Abstract
Objective
To examine changes in interhemispheric inhibition (IHI) during homologous muscle activation in healthy subjects and in people with hemiparesis.
Methods
IHI in the abductor pollicus brevis (APB) muscle was examined using paired transcranial magnetic stimulation. Stimuli were delivered while the target APB was at rest or activated, and while the non-target contralateral APB was at rest or activated.
Results
In control subjects, IHI in the resting target APB was enhanced during activation of the contralateral APB, and was greater from the dominant hemisphere to the non-dominant. In stroke subjects, IHI in the non-affected APB was not modulated during voluntary activation of the affected APB, but was influenced by the prior dominance of affected hemisphere. Bilateral muscle activation did not elicit any changes in IHI in either group.
Conclusions
IHI is asymmetrical between hemispheres but only when the target muscle is at rest. Subjects with stroke have an impaired ability to modulate IHI during unilateral muscle activation.
Significance
In people with stroke, the extent and modulation of interhemispheric transfer is influenced by the prior dominance of the affected hemisphere. This may impact on the efficacy of treatment interventions incorporating bilateral activation.
Keywords: hemiparesis, interhemispheric inhibition, transcranial magnetic stimulation, bilateral muscle activation
Introduction
Several studies have reported that motor pathway excitability to a resting target muscle is increased when the homologous muscle on the opposite side of the body is activated (Hess et al., 1986; Stedman et al., 1998; Tinazzi and Zanette, 1998; Muellbacher et al., 2000; Stinear et al., 2001; Woldag et al., 2004). This facilitation is thought to predominantly arise through neural pathways acting at the spinal cord (Muellbacher et al., 2000). In addition, Ferbert et al. (1992) demonstrated increased interhemispheric inhibition (IHI) directed to a target muscle during unilateral activation of the homologous muscle on the contralateral side. It has been suggested that this increase in inhibition functions to maintain a focal activation of the opposite hemisphere, so that homologous muscles are not activated simultaneously (Kobayashi et al., 2003).
Changes in neural excitability that occur during homologous muscle activation while the target muscle is also activated, i.e. bilateral muscle activation, have not been extensively studied. Such bilateral actions involving simultaneous contraction of homologous muscles have received support as an intervention to enhance motor performance in individuals with post-stroke hemiparesis (Stewart et al., 2006). In a study using single-pulse transcranial magnetic stimulation (TMS), Renner et al. (2005) reported that bilateral muscle activation gave rise to a facilitation of motor pathway excitability in people with stroke that was not seen in healthy control subjects. To further investigate this finding, the aim of the present study was to examine the excitability of interhemispheric pathways during unilateral and bilateral muscle activation in healthy subjects and in people with post-stroke hemiparesis. Dual-pulse TMS was used to measure the excitability of interhemispheric pathways when cortical motor areas in the opposite hemisphere are engaged voluntarily. It was hypothesized that activation of one cortical hemisphere would increase IHI directed to the opposite hemisphere, but that the extent of inhibition would be influenced by whether the test hemisphere was at rest or pre-activated. In addition, following the results of Renner et al. (2005), we predicted that IHI would be reduced in stroke subjects during bilateral activation.
We also investigated the influence of lesion location on IHI in the subjects with stroke. Previous studies have indicated a more substantial contribution of the dominant motor cortex in the control of bilateral motor actions (Leiguarda and Marsden, 2000; Verstynen et al., 2005) as well as asymmetries in the level of IHI between the two hemispheres (Netz et al., 1995; Kobayashi et al., 2003). Therefore, we hypothesized that, if interhemispheric transfer is implicated in bilateral control, modulation of IHI during bilateral activation would be reduced in individuals with lesions in the previously dominant hemisphere.
Materials and Methods
Subjects
Nine healthy subjects (mean age 62±8 years, range 47-74; one left-handed [Oldfield, 1971]) and 16 people with post-stroke hemiparesis volunteered to participate in the study. Subjects with stroke (see Table 1 and Figure 1 for details) were required to have had a single unilateral stroke at least one year previously and to have residual deficits in upper limb function. We focused on responses in the non-affected hand of the stroke subjects. This meant that we were not restricted to well-recovered subjects in whom responses to TMS could be elicited in the affected arm and, consequently, subjects with a range of functional recovery levels could be included. One individual with left hemiparesis was left-hand dominant prior to stroke (P10); the remainder were previously right-hand dominant. Subjects from both groups were excluded if they had contraindications to TMS or any orthopaedic limitations of either upper limb. Ethical approval was obtained from the Northwestern University Institutional Review Board and all subjects provided informed written consent prior to participation. The study was conducted in accordance with the Declaration of Helsinki.
Table 1.
Characteristics of the stroke subject group.
| ID | Age | Sex | Years PS | FMA | Affected Side |
|---|---|---|---|---|---|
| P1 | 59 | M | 6 | 43 | L |
| P2 | 66 | F | 16 | 34 | R |
| P3 | 60 | F | 6 | 43 | L |
| P4 | 54 | F | 10 | 47 | L |
| P5 | 35 | F | 15 | 27 | R |
| P6 | 70 | M | 8 | 43 | L |
| P7 | 76 | M | 12 | 30 | R |
| P8 | 56 | F | 8 | 10 | L |
| P9 | 65 | M | 13 | 24 | L |
| P10* | 49 | M | 4 | 20 | L |
| P11 | 61 | M | 6 | 28 | R |
| P12 | 63 | M | 25 | 14 | L |
| P13 | 47 | M | 6 | 24 | R |
| P14 | 67 | M | 11 | 49 | R |
| P15 | 48 | M | 2 | 31 | L |
| P16 | 61 | M | 3 | 29 | L |
| Average | 59±10 years | 11 M | 10±6 years | 31±12 | 6 R |
Years PS = years post-stroke; FMA = Fugl Meyer Assessment (out of 60); M = male; F = female; L = left; R = right.
left hand dominant prior to stroke
Figure 1.
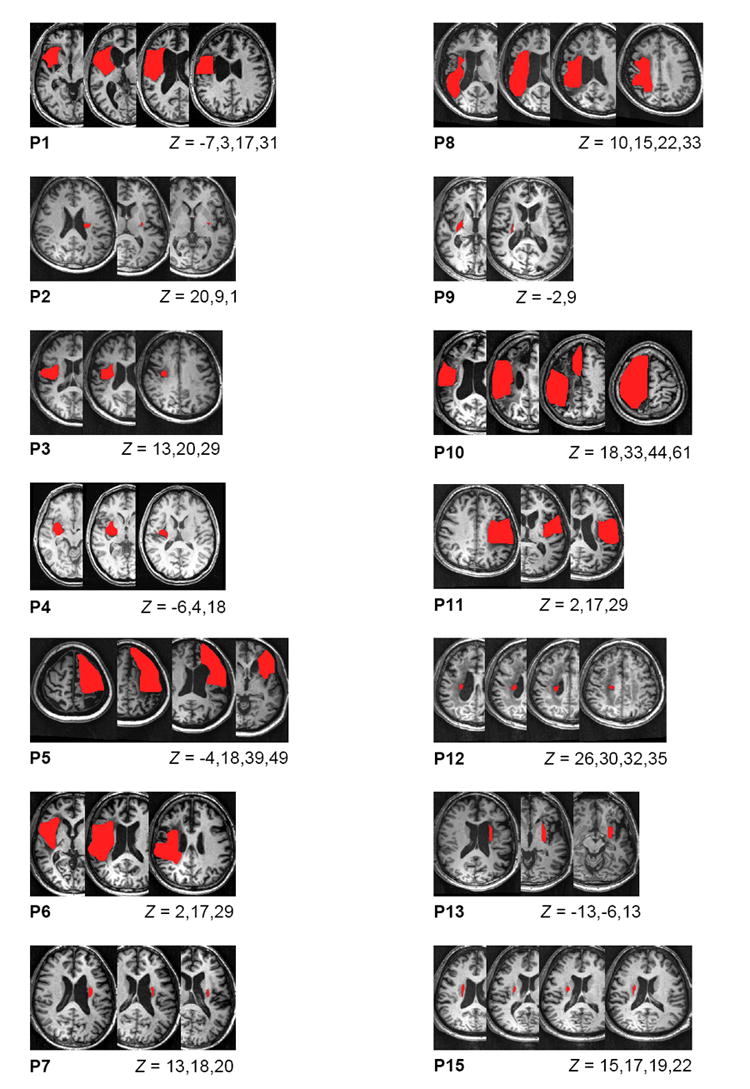
MR images showing lesion location (shaded area) for the stroke subjects. The Montreal Neurological Institute (MNI) coordinates for each slice are indicated below the figures. MRI scans were not available for subjects P14 and P16.
Electromyography
Self-adhesive dual electrodes (Noraxon USA Inc, AZ) were applied over the abductor pollicus brevis (APB) muscle in each hand following standard skin preparation techniques. Electromyographic (EMG) signals were amplified and conditioned using a Bortec AMT-8 (Bortec Biomedical Ltd, Canada) with high- and low-pass cut-off frequencies of 10 and 1000 Hz, respectively. The resulting signals were sampled at 5 kHz for subsequent analysis. At the start of the test session, a maximum voluntary contraction (MVC) of each APB was recorded by having the subjects perform three maximum isometric activations. The maximum EMG root mean square (rms) value from these activations was set as MVC.
Transcranial Magnetic Stimulation
TMS was applied using two Magstim 2002 (Magstim Company, Wales) units with two 70 mm figure-of-eight coils. The “hot spot” for the APB was located by moving the coil over the scalp until the site eliciting motor evoked potentials (MEPs) of the largest amplitude was determined. With the coil over the hot spot, resting motor threshold (RTh) was established as the lowest stimulus intensity that gave rise to a MEP greater than 50 μV in at least four of a train of eight stimuli. This was determined using intensity steps of 1% maximum stimulator output. To examine IHI, separate coils were held over the hot spots for the left and right APB muscles. Stimuli were delivered though the two coils with an inter-stimulus interval of 10 ms (Ferbert et al., 1992; Daskalakis et al., 2002).
Protocol
We examined IHI while the target APB muscle was at rest and while it was activated at 5% MVC. In both of these states, IHI was tested while the non-target APB was either at rest or activated at 5% MVC. Therefore, we examined the influence of voluntary activation of the non-target APB while the target APB was at rest and while it was pre-activated. In the stroke subjects, the test hemisphere was always the non-affected hemisphere and responses were recorded in the non-affected limb. In the control subjects the protocol was performed twice so that both hemispheres served as the test hemisphere.
During data collection subjects were seated comfortably on a chair in front of a computer monitor. Visual feedback of EMG activity from both APB muscles and the target levels of activation were displayed on the monitor. Stimuli were delivered when the APB muscles were at the required level of MVC for at least 100 ms. Conditioning stimulus intensity was set to 120% RTh. As the extent of IHI elicited is influenced by the size of the non-conditioned MEP (Daskalakis et al., 2002), individual test stimulus intensity was adjusted so that non-conditioned MEP amplitude was matched for the two conditions where the target APB was at rest and for the two conditions where the target APB was at 5% MVC. Table 2 displays the stimulus intensities required to match test MEP amplitude between relevant conditions. Ten conditioned and ten non-conditioned stimuli were randomly delivered in each muscle activation combination.
Table 2.
Rest threshold (RTh), stimulation intensities, and motor evoked potential (MEP) amplitudes for control and stroke groups. Intensities are reported as a percentage of maximum stimulator output. Muscle activation condition is shown as target:non-target muscle.
| Controls | Stroke | ||
|---|---|---|---|
| Dominant | Non-Dominant | ||
| RTh (%) | 42±8 | 46±9 | 52±11 |
| Conditioning Stimulus (%) | 58±9 | 53±10 | 76±10 |
| Test Stimulus (%) | |||
| Rest:Rest | 56±10 | 61±8 | 67±14 |
| Rest:Active | 54±10 | 56±9 | 65±14 |
| Active:Rest | 53±11 | 59±9 | 65±12 |
| Active:Active | 53±9 | 59±8 | 64±12 |
| Test MEP Amplitude (mV) | |||
| Rest:Rest | 0.83±0.61 | 1.3±0.7 | 1.7±1.9 |
| Rest:Active | 0.80±0.55 | 1.4±0.9 | 1.5±1.5 |
| Active:Rest | 2.0±0.9 | 2.8±1.0 | 3.8±4.7 |
| Active:Active | 2.2±1.1 | 2.8±1.0 | 4.0±4.7 |
Data Processing and Analysis
MEPs elicited in each experimental condition were averaged. From the averaged response, the maximum peak-to-peak MEP amplitude and the level of background EMG (rms amplitude) in a 30 ms window prior to stimulation were obtained. Conditioned MEP amplitude was expressed relative to non-conditioned to provide a measure of IHI. In the control subjects, IHI was analyzed using a two-way hand (dominant, non-dominant) × non-target muscle condition (relaxed, activated) ANOVA. Separate ANOVAs were completed for data obtained when the target APB was at rest and activated. IHI in the stroke group was compared between muscle activation conditions using paired t-tests. Significance is reported at P < 0.05. Results in the text are shown as mean±standard deviation.
Results
Interhemispheric inhibition directed to the target APB was influenced by activation of the non-target APB, but only in the healthy control subjects and only when the target muscle was at rest. Figures 2A and 3A show example averaged MEPs from individual control and stroke subjects. In the control subject, activation of the non-target APB increased the level of IHI when the target APB was at rest, in line with results of previous studies. The right side of Figure 2A illustrates that IHI was not modulated when the target APB was pre-activated. In the stroke subject, activation of the non-target APB had no effect on IHI whether the target APB was at rest or pre-activated. Group results are presented in Figures 2B and 3B.
Figure 2.
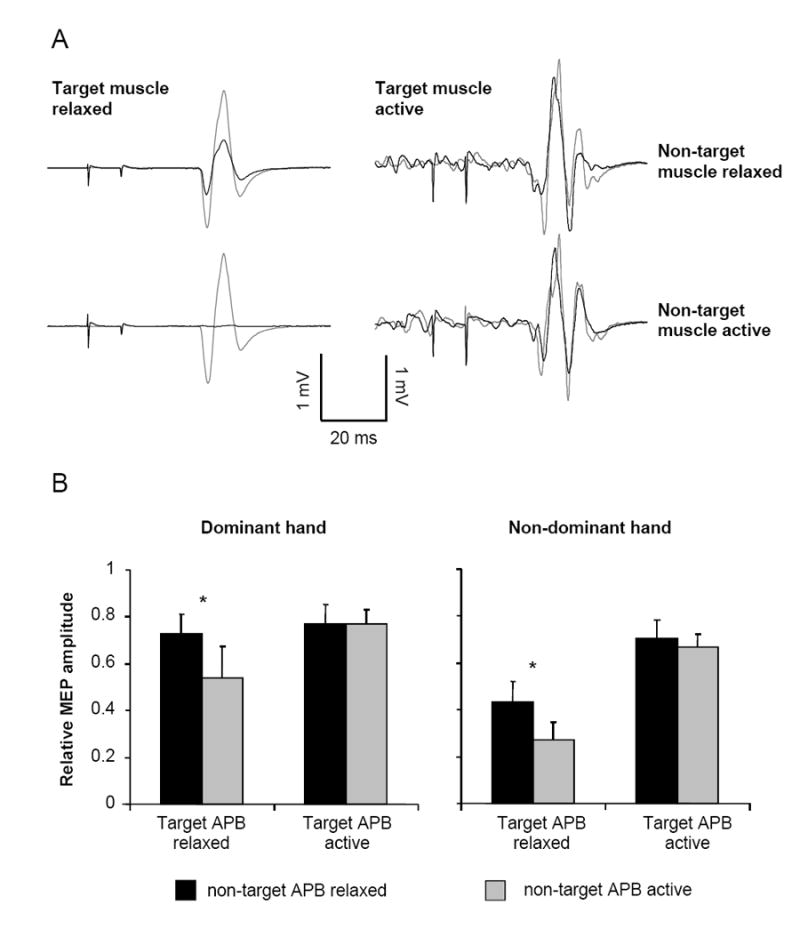
A. Example motor evoked potentials (MEPs; average of 10 stimuli) from the non-dominant APB of individual control subjects showing the response to non-conditioned (grey) and conditioned (black) stimulation. The responses on the left are with the target APB muscle at rest. The responses on the right are with the target APB at 5% maximum voluntary contraction (MVC). Responses obtained with the non-target (contralateral) APB at rest are in the top row and responses obtained with the non-target APB at 5% MVC are in the bottom row. B. Group data for the control subjects showing the extent of interhemispheric inhibition in the dominant and non-dominant hands. Relative MEP amplitude is determined as conditioned/non-conditioned MEP amplitude. Bars represent one standard error of the mean. * P < 0.05.
Figure 3.
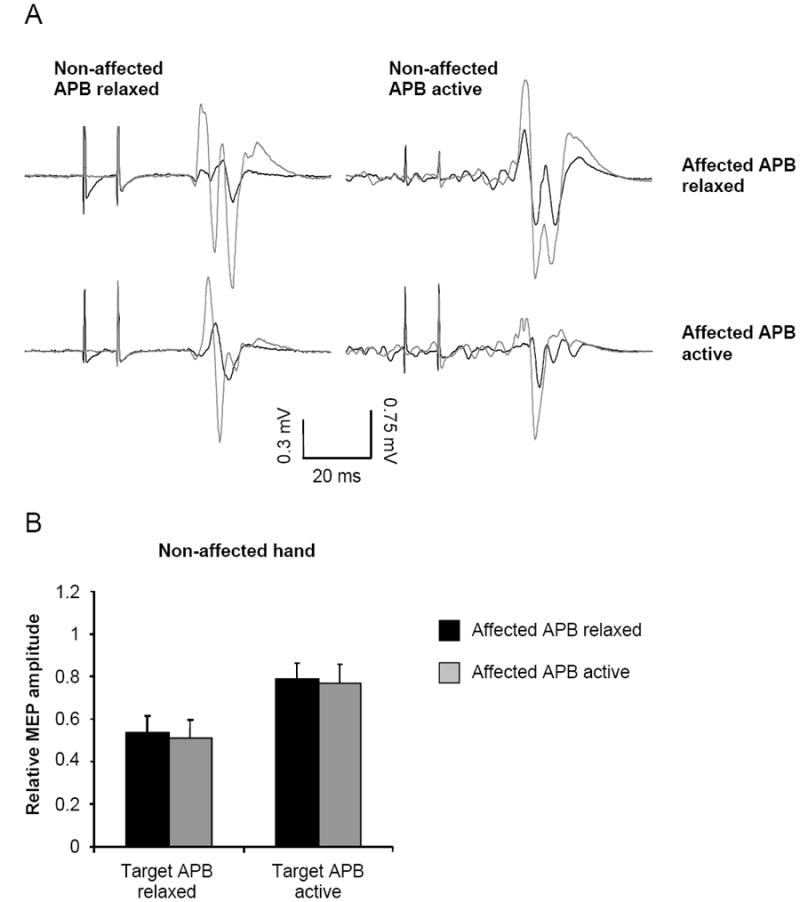
A. Example motor evoked potentials (MEPs; average of 10 stimuli) from a subject with stroke (P5) showing non-conditioned (grey) and conditioned (black) responses. The responses on the left are with the target APB muscle at rest. The responses on the right are with the target APB at 5% maximum voluntary contraction (MVC). Responses obtained with the affected APB at rest are in the top row and responses obtained with the affected APB at 5% MVC are in the bottom row. B. Group data for the stroke subjects showing the extent of interhemispheric inhibition. Relative MEP amplitude is determined as conditioned/non-conditioned MEP amplitude. Bars represent one standard error of the mean.
Control Subjects
Significant modulation of IHI occurred when the target APB was at rest but not when it was pre-activated. While at rest, the level of IHI in the target APB was increased during activation of the non-target APB compared to both APB at rest (F1,8 = 8.3, P = 0.01). That is, when the non-target APB was activated, inhibition directed to the resting target APB was increased. Also, a main effect of hand (F1,8 = 5.37, P = 0.03) revealed that the level of inhibition directed to the non-dominant APB was greater than that directed to the dominant APB. The interaction between hand and non-target muscle activation was not significant (F1,8 = 0.04, P = 0.8).
There were no significant main effects or interactions detected in the level of IHI when the target APB was pre-activated at 5% MVC (all P > 0.3). Therefore, there was no difference in IHI between unilateral or bilateral muscle activation, or between the dominant and non-dominant hands. Similar ANOVAs investigating non-conditioned MEP amplitude and background EMG did not reveal any significant effects in the rest or active conditions (all P > 0.3), indicating that test MEP amplitudes and background EMG were matched sufficiently.
Stroke Subjects
Responses to TMS were elicited in the non-affected APB of all stroke subjects. In the affected APB, responses in the resting muscle were elicited in four subjects at stimulus intensities < 70% of maximum stimulator output (MSO). In the remaining subjects we arbitrarily set the rest threshold at 70% MSO and conditioning stimulus intensity to 84% MSO (120% RTh). None of the subjects with stroke displayed overt mirror movements and all were able to complete the uni- and bi-lateral motor tasks as required.
Figure 3B shows that IHI was not modulated in the patient group during activation of the contralateral (affected) APB when the target APB was at rest or when it was activated (both P = 0.8). There were no significant differences in the test MEP amplitude or background EMG between conditions of comparison (all P > 0.2). Overall, the level of IHI in the stroke subjects (rest = 0.52±0.32; active = 0.78±0.32) was comparable to that in the control subjects (rest = 0.49±0.30; active = 0.73±0.20).
Effect of Lesion Location
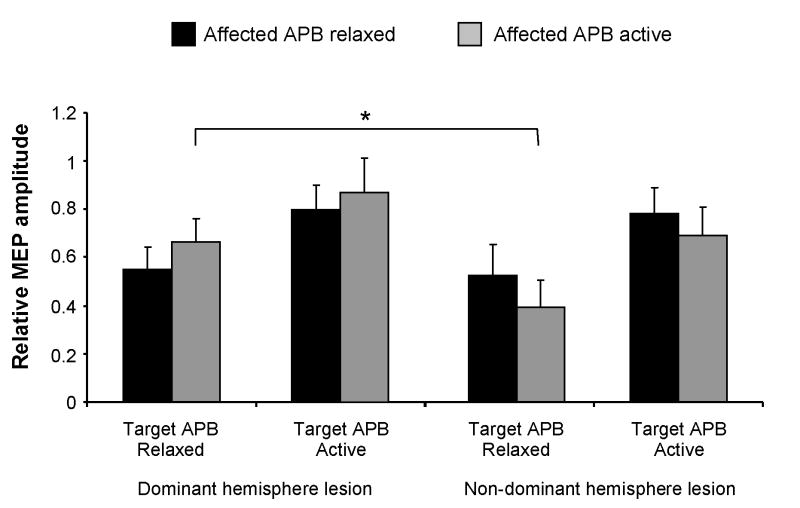
We analyzed the level of IHI with the stroke subjects separated into those with lesions in the dominant (n = 7) and non-dominant hemisphere (n = 9). Figure 4A shows the level of IHI when the target APB was at rest. IHI was comparable in subjects with lesions in the dominant and non-dominant hemispheres when the affected APB was also at rest (P = 0.8). When the affected APB was activated unilaterally, IHI was significantly greater in subjects with lesions in the non-dominant hemisphere (P = 0.04). Figure 4B shows the level of IHI when the target APB was activated. There was no difference in IHI between the two sub-populations when the affected APB was at rest (P = 0.9) or during bilateral APB activation (P = 0.4).
Figure 4.
Group data showing interhemispheric inhibition in the stroke subjects separated into those with lesions in the dominant and non-dominant hemispheres. Relative motor evoked potential (MEP) amplitude is determined as conditioned/non-conditioned response amplitude. Bars are one standard error of the mean. * P < 0.05.
Correlations with Motor Impairment
We correlated the level of IHI elicited at rest with the FMA to examine if the degree of motor impairment influenced extent of interhemispheric transfer. This relationship was not significant (R = -0.24, P = 0.4). As the modulation of IHI during activation of the affected APB was altered in the stroke group, we also correlated the individual change in IHI with the FMA. Again, this relationship did not reach significance (R = -0.12, P = 0.7).
Discussion
The purpose of this study was to investigate the excitability of interhemispheric pathways during voluntary activation of muscles on the opposite side of the body, and to examine how these pathways are influenced by lesion location in individuals with hemiparesis following stroke. In subjects with a healthy nervous system, we found that: 1) activation of the non-target APB increased IHI directed to the homologous target APB, but only when the target muscle was at rest; and 2) greater IHI was directed to the non-dominant APB compared to the dominant, but, again, only when the target muscle was at rest. In the non-affected APB of the stroke subjects: 1) modulation of IHI with activation of the non-target (affected) APB was not detected; and 2) during activation of the affected (contralateral) APB, greater IHI was elicited in stroke subjects with an intact dominant hemisphere, but only when the target muscle was at rest.
Effect of Voluntary Muscle Activation in Control Subjects
In one of the pioneering studies using paired-pulse TMS to examine IHI, Ferbert et al. (1992) demonstrated that the level of IHI directed to a resting target muscle was modulated by voluntary activation of the homologous muscle on the opposite side of the body. In the current study, we re-examined these findings with more controlled muscle activation and also matched test MEP amplitudes between conditions of comparison. With the target muscle at rest, activation of the opposite APB enhanced IHI directed to the target hemisphere, in accordance with the earlier findings. In contrast to these results at rest, we report that activation of the non-target APB did not increase IHI when the target muscle itself was activated. Thus, bilateral activation did not elicit any changes to the level of inhibition between hemispheres. Potentially, the larger test MEP amplitudes in the active target muscle conditions may have reduced the level of IHI that could be achieved compared to the resting muscle conditions (Daskalakis et al., 2002). However, there is no evidence to suggest that the modulation of IHI would be reduced with these larger responses. If the target muscle is pre-activated, it would be inappropriate to enhance inhibition directed to this muscle when the opposite hemisphere is voluntarily engaged. The lack of change in IHI during bilateral activation may serve to facilitate output from the two cortical hemispheres and promote symmetric activation.
We noted asymmetries in the extent of IHI that could be elicited in the dominant and non-dominant hemispheres while the target muscle was at rest. Similar to previous studies, we found that inhibition from the dominant to the non-dominant hemisphere was greater than vice versa (Netz et al., 1995; Kobayashi et al., 2003; Duque et al., 2007). Duque et al. (2007) proposed that this asymmetry in interhemispheric transfer may contribute to intermanual differences in dexterity in healthy subjects. All of these previous studies examined IHI while the target muscle was at rest. In the current study we show that the asymmetry in IHI is not present when the target muscle is pre-activated. This finding may question the relevance of asymmetries in interhemispheric transfer to manual dexterity differences between the hands when performing active motor tasks.
IHI in Subjects with Stroke
While the overall level of IHI in the stroke subjects was similar to the controls, there was no modulation in IHI during activation of the affected hand. Other studies have reported that IHI from the affected to non-affected hemisphere in stroke subjects is comparable to healthy control subjects while at rest (Boroojerdi et al., 1996; Duque et al., 2005) or during a pre-movement period (Murase et al., 2004). This is the first study to show a lack of change in IHI during activation of the homologous muscle in a stroke population. That this change in IHI is deficient in the stroke subjects indicates that the modulation seen in controls is likely generated by the hemisphere controlling voluntary activation, which was the affected hemisphere in our study. This supports the idea that, during activation of the homologous muscle, transcallosal inhibition is passed from the activated hemisphere to the opposite, resting hemisphere to facilitate suppression of unwanted motor activity (Muellbacher et al., 2000). Our findings in the stroke subjects could be influenced by the level of activation achieved by the affected APB. We attempted to standardize activation by using a percentage of MVC. It is likely that the absolute levels of activation varied among the stroke group and were reduced in comparison to controls. The lack of correlation between clinical scores and our measures of IHI suggests that this was not a major influencing factor. Also, the interstimulus-interval between the conditioning and test stimuli may not have been optimal for all stroke subjects. For example, some subjects may have delayed conduction times in transcallosal pathways. Following previous studies (Murase et al., 2004; Duque et al., 2005), we decided to standardize the inter-stimulus interval for all subjects rather than attempting to optimize this individually.
A further novel finding in the present study was that the extent of IHI in the stroke subjects was influenced by the prior dominance of the affected hemisphere. Subjects with lesions in the non-dominant hemisphere displayed greater inhibition to the target APB when the affected APB was activated. Therefore, in the control subjects we found greater IHI from the dominant to the non-dominant hemisphere, and in the stroke subjects there was greater IHI in individuals with an intact dominant hemisphere. These findings strongly suggest that the side of lesion will influence the extent of interhemispheric transfer following stroke. As bilateral training has been promoted as an intervention following stroke, it would therefore be useful to determine how side of lesion may influence the outcome of bilateral-based interventions (McCombe Waller and Whitall, 2005). We did not detect any hemispheric differences in IHI in control subjects when the target muscle was pre-activated, and we also did not detect any differences in IHI between stroke subjects with lesions in their dominant and non-dominant hemispheres when the target muscle was pre-activated. This supports our contention that asymmetries in interhemispheric transfer are less influential during voluntary muscle activation.
Similar to control subjects, there was no change in IHI during bilateral compared to unilateral activation of the target APB alone. This outcome did not support our hypothesis that IHI would be reduced in the stroke group when the homologous muscles were activated simultaneously. This prediction was based on the facilitation of MEP amplitude in stroke subjects during bilateral activation reported by Renner at al (2005). Reduced corticospinal excitability is a standard finding following stroke (Turton et al., 1996; Catano et al., 1997; Cicinelli et al., 1997; Byrnes et al., 1999; Turton and Lemon, 1999). Therefore, interventions that increase motor pathway excitability have the potential to be beneficial for stroke rehabilitation. Our findings suggest that the reported facilitation of MEP amplitude during bilateral activation is not mediated by alterations in IHI. It should be noted that we used the non-affected limb as the target limb in our study whereas Renner and colleagues (2005) recorded responses in the affected limb. Previous studies have reported abnormally enhanced IHI directed from the non-affected to the affected hemisphere in a pre-movement period in subjects with stroke (Murase et al., 2004; Duque et al., 2005). These findings lead us to speculate that modulation of IHI directed to the affected hemisphere also may be compromised in stroke subjects during activation of the homologous non-affected muscle. We suggest that further studies investigate IHI in the affected limb during unilateral and bilateral activation.
Acknowledgments
We would like to acknowledge Dr James Stinear, Dr Jonathon Shemmell, Dr Tatyana Gerachshenko, Gowri Jayaram, and Michelle Prior for their assistance in data collection. Funding for this project was received from NIH grant 1 R21 HD049883-01 to GN Lewis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boroojerdi B, Diefenbach K, Ferbert A. Transcallosal inhibition in cortical and subcortical cerebral vascular lesions. J Neurol Sci. 1996;144:160–170. doi: 10.1016/s0022-510x(96)00222-5. [DOI] [PubMed] [Google Scholar]
- Byrnes ML, Thickbroom GW, Phillips BA, Wilson SA, Mastaglia FL. Physiological studies of the corticomotor projection to the hand after subcortical stroke. Clin Neurophys. 1999;110:487–498. doi: 10.1016/s1388-2457(98)00044-3. [DOI] [PubMed] [Google Scholar]
- Catano A, Houa M, Noel P. Magnetic transcranial stimulation: clinical interest of the silent period in acute and chronic stages of stroke. Electroencephalogr Clin Neurophysiol. 1997;105:290–296. doi: 10.1016/s0924-980x(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Cicinelli P, Traversa R, Rossini PM. Post-stroke reorganisation of brain motor output to the hand: a 2-4 month follow-up with focal magnetic transcranial stimulation. Electroencephalogr Clin Neurophysiol. 1997;105:438–450. doi: 10.1016/s0924-980x(97)00052-0. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol. 2002;543:317–326. doi: 10.1113/jphysiol.2002.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG. Transcallosal inhibition in chronic subcortical stroke. NeuroImage. 2005;28:940–946. doi: 10.1016/j.neuroimage.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Duque J, Murase N, Celnik P, Hummel F, Harris-Love M, Mazzocchio R, Olivier E, Cohen LG. Intermanual differences in movement-related interhemispheric inhibition. J Cogn Neurosci. 2007;19:204–213. doi: 10.1162/jocn.2007.19.2.204. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess CW, Mills KR, Murray NM. Magnetic stimulation of the human brain: facilitation of motor responses by voluntary contraction of ipsilateral and contralateral muscles with additional observations on an amputee. Neurosci Lett. 1986;71:235–240. doi: 10.1016/0304-3940(86)90565-3. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Hutchinson S, Schlaug G, Pascual-Leone A. Ipsilateral motor cortex activation on functional magnetic resonance imaging during unilateral hand movements is related to interhemispheric interactions. NeuroImage. 2003;20:2259–2270. doi: 10.1016/s1053-8119(03)00220-9. [DOI] [PubMed] [Google Scholar]
- Leiguarda RC, Marsden CD. Higher-order disorders of sensorimotor integration. Brain. 2000;123:860–879. doi: 10.1093/brain/123.5.860. [DOI] [PubMed] [Google Scholar]
- McCombe Waller S, Whitall J. Hand dominance and side of stroke affect rehabilitation in chronic stroke. Clin Rehabil. 2005;19:544–551. doi: 10.1191/0269215505cr829oa. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Facchini S, Boroojerdi B, Hallett M. Changes in motor cortex excitability during ipsilateral hand muscle activation in humans. Clin Neurophys. 2000;111:344–349. doi: 10.1016/s1388-2457(99)00243-6. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen L. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Netz J, Ziemann U, Homberg V. Hemispheric asymmetry of transcallosal inhibition in man. Exp Brain Res. 1995;104:527–533. doi: 10.1007/BF00231987. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Renner CIE, Woldag H, Atanasova R, Hummelsheim H. Change of facilitation during voluntary bilateral hand activation after stroke. J Neurol Sci. 2005:25–30. doi: 10.1016/j.jns.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Stedman A, Davey NJ, Ellaway PH. Facilitation of human first dorsal interosseous muscle responses to transcranial magnetic stimulation during voluntary contraction of the contralateral homonymous muscle. Muscle Nerve. 1998;21:1033–1039. doi: 10.1002/(sici)1097-4598(199808)21:8<1033::aid-mus7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Stewart KC, Cauraugh JH, Summers JJ. Bilateral movement training and stroke rehabilitation: A systematic review and meta-analysis. J Neurol Sci. 2006 doi: 10.1016/j.jns.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Walker KS, Byblow WD. Symmetric facilitation between motor cortices during contraction of ipsilateral hand muscles. Exp Brain Res. 2001;139:101–105. doi: 10.1007/s002210100758. [DOI] [PubMed] [Google Scholar]
- Tinazzi M, Zanette G. Modulation of ipsilateral motor cortex in man during unimanual finger movements of different complexities. Neurosci Lett. 1998;244:121–124. doi: 10.1016/s0304-3940(98)00150-5. [DOI] [PubMed] [Google Scholar]
- Turton A, Lemon RN. The contribution of fast corticospinal input to the voluntary activation of proximal muscles in normal subjects and in stroke patients. Exp Brain Res. 1999;129:559–572. doi: 10.1007/s002210050926. [DOI] [PubMed] [Google Scholar]
- Turton A, Wroe S, Trepte N, Fraser C, Lemon RN. Contralateral and ipsilateral EMG responses to transcranial magnetic stimulation during recovery of arm and hand function after stroke. Electroencephalogr Clin Neurophysiol. 1996;101:316–328. doi: 10.1016/0924-980x(96)95560-5. [DOI] [PubMed] [Google Scholar]
- Verstynen T, Diedrichsen J, Albert N, Aparicio P, Ivry RB. Ipsilateral motor cortex activity during unimanual hand movements relates to task complexity. J Neurophysiol. 2005;93:1209–1222. doi: 10.1152/jn.00720.2004. [DOI] [PubMed] [Google Scholar]
- Woldag H, Lukhaup S, Renner CIE, Hummelsheim H. Enhanced motor cortex excitability during ipsilateral voluntary hand activation in healthy subjects and stroke patients. Stroke. 2004;35:2556–2559. doi: 10.1161/01.STR.0000144651.07122.da. [DOI] [PubMed] [Google Scholar]