control monocytes, although they express surface HLA-G at the same levels as monocytes from age- and gender-matched healthy donors. It is likely that the synthesis and release of HLA-G by monocytes from NB patients are maximal under basal conditions, since the number of sHLA-G secreting monocytes was not increased following incubation with IFN-γ. In contrast an increase was observed when monocytes from healthy donors were incubated with IFN-γ [17]. These data altogether suggest that NB patients' monocytes are in an activated state. This possibility is supported by the results of flow cytometry analysis of freshly isolated monocytes from NB patients showing that these cells express de novo CD69, a marker of recently activated cells [17].
The above set of experiments points to monocytes as a major source of sHLA-G in NB patients, but the trigger responsible for their activation is not known. It is our working hypothesis that tumor cells “instruct” monocytes to produce increased levels of sHLA-G that, in turn, protects malignant cells from the attack of the host immune system. Two lines of evidence suggest that this interaction between tumor cells and monocytes takes place through soluble factors released by the former cells rather than through direct cell-to-cell contact. First, monocytes are found in the circulation and their migration to tissues is associated with irreversible differentiation into resident macrophages or dendritic cells. Thus, they have virtually no chance to encounter NB cells. Second, the results of transwell experiments in which monocytes from normal subjects are separated from NB cell lines by a permeable filter demonstrate that sHLA-G production by monocytes takes place in the absence of physical interactions with tumor cells. Accordingly, NB cell line supernatants are able to instruct normal monocytes to produce increased amounts of sHLA-G [17].
In our own experiments, following incubation with pooled supernatants from four NB cell lines, the proportions of sHLA-G secreting monocytes was doubled and a number of immunophenotypic changes took place. They include induction of the CD69 and CD71 activation markers, and up-regulation of HLA class II molecules, of the macrophage marker CD68, and of the costimulatory molecule CD86 [17]. Taken together, these findings indicate that normal monocytes incubated with NB cell supernatants undergo activation and increase their sHLA-G producing capacity. Futhermore, the latter cells are larger in size than control monocytes, undergo spreading on plastic surfaces and display pseudopodia-like structures projecting from the cell surface. All of these changes are consistent with a macrophage-like differentiation of monocytes.
Since tumor cells produce the immunosuppressive cytokines IL-10 and TGF-β1, that are among the best inducers of sHLA-G production [68], one might postulate that these molecules released by NB cells could mediate the effects of NB cells on normal monocytes. However, the results of experiments in which NB cell line supernatants were incubated with neutralizing anti-IL10 or anti-TGFβ1 antibodies before being tested on monocytes did not support this working hypothesis. Likewise, neutralization of the ganglioside GD2, that is released constitutively by NB cells and possesses immunosuppressive activity, was unsuccessful [17]. Thus, for the time being, the identity of the soluble factor(s) produced by NB cells and endowed with “arming” activity on monocytes remains unknown.
IL-12 is a pleiotropic cytokine produced predominantly by macrophages and mature myeloid dendritic cells that mediates potent anti-tumor activity by promoting Th1 cell differentiation, and activating cytotoxic effector functions of CTL and NK cells [69]. IL-10 is the antagonistic cytokine to IL-12; it is produced mainly by macrophages, plasmacytoid dendritic cells and Th2 cells, and, as mentioned above, anergizes the host immune system thus facilitating tumor growth and spreading [70].
A series of studies has demonstrated the existence of two distinct lineages of macrophages, named M1 and M2, that exhibit discrete transcriptional profiles. M1 macrophages are potent producers of IL-12 and low producers of IL-10, whereas M2 macrophages display a specular behaviour, i.e. high IL-10 and low IL-12 production. These latter macrophages, that are frequently detected within tumor infiltratates, promote tumor progression, tissue repair and remodeling [71].
NB tumors usually contain scarce lymphoid infiltrates, that are detected occasionally in stroma poor tumors [62] and consistently in stroma rich tumors [63]. Tumor infiltrating cells are predominantly T and B lymphocytes admixed with macrophages whose M1/M2 profile has not yet been investigated.
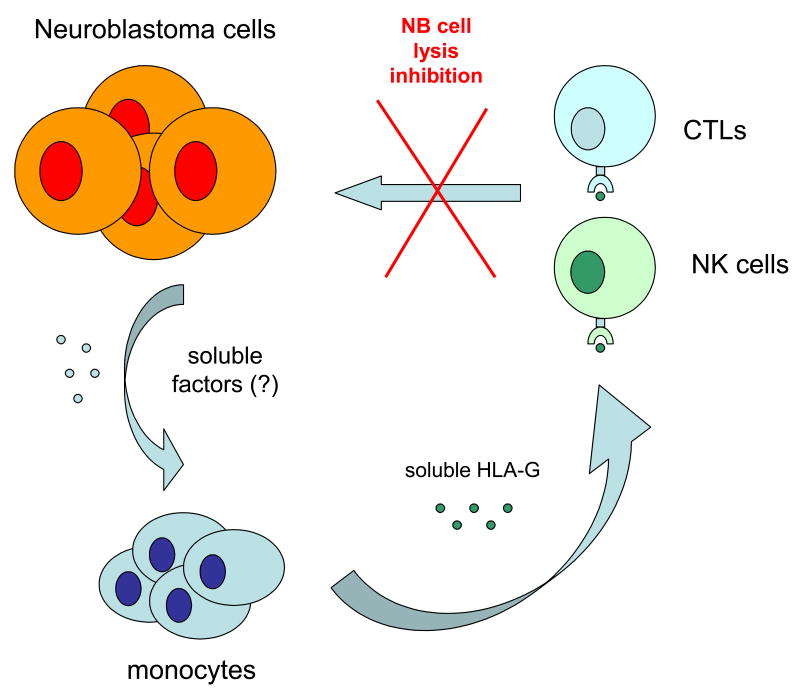
In view of the ability of NB cells to activate normal monocytes, it is noteworthy that when normal peripheral blood monocytes were incubated with NB cell line supernatants, IL-10 was not detected under any of the experimental conditions tested, whereas IL-12 was down-regulated in cells exposed to tumor cell supernatants as compared to cells cultured with medium alone [17]. Although this cytokine profile is unrelated to that of M1/M2 macrophages, NB cell induced downregulation of IL-12 production by monocytes may represent an additional immnosuppressive mechanism utilized by tumor cells to avoid immune recognition and destruction by the host's immune system. Thus, it is our working hypothesis that tumor cell mediated monocyte activation is a sort of “frustrated activation”, characterized simultaneously by activated immunophenotype and diminished IL-12 production. A cartoon depicting this novel mechanism is shown in Fig. 4.
Figure 4.
Schematic representation of a novel mechanism utilized by human neuroblastoma cells to elude the control of the host's immune system. Neuroblastoma cells release soluble factors that activate monocytes to upregulate synthesis and release of soluble HLA-G. This, in turn, inhibits CTL and NK cell mediated cytotoxicity against tumour cells [16].
Conclusions
The data we have reviewed clearly indicate that a significant amount of information has been gathered during the last few years about the functional characteristics of serum sHLA-G and its potential clinical relevance. Furthermore, the availability of standardized reagents and assays has eliminated the variability of the results obtained in different laboratories. This progress is expected to facilitate the comparison of results from various groups and to contribute significantly to a better understanding of the functional properties of sHLA-G. A number of questions remain to be addressed in order to define the role of sHLA-G in the regulation of immune responses and in the pathogenesis and clinical course of diseases with an immunological basis, as well as the usefulness of sHLA-G as a diagnostic and prognostic biomarker in pathological conditions. Therefore in this section we will summarize the most important conclusions derived from the review of the literature as well as from our own work and we will discuss some of the questions which in our opinion should be addressed.
Like other types of histocompatibility antigens (18,19), HLA-G are expressed not only on the cell surface, but also in body fluids. sHLA-G has been described thus far in serum, in amniotic fluid and in CSF. Whether it is also present in urine, tears and milk, like classical HLA class I antigens (18,19), remains to be determined. Several variables have been found to influence the level of sHLA-G in serum. However it is not known whether such level is correlated with that of other histocompatibility antigens and/or other molecule(s) with immunological function. Furthermore, do the differences in the serum level of sHLA-G have a functional significance? Which mechanism(s) regulate the serum level of sHLA-G? Especially answers to the latter question may suggest the design of strategies aimed at manipulating the serum level of sHLA-G in order to modulate the function of the immune system.
Serum levels of sHLA-G are increased in a number of pathological conditions and in some of them have been shown to be associated with the clinical course of the disease. These intriguing findings provide the rationale to investigate whether the changes in sHLA-G serum level play a role in the associated pathological condition or represent a non specific epiphenomenon without functional significance. Furthermore the suggestive evidence derived from studies performed in one center with a limited number of patients that sHLA-G serum levels may have diagnostic or prognostic significance emphasizes the need to implement multicentric studies with large numbers of patients in order to obtain conclusive results about the value of sHLA-G level in serum as a biomarker.
Convincing evidence derived from in vitro experiments indicates that like surface membrane bound HLA-G, sHLA-G in serum may provide tumor cells with multiple escape mechanisms. As discussed in this paper, our own studies have identified a novel escape mechanism that NB cells can utilize: it relies on the instruction of monocytes to release sHLA-G, thus downregulating tumor antigen-specific cell mediated immunity [17]. However, it will be important to determine the clinical significance of these in vitro findings. Besides contributing to our understanding of the mechanisms underlying tumor growth and disease progression in spite of a tumor antigen-specific immune response, this information may suggest the design of rational strategies to counteract the escape mechanisms utilized by tumor cells.
Acknowledgments
This study has been supported by grants from Fondazione Carige, Genova, and Ministero dells Salute, Progetti di ricerca corrente and by PHS grants RO1CA67108, RO1CA110249 and PO1CA109688 awarded by the National Cancer Institute, DHHS. F.M. is the recipient of a fellowship from Fondazione Italiana Ricerca sul Cancro (F.I.R.C.).
Abbreviations
- ALL
acute lymphoblastic leukemia
- AML
acute myeloid leukemia
- B-CLL
B cell-chronic lymphocytic leukemia
- CSF
cerebrospinal fluid
- GB
glioblastoma
- MM
multiple myeloma
- MS
multiple sclerosis
- NB
neuroblastoma
- NIND
non-inflammatory neurological disorders
- RA
rheumatoid arthritis
- SHLA-G
soluble HLA-G
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chang CC, Campoli M, Ferrone S. Classical and non-classical HLA class I antigen and NK cell activating ligand changes in malignant cells: current challenges and future directions. Adv Cancer Res. 2005;93:189–234. doi: 10.1016/S0065-230X(05)93006-6. [DOI] [PubMed] [Google Scholar]
- 2.Davidson B, Elstrand MB, McMaster MT, Berner A, Kurman RJ, Risberg B, et al. HLA-G expression in effusions is a possible marker of tumor susceptibility to chemotherapy in ovarian carcinoma. Gynecol Oncol. 2005;96:42–7. doi: 10.1016/j.ygyno.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 3.Rouas-Freiss N, Moreau P, Ferrone S, Carosella ED. HLA-G proteins in cancer: do they provide tumor cells with an escape mechanism? Cancer Res. 2005;65:10139–44. doi: 10.1158/0008-5472.CAN-05-0097. [DOI] [PubMed] [Google Scholar]
- 4.Barrier BF, Kendall BS, Sharpe-Timms KL, Kost ER. Characterization of human leukocyte antigen-G (HLA-G) expression in endometrial adenocarcinoma. Gynecol Oncol. 2006;103:25–30. doi: 10.1016/j.ygyno.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 5.Ishigami S, Natsugoe S, Miyazono F, Nakajo A, Tokuda K, Matsumoto M, et al. HLA-G expression in gastric cancer. Anticancer Res. 2006;26:2467–72. [PubMed] [Google Scholar]
- 6.Kleinberg L, Florenes VA, Skrede M, Dong HP, Nielsen S, McMaster MT, et al. Expression of HLA-G in malignant mesothelioma and clinically aggressive breast carcinoma. Virchows Arch. 2006;449:31–9. doi: 10.1007/s00428-005-0144-7. [DOI] [PubMed] [Google Scholar]
- 7.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T- cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 8.Rouas-Freiss N, Marchal RE, Kirszenbaum M, Dausset J, Carosella ED. The alpha1 domain of HLA-G1 and HLA-G2 inhibits cytotoxicity induced by natural killer cells: is HLA-G the public ligand for natural killer cell inhibitory receptors? Proc Natl Acad Sci USA. 1997;94:5249–54. doi: 10.1073/pnas.94.10.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Gal FA, Riteau B, Sedlik C, Khalil-Daher I, Menier C, Dausset J, et al. HLA-G-mediated inhibition of antigen-specific cytotoxic T lymphocytes. Int Immunol. 1999;11:1351–6. doi: 10.1093/intimm/11.8.1351. [DOI] [PubMed] [Google Scholar]
- 10.Bainbridge DR, Ellis SA, Sargent IL. HLA-G suppresses proliferation of CD4(+) T-lymphocytes. J Reprod Immunol. 2000;48:17–26. doi: 10.1016/s0165-0378(00)00070-x. [DOI] [PubMed] [Google Scholar]
- 11.van der Meer A, Lukassen HG, van Cranenbroek B, Weiss EH, Braat DD, van Lierop MJ, et al. Soluble HLA-G promotes Th1-type cytokine production by cytokine-activated uterine and peripheral natural killer cells. Mol Hum Reprod. 2007;13:123–33. doi: 10.1093/molehr/gal100. [DOI] [PubMed] [Google Scholar]
- 12.Bahri R, Hirsch F, Josse A, Rouas-Freiss N, Bidere N, Vasquez A, et al. Soluble HLA-G inhibits cell cycle progression in human alloreactive T lymphocytes. J Immunol. 2006;176:1331–9. doi: 10.4049/jimmunol.176.3.1331. [DOI] [PubMed] [Google Scholar]
- 13.LeMaoult J, Caumartin J, Daouya M, Favier B, Le Rond S, Gonzalez A, et al. Immune regulation by pretenders: cell-to-cell transfers of HLA-G make effector T cells act as regulatory cells. Blood. 2007;109:2040–8. doi: 10.1182/blood-2006-05-024547. [DOI] [PubMed] [Google Scholar]
- 14.Ristich V, Liang S, Zhang W, Wu J, Horuzsko A. Tolerization of dendritic cells by HLA-G. Eur J Immunol. 2005;35:1133–42. doi: 10.1002/eji.200425741. [DOI] [PubMed] [Google Scholar]
- 15.Kanai T, Fujii T, Kozuma S, Yamashita T, Miki A, Kikuchi A, et al. Soluble HLA-G influences the release of cytokines from allogeneic peripheral blood mononuclear cells in culture. Mol Hum Reprod. 2001;7:195–200. doi: 10.1093/molehr/7.2.195. [DOI] [PubMed] [Google Scholar]
- 16.Nückel H, Rebmann V, Dürig J, Dührsen U, Grosse-Wilde H. HLA-G expression is associated with an unfavorable outcome and immunodeficiency in chronic lymphocytic leukemia. Blood. 2005;105:1694–8. doi: 10.1182/blood-2004-08-3335. [DOI] [PubMed] [Google Scholar]
- 17.Morandi F, Levreri I, Bocca P, Galleni B, Raffaghello L, Ferrone S, et al. Human neuroblastoma cells trigger an immunosuppressive program in monocytes by stimulating soluble HLA-G release. Cancer Res. 2007;67:6433–41. doi: 10.1158/0008-5472.CAN-06-4588. [DOI] [PubMed] [Google Scholar]
- 18.Puppo F, Scudeletti M, Indiveri F, Ferrone S. Serum HLA class I antigens: markers and modulators of an immune response? Immunol Today. 1995;16:124–7. doi: 10.1016/0167-5699(95)80127-8. [DOI] [PubMed] [Google Scholar]
- 19.Puppo F, Indiveri F, Scudeletti M, Ferrone S. Soluble HLA antigens: new roles and uses. Immunol Today. 1997;18:154–155. doi: 10.1016/s0167-5699(97)84660-9. [DOI] [PubMed] [Google Scholar]
- 20.Ishitani A, Geraghty DE. Alternative splicing of HLA-G transcripts yields proteins with primary structures resembling both class I and class II antigens. Proc Natl Acad Sci USA. 1992;89:3947–51. doi: 10.1073/pnas.89.9.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujii T, Ishitani A, Geraghty DE. A soluble form of the HLA-G antigen is encoded by a messenger ribonucleic acid containing intron 4. J Immunol. 1994;153:5516–24. [PubMed] [Google Scholar]
- 22.Kirszenbaum M, Moreau P, Gluckman E, Dausset J, Carosella E. An alternatively spliced form of HLA-G mRNA in human trophoblasts and evidence for the presence of HLA-G transcript in adult lymphocytes. Proc Natl Acad Sci USA. 1994;91:4209–13. doi: 10.1073/pnas.91.10.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paul P, Cabestre FA, Ibrahim EC, Lefebvre S, Khalil-Daher I, Vazeux G, Quiles RM, Bermond F, Dausset J, Carosella ED. Identification of HLA-G7 as a new splice variant of the HLA-G mRNA and expression of soluble HLA-G5, -G6, and -G7 transcripts in human transfected cells. Hum Immunol. 2000;61:1138–49. doi: 10.1016/s0198-8859(00)00197-x. [DOI] [PubMed] [Google Scholar]
- 24.Rebmann V, Busemann A, Lindemann M, Grosse-Wilde H. Detection of HLA-G5 secreting cells. Hum Immunol. 2003;64:1017–24. doi: 10.1016/j.humimm.2003.08.354. [DOI] [PubMed] [Google Scholar]
- 25.Le Friec G, Laupeze B, Fardel O, Sebti Y, Pangault C, Guilloux V, et al. Soluble HLA-G inhibits human dendritic cell-triggered allogeneic T-cell proliferation without altering dendritic differentiation and maturation processes. Hum Immunol. 2003;64:752–61. doi: 10.1016/s0198-8859(03)00091-0. [DOI] [PubMed] [Google Scholar]
- 26.Le Rond S, Azema C, Krawice-Radanne I, Durrbach A, Guettier C, Carosella ED, et al. Evidence to support the role of HLA-G5 in allograft acceptance through induction of immunosuppressive/ regulatory T cells. J Immunol. 2006;176:3266–76. doi: 10.4049/jimmunol.176.5.3266. [DOI] [PubMed] [Google Scholar]
- 27.Zavazava N, Krönke M. Soluble HLA class I molecules induce apoptosis in alloreactive cytotoxic T lymphocytes. Nat Med. 1996;2:1005–10. doi: 10.1038/nm0996-1005. [DOI] [PubMed] [Google Scholar]
- 28.Puppo F, Contini P, Ghio M, Brenci S, Scudeletti M, Filaci G, Ferrone S, Indiveri F. Soluble human MHC class I molecules induce soluble Fas Ligand secretion and trigger apoptosis in activated CD8 (+) Fas (CD95) (+) T lymphocytes. Int Immunol. 2000;12:195–203. doi: 10.1093/intimm/12.2.195. [DOI] [PubMed] [Google Scholar]
- 29.Fournel S, Aguerre-Girr M, Huc X, Lenfant F, Alam A, Toubert A, et al. Cutting edge: soluble HLA-G1 triggers CD95/CD95 ligand-mediated apoptosis in activated CD8+ cells by interacting with CD8. J Immunol. 2000;164:6100–4. doi: 10.4049/jimmunol.164.12.6100. [DOI] [PubMed] [Google Scholar]
- 30.Contini P, Ghio M, Poggi A, Filaci G, Indiveri F, Ferrone S, et al. Soluble HLA-A,-B,-C and -G molecules induce apoptosis in T and NK CD8+ cells and inhibit cytotoxic T cell activity through CD8 ligation. Eur J Immunol. 2003;33:125–34. doi: 10.1002/immu.200390015. [DOI] [PubMed] [Google Scholar]
- 31.Hunt JS. Stranger in a strange land. Immunol Rev. 2006;213:36–47. doi: 10.1111/j.1600-065X.2006.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rouas-Freiss N, Khalil-Daher I, Riteau B, Menier C, Paul P, Dausset J, et al. The immunotolerance role of HLA-G. Semin Cancer Biol. 1999;9:3–12. doi: 10.1006/scbi.1998.0103. [DOI] [PubMed] [Google Scholar]
- 33.Rajagopalan S, Bryceson YT, Kuppusamy SP, Geraghty DE, van der Meer A, Joosten I, et al. Activation of NK cells by an endocytosed receptor for soluble HLA-G. PLoS Biol. 2006;4:e9. doi: 10.1371/journal.pbio.0040009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fons P, Chabot S, Cartwright JE, Lenfant F, L'Faqihi F, Giustiniani J, et al. Soluble HLA-G1 inhibits angiogenesis through an apoptotic pathway and by direct binding to CD160 receptor expressed by endothelial cells. Blood. 2006;108:2608–15. doi: 10.1182/blood-2005-12-019919. [DOI] [PubMed] [Google Scholar]
- 35.Le Bouteiller P, Fons P, Herault JP, Bono F, Chabot S, Cartwright JE, et al. Soluble HLA-G and control of angiogenesis. J Reprod Immunol. 2007 doi: 10.1016/j.jri.2007.03.007. in press. [DOI] [PubMed] [Google Scholar]
- 36.Rebmann V, Lemaoult J, Rouas-Freiss N, Carosella ED, Grosse-Wilde H. Report of the wet workshop for quantification of soluble HLA-G in Essen, 2004. Hum Immunol. 2005;66:853–63. doi: 10.1016/j.humimm.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Hackmon R, Hallak M, Krup M, Weitzman D, Sheiner E, Kaplan B, et al. HLA-G antigen and parturition: maternal serum, fetal serum and amniotic fluid levels during pregnancy. Fetal Diagn Ther. 2004;19:404–9. doi: 10.1159/000078992. [DOI] [PubMed] [Google Scholar]
- 38.Rebmann V, LeMaoult J, Rouas-Freiss N, Carosella ED, Grosse-Wilde H. Quantification and identification of soluble HLA-G isoforms. Tissue Antigens. 2007;69 1:143–9. doi: 10.1111/j.1399-0039.2006.763_5.x. [DOI] [PubMed] [Google Scholar]
- 39.Rudstein-Svetlicky N, Loewenthal R, Horejsi V, Gazit E. HLA-G levels in serum and plasma. Tissue Antigens. 2007;69 1:140–2. doi: 10.1111/j.1399-0039.2006.763_4.x. [DOI] [PubMed] [Google Scholar]
- 40.Hviid TV, Rizzo R, Christiansen OB, Melchiorri L, Lindhard A, Baricordi OR. HLA-G and IL-10 in serum in relation to HLA-G genotype and polymorphisms. Immunogenetics. 2004;56:135–41. doi: 10.1007/s00251-004-0673-2. [DOI] [PubMed] [Google Scholar]
- 41.Rizzo R, Hviid TV, Stignani M, Balboni A, Grappa MT, Melchiorri L, et al. The HLA-G genotype is associated with IL-10 levels in activated PBMCs. Immunogenetics. 2005;57:172–81. doi: 10.1007/s00251-005-0788-0. [DOI] [PubMed] [Google Scholar]
- 42.Hunt JS, Jadhav L, Chu W, Geraghty DE, Ober C. Soluble HLA-G circulates in maternal blood during pregnancy. Am J Obstet Gynecol. 2000;183:682–8. doi: 10.1067/mob.2000.106762. [DOI] [PubMed] [Google Scholar]
- 43.Sher G, Keskintepe L, Nouriani M, Roussev R, Batzofin J. Expression of sHLA-G in supernatants of individually cultured 46-h embryos: a potentially valuable indicator of ‘embryo competency’ and IVF outcome. Reprod Biomed Online. 2004;9:74–8. doi: 10.1016/s1472-6483(10)62113-x. [DOI] [PubMed] [Google Scholar]
- 44.Rebmann V, Switala M, Eue I, Schwahn E, Merzenich M, Grosse-Wilde H. Rapid evaluation of soluble HLA-G levels in supernatants of in vitro fertilized embryos. Hum Immunol. 2007;68:251–8. doi: 10.1016/j.humimm.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Amato MP, Zipoli V, Portaccio E. Multiple sclerosis-related cognitive changes: a review of cross-sectional and longitudinal studies. J Neurol Sci. 2006;245:41–6. doi: 10.1016/j.jns.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 46.Wiendl H, Feger U, Mittelbronn M, Jack C, Schreiner B, Stadelmann C, et al. Expression of the immune-tolerogenic major histocompatibility molecule HLA-G in multiple sclerosis: implications for CNS immunity. Brain. 2005;128:2689–704. doi: 10.1093/brain/awh609. [DOI] [PubMed] [Google Scholar]
- 47.Fainardi E, Rizzo R, Melchiorri L, Vaghi L, Castellazzi M, Marzola A, et al. Presence of detectable levels of soluble HLA-G molecules in CSF of relapsing-remitting multiple sclerosis: relationship with CSF soluble HLA-I and IL-10 concentrations and MRI findings. J Neuroimmunol. 2003;142:149–58. doi: 10.1016/s0165-5728(03)00266-2. [DOI] [PubMed] [Google Scholar]
- 48.Fainardi E, Rizzo R, Melchiorri L, Castellazzi M, Paolino E, Tola MR, et al. Intrathecal synthesis of soluble HLA-G and HLA-I molecules are reciprocally associated to clinical and MRI activity in patients with multiple sclerosis. Mult Scler. 2006;12:2–12. doi: 10.1191/1352458506ms1241oa. [DOI] [PubMed] [Google Scholar]
- 49.Verbruggen LA, Rebmann V, Demanet C, De Cock S, Grosse-Wilde H. Soluble HLA-G in rheumatoid arthritis. Hum Immunol. 2006;67:561–7. doi: 10.1016/j.humimm.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 50.Tahan F, Patiroglu T. Plasma soluble human leukocyte antigen G levels in asthmatic children. Int Arch Allergy Immunol. 2006;141:213–6. doi: 10.1159/000095290. [DOI] [PubMed] [Google Scholar]
- 51.Torres MI, Lopez-Casado MA, Luque J, Pena J, Rios A. New advances in coeliac disease: serum and intestinal expression of HLA-G. Int Immunol. 2006;18:713–8. doi: 10.1093/intimm/dxl008. [DOI] [PubMed] [Google Scholar]
- 52.Qiu J, Terasaki PI, Miller J, Mizutani K, Cai J, Carosella ED. Soluble HLA-G expression and renal graft acceptance. Am J Transplant. 2006;6:2152–6. doi: 10.1111/j.1600-6143.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- 53.Lila N, Amrein C, Guillemain R, Chevalier P, Latremouille C, Fabiani JN, et al. Human leukocyte antigen-G expression after heart transplantation is associated with a reduced incidence of rejection. Circulation. 2002;105:1949–54. doi: 10.1161/01.cir.0000015075.89984.46. [DOI] [PubMed] [Google Scholar]
- 54.Basturk B, Karakayali F, Emiroglu R, Sozer O, Haberal A, Bal D, et al. Human leukocyte antigen-G, a new parameter in the follow-up of liver transplantation. Transplant Proc. 2006;38:571–4. doi: 10.1016/j.transproceed.2005.12.108. [DOI] [PubMed] [Google Scholar]
- 55.Luque J, Torres MI, Aumente MD, Marin J, Garcia-Jurado G, Gonzalez R, et al. Soluble HLA-G in heart transplantation: their relationship to rejection episodes and immunosuppressive therapy. Hum Immunol. 2006;67:257–63. doi: 10.1016/j.humimm.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 56.Wiendl H, Mitsdoerffer M, Hofmeister V, Wischhusen J, Bornemann A, Meyermann R, et al. A functional role of HLA-G expression in human gliomas: an alternative strategy of immune escape. J Immunol. 2002;168:4772–80. doi: 10.4049/jimmunol.168.9.4772. [DOI] [PubMed] [Google Scholar]
- 57.Rebmann V, Regel J, Stolke D, Grosse-Wilde H. Secretion of sHLA-G molecules in malignancies. Semin Cancer Biol. 2003;13:371–7. doi: 10.1016/s1044-579x(03)00028-2. [DOI] [PubMed] [Google Scholar]
- 58.Amiot L, Le Friec G, Sebti Y, Drenou B, Pangault C, Guilloux V, et al. HLA-G and lymphoproliferative disorders. Semin Cancer Biol. 2003;13:379–85. doi: 10.1016/s1044-579x(03)00029-4. [DOI] [PubMed] [Google Scholar]
- 59.Gros F, Sebti Y, de Guibert S, Branger B, Bernard M, Fauchet R, et al. Soluble HLA-G molecules increase during acute leukemia, especially in subtypes affecting monocytic and lymphoid lineages. Neoplasia. 2006;8:223–30. doi: 10.1593/neo.05703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ugurel S, Rebmann V, Ferrone S, Tilgen W, Grosse-Wilde H, Reinhold U. Soluble human leukocyte antigen--G serum level is elevated in melanoma patients and is further increased by interferon-alpha immunotherapy. Cancer. 2001;92:369–76. doi: 10.1002/1097-0142(20010715)92:2<369::aid-cncr1332>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 61.Leleu X, Le Friec G, Facon T, Amiot L, Fauchet R, Hennache B, et al. Total soluble HLA class I and soluble HLA-G in multiple myeloma and monoclonal gammopathy of undetermined significance. Clin Cancer Res. 2005;11:7297–303. doi: 10.1158/1078-0432.CCR-05-0456. [DOI] [PubMed] [Google Scholar]
- 62.Prigione I, Facchetti P, Lanino E, Garaventa A, Pistoia V. Clonal analysis of peripheral blood lymphocytes from three patients with advanced neuroblastoma receiving recombinant interleukin-2 and interferon alpha. Cancer Immunol Immunother. 1993;37:40–6. doi: 10.1007/BF01516940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gambini C, Conte M, Bernini G, Angelini P, Pession A, Paolucci P, et al. Neuroblastic tumors associated with opsoclonus-myoclonus syndrome: histological, immunohistochemical and molecular features of 15 Italian cases. Virchows Arch. 2003;442:555–62. doi: 10.1007/s00428-002-0747-1. [DOI] [PubMed] [Google Scholar]
- 64.Raffaghello L, Prigione I, Airoldi I, Camoriano M, Levreri I, Gambini C, et al. Downregulation and/or release of NKG2D ligands as immune evasion strategy of human neuroblastoma. Neoplasia. 2004;6:558–68. doi: 10.1593/neo.04316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raffaghello L, Prigione I, Airoldi I, Camoriano M, Morandi F, Bocca P, et al. Mechanisms of immune evasion of human neuroblastoma. Cancer Lett. 2005;228:155–61. doi: 10.1016/j.canlet.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 66.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–16. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 67.Moreau P, Mouillot G, Rousseau P, Marcou C, Dausset J, Carosella ED. HLA-G gene repression is reversed by demethylation. Proc Natl Acad Sci USA. 2003;100:1191–6. doi: 10.1073/pnas.0337539100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moreau P, Adrian-Cabestre F, Menier C, Guiard V, Gourand L, Dausset J, et al. IL-10 selectively induces HLA-G expression in human trophoblasts and monocytes. Int Immunol. 1999;11:803–11. doi: 10.1093/intimm/11.5.803. [DOI] [PubMed] [Google Scholar]
- 69.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 70.Steinbrink K, Jonuleit H, Muller G, Schuler G, Knop J, Enk AH. Interleukin-10-treated human dendritic cells induce a melanoma-antigen-specific anergy in CD8(+) T cells resulting in a failure to lyse tumor cells. Blood. 1999;93:1634–42. [PubMed] [Google Scholar]
- 71.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]