Abstract
This paper attempts to illustrate both the need for new approaches to biomaterials discovery as well as the significant promise inherent in the use of combinatorial and computational design strategies. The key observation of this Leading Opinion Paper is that the biomaterials community has been slow to embrace advanced biomaterials discovery tools such as combinatorial methods, high throughput experimentation, and computational modeling in spite of the significant promise shown by these discovery tools in materials science, medicinal chemistry and the pharmaceutical industry. It seems that the complexity of living cells and their interactions with biomaterials has been a conceptual as well as a practical barrier to the use of advanced discovery tools in biomaterials science. However, with the continued increase in computer power, the goal of predicting the biological response of cells in contact with biomaterials surfaces is within reach. Once combinatorial synthesis, high throughput experimentation, and computational modeling are integrated into the biomaterials discovery process, a significant acceleration is possible in the pace of development of improved medical implants, tissue regeneration scaffolds, and gene/drug delivery systems.
Keywords: Biomaterials design, computational modeling, combinatorial synthesis, high throughput experimentation
Introduction
Rapid advances in our understanding of cell and stem cell biology [1-3] provide the basis for a revolutionary and far-reaching change in the treatment of trauma and aging related tissue loss: Instead of using permanently implanted prostheses to replace damaged tissue, the future goal of surgical intervention will be to implant a reconstructive or regenerative temporary scaffold that enables the body to heal itself [4, 5]. The most commonly studied tissue scaffold is the cell-material hybrid, a porous implant that has to support the growth of specific cells. Once implanted, the scaffold must safely resorb while enabling cells to differentiate into functional tissue [6, 7]. Numerous reviews in the field describe the need to develop resorbable polymer scaffolds [8-12] and tissue engineers advocate the use of increasingly complex polymer structures that can be tailored to specific tissue applications [13-15]. On the other hand, an analysis of the literature conducted by the authors indicates that simple copolymers of lactic and glycolic acid remain the most commonly used scaffold material in all tissue engineering research.
It therefore appears that the imagination of biomedical engineers and clinicians has outpaced the ability of material scientists to provide the next generation of biomaterials that is critically needed for the full clinical implementation of the tissue engineering approach.
In 1995, Frost and Sullivan, leading US-based market analysts, predicted that the tissue engineering market would grow to about $3 billion by 2002. This prediction proved to be inaccurate: The total tissue engineering market in 2002 was less than $50 million [16]. Repeated analyses of the commercial impact of tissue engineering performed by Lysaght et al. [16-18] revealed many reasons for the failure of tissue engineering to advance from the laboratory into the clinic, including regulatory and legal hurdles, problems with the availability of cells suitable for seeding the porous scaffolds, and the inability to generate a functional blood supply within the newly growing tissue. However, one of the most fundamental stumbling blocks has been the inability of the biomaterials community to move from simple poly(hydroxy acid)s (which are at best a biologically inert cell growth substrate) to bioactive polymers that can support the desired formation of functional tissue.
Despite the substantial research effort on new polymeric biomaterials, only a handful of distinct classes of synthetic biodegradable polymers are being used in FDA-approved medical devices in the USA (Table 1). There are very few polymers under consideration that have “complex structures”, and attempts to tailor the properties of polymers to specific applications are mostly based on “trial and error” [19].
Table 1.
Major classes of synthetic degradable polymers used in FDA-approved medical devices in the USAa
| Polymer classb | Year of device approval by the FDA |
|---|---|
| Polyesters containing glycolic acid | 1969 (suture) |
| Polyesters containing lactic acid | 1971 (suture) |
| Poly(trimethylene carbonate) and copolymers thereof | 1974 (suture) |
| Polyesters containing dioxanone | 1981 (suture, bone fixation device) |
| Polyanhydrides containing sebacic acid | 1996 (drug delivery system) |
| Polyesters containing caprolactone | 1997 (coating for DEXON H® suture) |
| Photocrosslinkable copolymers of lactic acid and PEG | 1998 (lung and tissue sealant) |
| Tyrosine-derived polyarylate | 2006 (hernia repair device) |
The US Food and Drug Administration (FDA) does not approve polymers. The commonly made statement “the polymer is FDA approved” is incorrect. Instead, it is best to refer to the approval history of a given medical device which contained a specific polymer. For example, the first FDA-approved synthetic degradable sutures were made of poly(glycolic acid). The FDA cleared the suture for marketing, the FDA did not approve poly(glycolic acid).
This table only lists key polymers or major polymer classes, but does not include copolymers thereof or minor polymer structure modifications.
In the pharmaceutical industryadvanced tools of drug discovery (combinatorial synthesis, high-throughput experimentation, computational modeling) have revolutionized the way lead compounds are identified [20-23]. These general concepts of advanced drug discovery are in principle applicable to materials discovery as well. Combinatorial methods, high-throughput experimentation, and computational modeling have been applied to a wide range of materials design challenges, including the discovery of semiconductors [24], coatings [25], catalysts [26], and light emitting phosphors [27]. Based on this, Gao et al. concluded that advanced materials discovery tools will play an important role in the emerging nano- and biomaterials fields [28]. In fact, it is reasonable to assume that the complexity of biological systems can only be overcome when experimentation is effectively combined with combinatorial approaches and computational modeling [28].
Early results indicate that the general concepts of advanced drug discovery can indeed by applied to the discovery of biomaterials. Noteworthy is the demonstration that the first combinatorially designed library of biomaterials [29, 30] has yielded structure-performance correlations that enabled a New Jersey based start-up company to apply one of the library polymers to the design of a new hernia repair device that was recently cleared by the FDA for marketing in the USA [31]. The most significant benefit to the company of using a combinatorial discovery approach was a shortening of the product development cycle.
Does polymer composition really matter?
Polymer composition is the key parameter determining both the surface properties as well as the physicomechanical bulk properties of an implant [32]. There are clearly other factors that influence the clinical performance of an implant, ranging from various surface treatments to the skill of the surgeon performing the implantation. However, these secondary factors cannot overcome the simple fact that the wrong material in the wrong place will always lead to poor clinical outcomes. The importance of careful materials design is illustrated by the outcome of two recent in vivo studies. In one study, the in vivo performance of two types of bone implants was followed over 4 years in a rabbit transcortical bone pin model [33]. The pins were identical in every aspect, except that one was made of poly(L-lactic acid) while the other was made from poly(DTE carbonate), a tyrosine-derived polymer with virtually identical initial strength and strength retention profile to poly(L-lactic acid). Figure 1 illustrates the histological outcome, 900 days post implantation: poly(L-lactic acid) had degraded, the deformed shape of the residual pin was caused by swelling of the pin due to water uptake, the surrounding bone shows clear evidence of bone resorption, an inflammatory response, and possibly even tissue necrosis possibly related to the release of acidic degradation products. In contrast, the poly(DTE carbonate) pin was surrounded by normal bone devoid of any signs of inflammation, device swelling, or degradation-induced tissue changes. The most noteworthy finding is the ribbon of bone growing right through the middle of the degrading pin. It appears that poly(DTE carbonate) was replaced by newly grown bone as the polymer degraded.
Figure 1.

In vivo performance of a poly(L-lactic acid) bone pin (LEFT) and an identical poly(DTE carbonate) pin (RIGHT). Both pins were round with smooth surfaces and had exactly matched implant dimensions. The histology images were obtained 900 days post implantation and represent cross sections of the pin, showing the bone response around the implant. See text for additional details. Copyright ©2006 New Jersey Center for Biomaterials. Reproduced with permission.
While Figure 1 illustrates the importance of choosing an appropriate material for a given application, Figure 2 makes a significant point [34] by showing the different bone response to poly(DTE carbonate) and poly(DTB carbonate) where “E” indicates an ethyl (2 carbon atoms) ester pendent chain and “B” indicates a butyl ester (4 carbon atoms) pendent chain (Figure 3). These two polymers are virtually indistinguishable in their physicomechanical properties, the sole difference being the addition of two carbon atoms to the length of a pendent chain present in the polymer structure. Yet, about 70% of all poly(DTB carbonate) implants developed a fibrous tissue layer at the bone-implant interface (foreign body response) while for poly(DTE carbonate) the predominant tissue response was bone apposition, i.e., direct contact between the implant and bone without foreign body response. The superior bone response to poly(DTE carbonate) over poly(DTB carbonate) illustrates the potential benefits of a very detailed, fine-tuning of the polymer composition to achieve superior clinical outcomes. It is difficult to discover such optimized polymer structures by a random walk through design space. In fact, the more complex the polymer structure the greater the need for a systematic discovery process that implements a computational modeling component to reduce the time and cost associated with exhaustive testing of hundreds of candidate polymer structures [35].
Figure 2.

Histological evaluation of the implant-bone interface. LEFT: poly(DTB carbonate) showing predominantly (79% of implants) a foreign body response with fibrous capsule (arrow, blue tissue layer). RIGHT: poly(DTE carbonate), showing predominantly bone apposition (72% of all implants) indicated by the absence of any fibrous tissue at the bone-implant interface. In these cross sections perpendicular to the long axis of the pin, bone is stained orange, fibrous tissue is blue. The implant is unstained. Copyright ©2006 New Jersey Center for Biomaterials. Reproduced with permission.
Figure 3.
Chemical structures of poly(DTE carbonate) and poly(DTB carbonate), two experimental biomaterials used to explore the effect of polymer structure on the tissue response in vivo. The two polymers have identical structures except for two -CH2- groups at the pendent chain.
Combinatorial, parallel synthesis of libraries of polymeric biomaterials
Combinatorial chemistry has led to dramatic changes in the way lead compounds for the discovery of new drugs are identified [23]. Over the last 20 years, this so-called “Combichem” approach, based on the parallel synthesis of millions of random moieties followed by identification of the most active compounds by selective bioassay and advanced analytical techniques, has led to the development of highly sophisticated methods for the simultaneous synthesis of large numbers of drug candidates, specialized robotic instruments for synthesis and analysis of complex mixtures, and computational approaches for data mining and design optimization [20, 36, 37].
There are several conceptual and experimental difficulties in the application of the traditional “Combichem” approach to materials discovery. One key point is that in drug discovery it is possible to identify useful lead compounds contained within a complex mixture. In polymer discovery, it is impossible to screen for useful material properties unless the test polymer can be obtained in a state of high purity. Another key difference is that the chemical structure of a low-molecular weight drug candidate determines its biological activity. For a polymer, the chemical structure is only one of many parameters that affect its ultimate utility. Other, equally important parameters are the polymer molecular weight, molecular weight distribution and the way in which a particular test specimen was fabricated. The concept that the properties of a polymer are dramatically affected by the way in which it is shaped into an object is often not appreciated by chemists and biologists who are key participants in the biomaterials design process. For all of these reasons, the concept of synthesizing many different polymers within one reaction vessel followed by some assay to “fish” for a desirable polymer will most probably remain impractical.
However, an alternative to simultaneous synthesis of many species within the same reaction vessel is to implement a combinatorial search for optimized polymer structures through parallel synthesis, i.e., the synthesis of a larger number of individual polymers at the same time, but each one within its own reaction vessel. In this way each individual material of the library is obtained in pure form [29, 38].
Rapid screening and high-throughput evaluation
Irrespective of the way in which the test polymers are obtained, the unavoidable next step in the materials discovery paradigm is a thorough characterization of most (perhaps even all) the individual polymers contained within a library. This requirement poses the most significant hurdle for materials scientists, as there are very few good equivalents to high-throughput assays when it comes to polymer characterization. While some commercial solutions are available (see for example: Symyx, Inc. at http://www.symyx.com/), smaller companies and academic laboratories cannot easily afford expensive commercial tools, and building the necessary high-throughput experimental infrastructure “in house” is a time and labor intensive effort. Therefore, any progress that makes high-throughput screening and characterization of polymers more widely available can have substantial impact on accelerating the overall pace at which new materials are discovered.
The lack of suitable high-throughput assays is particularly problematic in the field of biomaterials research where a wide range of specialized tests are required. Examples of key biomaterial properties include surface protein adsorption, rate and mechanism of degradation, specific biological responses of cells contacting the material surface, cytotoxicity, and degree of biocompatibility in vivo. Each of the above mentioned properties must be explored through rather tedious experiments. This is a critical bottleneck in the implementation of a combinatorial biomaterials discovery process.
There are, however, ways around this problem. Several laboratories are now developing accelerated screening assays for protein adsorption and cell growth [39], transfection efficacy [40], and the effect of substratum chemistry on stem cell differentiation [41]. In addition, there are significantly more rapid material test platforms available that do come close to the “high throughput” concept as it is understood in the pharmaceutical industry. One possible solution is provided by spatially resolved libraries: When a test library is spread within a grid of x,y coordinates such that the material composition changes along the x and y coordinates, millions of individual material compositions can be assayed by simply scanning over the film surface with an appropriate analytical technique. This approach has been used by Amis and his coworkers at the National Institute of Standards and Technology (NIST) to investigate the properties of polymer blends whereby all possible blend compositions were represented by a pair of x,y coordinates [42, 43].
The Combinatorial-Computational Method
One of the most noteworthy features of 20th Century science and engineering was the emergence of computational modeling as the third element of the research triad, complementing experiment and theory. In nearly every field of engineering, computational modeling developed from a virtually unknown research area in the 1950s to a dominant role by the year 2000. Examples are numerous. Aircraft design was revolutionized by the development of Computational Fluid Dynamics (CFD) in the 1970s and 1980s and quantum simulation techniques are routinely used to design molecular materials such as nanotubes and pharmaceutical drugs [28].
The field of biomaterials has lagged behind in embracing computational modeling. Several reasons may be suggested for this situation including the innate complexity of cells and tissues and their response to contact with biomaterials, and the difficulty of generating biologically relevant in vitro data or clinically relevant in vivo data for large sets of biomaterials. Another reason is related to the fact that semi-empirical modeling approaches work best with data sets that contain a large number of data points, yet in biomaterials science, only a few polymers are investigated by any one laboratory and rarely do researchers produce large enough data sets to warrant a semi-empirical modeling effort. However, advances in high throughput combinatorial synthesis have made it easier to generate libraries of polymers. Once the first library of biomaterials had been reported in 1997 [29], other laboratories started to apply advanced methods of discovery to biomaterials research [40, 44]. A notable attempt to use a computational modeling approach to understand the attachment of cells to biomaterials was made by Dobkowski et al. in 2000 [45]. The major shortcoming of Dobkowski's approach was not in the concept, but in the limited computing power available to this group of researchers.
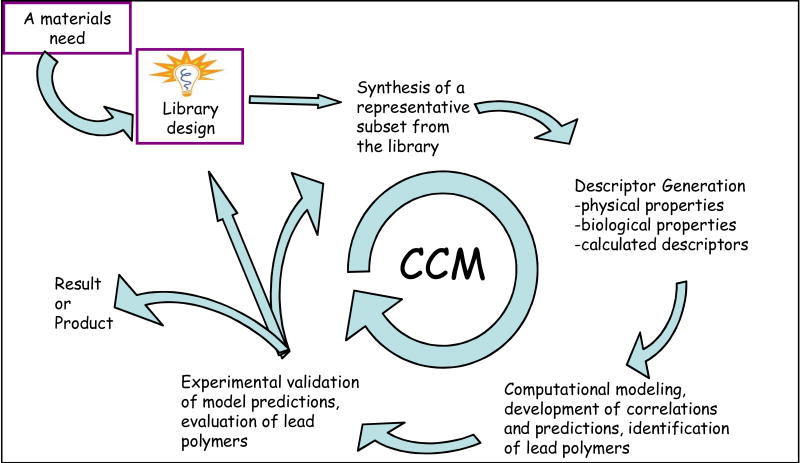
The emergence of combinatorial synthesis, rapid screening, and computational modeling as materials discovery tools can lead to a revolution in biomaterials science. Each of these discovery tools has sufficiently developed to enable a synergy amongst all three that has heretofore been impossible, leading to the formulation of the Combinatorial-Computational Method (CCM) (Figure 4) as a new paradigm for biomaterials discovery [46, 47]. This approach integrates fabrication, experiment and modeling to achieve dramatic breakthroughs in predicting important biomaterials properties. Following the outline shown in Figure 4, a multivariate model was constructed of cellular proliferation on the surfaces of polymers from a library of polyarylates. The model then accurately predicted cellular proliferation for five of six new and untested polymers [47]. Since the six untested polymers spanned the full range of cellular proliferation for the polyarylate library, this seminal experiment constituted a realistic test of the approach. The Combinatorial-Computational Method uses the most advanced computational modeling techniques including Molecular Dynamics simulations, semi-empirical models (e.g., Artificial Neural Networks, Support Vector Machines, etc) and Data Mining. With the continued growth of computational power and more sophisticated algorithms, the prospect to predict key bioresponse characteristics such as cell growth, differentiation and migration for cells being cultured on thousands or even tens of thousands of virtual polymer surfaces appears to be within reach.
Figure 4.
One of many possible implementations of the combinatorial-computational method (CCM) for biomaterials discovery: The CCM usually starts from a well-defined materials need. Based on this materials need, a virtual library of polymers is designed. This library can contain thousands of polymers as long as all polymers in the library share some common structural features. Using parallel synthesis, a small subset (usually less than 100) of library polymers are synthesized and characterized. This characterization leads to the collection of both experimentally measured “data” as well as calculated “descriptors” of specific polymer properties. Experimental data and descriptors are then fed into a series of computational models, leading to predictions of polymer properties and performance for all members of the original polymer library. Based on these predictions a small number of “lead polymers” can be selected for synthesis and detailed characterization. This last step can have three principal outcomes: (a) the predictions were validated by experiment and the newly discovered “lead polymers” fulfill the initially identified materials need; (b) correlations between predictions and validation are not sufficiently accurate, requiring a second iteration of the CCM process to discover promising lead polymers; and finally (c) the predicted lead polymers perform so poorly that one can conclude that no appropriate materials are contained within this particular library, requiring the development of a new library concept. Copyright ©2007 New Jersey Center for Biomaterials. Reproduced with permission.
Conclusion
Biomaterials are being used under complex, demanding conditions and must fulfill stringent requirements relating to their chemical, biological, and physicomechanical properties. Considering the vision of the biomedical community for some of the future uses of biomaterials as tissue regeneration scaffolds or as delivery systems for drugs, genes, and cells, there is an urgent need for new biomaterials that can satisfy the demanding performance requirements and standards required for these applications.
There is strong evidence that the careful optimization of the chemical structure of polymers used in medical implants can result in better clinical outcomes for the implant recipients. There is also ample evidence that the development of innovative biomaterials can be an engine of innovation for new medical treatments and therapies. However, to develop a new generation of optimal biomaterials in a timely and cost effective way, the biomaterials community could benefit from (i) a more quantitative understanding of cell-material interactions and (ii) a more efficient biomaterials discovery paradigm. These challenges can be addressed through the greater use of combinatorial and computational methods as part of a new biomaterials discovery paradigm.
Acknowledgments
The work on combinatorial and computational methods in the laboratories of the authors were supported by “RESBIO”, the NIH-funded Resource for Polymeric Biomaterials (NIH Grant EB001046). The experimental results used to develop the vision presented in this Leading Opinion Paper were obtained through the collaboration of the scientists of the RESBIO team.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vats A, Tolley NS, Bishop AE, Polak JM. Embryonic stem cells and tissue engineering: delivering stem cells to the clinic. J Royal Soc Med. 2005 Aug;98(8):346–350. doi: 10.1258/jrsm.98.8.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polak JM, Bishop AE. Stem cells and tissue engineering: past, present, and future. Ann New York Acad Sci. 2006 Apr;1068:352–366. doi: 10.1196/annals.1346.001. [DOI] [PubMed] [Google Scholar]
- 3.Greenberger JS, Goff JP, Bush J, Bahnson A, Koebler D, Athanassiou H, et al. Expansion of hematopoietic stem cells in vitro as a model system for human tissue engineering. Orthop Clin North Am. 2000 Jul;31(3):499–510. doi: 10.1016/s0030-5898(05)70167-x. [DOI] [PubMed] [Google Scholar]
- 4.Tabata Y. Tissue regeneration based on tissue engineering technology. Congenit Anom (Kyoto) 2004 Sep;44(3):111–124. doi: 10.1111/j.1741-4520.2004.00024.x. [DOI] [PubMed] [Google Scholar]
- 5.Giannoudis PV, Pountos I. Tissue regeneration. The past, the present and the future. Injury. 2005 Nov;36 4:S2–5. doi: 10.1016/j.injury.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Hutmacher DW, Sittinger M, Risbud MV. Scaffold-based tissue engineering: rationale for computer-aided design and solid free-form fabrication systems. Trends Biotechnol. 2004 Jul;22(7):354–362. doi: 10.1016/j.tibtech.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005 Jul;4(7):518–524. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 8.Tatard VM, Menei P, Benoit JP, Montero-Menei CN. Combining polymeric devices and stem cells for the treatment of neurological disorders: a promising therapeutic approach. Curr Drug Targets. 2005 Feb;6(1):81–96. doi: 10.2174/1389450053344885. [DOI] [PubMed] [Google Scholar]
- 9.Yarlagadda PK, Chandrasekharan M, Shyan JY. Recent advances and current developments in tissue scaffolding. Biomed Mater Eng. 2005;15(3):159–177. [PubMed] [Google Scholar]
- 10.Hammond JS, Beckingham IJ, Shakesheff KM. Scaffolds for liver tissue engineering. Expert Rev Med Devices. 2006 Jan;3(1):21–27. doi: 10.1586/17434440.3.1.21. [DOI] [PubMed] [Google Scholar]
- 11.Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006 Jun;27(18):3413–3431. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 12.Shi C, Zhu Y, Ran X, Wang M, Su Y, Cheng T. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J Surg Res. 2006 Jun 15;133(2):185–192. doi: 10.1016/j.jss.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Hubbell JA. Bioactive biomaterials. Curr Opin Biotechnol. 1999 Apr;10(2):123–129. doi: 10.1016/s0958-1669(99)80021-4. [DOI] [PubMed] [Google Scholar]
- 14.Hubbell JA. Materials as morphogenetic guides in tissue engineering. Curr Opin Biotechnol. 2003 Oct;14(5):551–558. doi: 10.1016/j.copbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005 Jan;23(1):47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 16.Lysaght MJ, Hazlehurst AL. Tissue engineering: the end of the beginning. Tissue Eng. 2004 Jan-Feb;10(12):309–320. doi: 10.1089/107632704322791943. [DOI] [PubMed] [Google Scholar]
- 17.Lysaght MJ, Nguy NA, Sullivan K. An economic survey of the emerging tissue engineering industry. Tissue Eng. 1998 Fall;4(3):231–238. doi: 10.1089/ten.1998.4.231. [DOI] [PubMed] [Google Scholar]
- 18.Lysaght MJ, Reyes J. The growth of tissue engineering. Tissue Eng. 2001 Oct;7(5):485–493. doi: 10.1089/107632701753213110. [DOI] [PubMed] [Google Scholar]
- 19.Eisenberger P. NIH Workshop: Biomaterials and Medical Implant Science. Bethesda, MD: National Institutes of Health; 1995. Oct 16-17, Executive Summary of the NIH Workshop on Biomaterials and Medical Implant Science. 1995. 1995. [Google Scholar]
- 20.Eksterowicz JE, Evensen E, Lemmen C, Brady GP, Lanctot JK, Bradley EK, et al. Coupling structure-based design with combinatorial chemistry: application of active site derived pharmacophores with informative library design. J Mol Graph Model. 2002 Jun;20(6):469–477. doi: 10.1016/s1093-3263(01)00148-6. [DOI] [PubMed] [Google Scholar]
- 21.Stanton RV, Mount J, Miller JL. Combinatorial library design: maximizing model-fitting compounds within matrix synthesis constraints. J Chem Inf Comput Sci. 2000 May;40(3):701–705. doi: 10.1021/ci990183c. [DOI] [PubMed] [Google Scholar]
- 22.Kirkpatrick DL, Watson S, Ulhaq S. Structure-based drug design: combinatorial chemistry and molecular modeling. Comb Chem High Throughput Screen. 1999 Aug;2(4):211–221. [PubMed] [Google Scholar]
- 23.Lowe G. Combinatorial chemistry. JCS Reviews. 1995:309–317. [Google Scholar]
- 24.Simon U, Sanders D, Jockel J, Heppel C, Brinz T. Design strategies for multielectrode arrays applicable for high-throughput impedance spectroscopy on novel gas sensor materials. J Comb Chem. 2002 Sep-Oct;4(5):511–515. doi: 10.1021/cc020025p. [DOI] [PubMed] [Google Scholar]
- 25.Potyrailo RA, Ezbiansky K, Chisholm BJ, Morris WG, Cawse JN, Hassib L, et al. Development of combinatorial chemistry methods for coatings: high-throughput weathering evaluation and scale-up of combinatorial leads. J Comb Chem. 2005 Mar-Apr;7(2):190–196. doi: 10.1021/cc049920u. [DOI] [PubMed] [Google Scholar]
- 26.Watkins K. Cover story: Strength in numbers. C&EN (Chemical & Engineering News) 2001;79(43):30–34. [Google Scholar]
- 27.Wang J, Yoo Y, Gao C, Takeuchi II, Sun X, Chang H, et al. Identification of a blue photoluminescent composite material from a combinatorial library. Science. 1998 Mar 13;279(5357):1712–1714. doi: 10.1126/science.279.5357.1712. [DOI] [PubMed] [Google Scholar]
- 28.Gao H. Modelling strategies for nano- and biomaterials. In: Rühle M, Dosch H, Mittemeijer EJ, Van de Voorde MH, editors. European White Book on Fundamental Research in Materials Science. Stuttgart (Germany): Max Planck Gesellschaft, GMBH; 2001. pp. 144–148. [Google Scholar]
- 29.Brocchini S, James K, Tangpasuthadol V, Kohn J. A combinatorial approach for polymer design. J Amer Chem Soc. 1997;119(19):4553–4554. [Google Scholar]
- 30.Brocchini S, James K, Tangpasuthadol V, Kohn J. Structure-property correlations in a combinatorial library of degradable biomaterials. J Biomed Mater Res. 1998;42:66–75. doi: 10.1002/(sici)1097-4636(199810)42:1<66::aid-jbm9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 31.Kohn J. Implants: The biodegradable future. Medical Device Development (MDD) 2006 February;:35–36. 2006. [Google Scholar]
- 32.Castner DG, Ratner BD. Biomedical surface science: Foundations to frontiers. Surf Sci. 2002;500(13):28–60. [Google Scholar]
- 33.Abramson SD, James K, Levene H, Parsons JR, Kohn J. Long-term comparative study of the bone response to PLLA and a tyrosine-derived polycarbonate in a rabbit transcortical pin model: Results of a 4 year study. J Biomed Mat Res. 2006 manuscript in preparation. [Google Scholar]
- 34.James K, Levene H, Parsons JR, Kohn J. Small changes in polymer chemistry have a large effect on the bone-implant interface: Evaluation of a series of degradable tyrosine-derived polycarbonates in bone defects. Biomaterials. 1999;20(2324):2203–2212. doi: 10.1016/s0142-9612(99)00151-9. [DOI] [PubMed] [Google Scholar]
- 35.Smith JR, Seyda A, Weber N, Knight D, Abramson S, Kohn J. Integration of combinatorial synthesis, rapid screening, and computational modeling in biomaterials development. Macromol Rapid Commun. 2004;25:127–140. [Google Scholar]
- 36.Garcia JG. Scavenger resins in solution-phase CombiChem. Methods Enzymol. 2003;369:391–412. doi: 10.1016/S0076-6879(03)69021-X. [DOI] [PubMed] [Google Scholar]
- 37.Lemmen C, Lengauer T. Computational methods for the structural alignment of molecules. J Comput Aided Mol Des. 2000 Mar;14(3):215–232. doi: 10.1023/a:1008194019144. [DOI] [PubMed] [Google Scholar]
- 38.Brocchini S, James KS, Tangpasuthadol V, Pendharkar SM, Tong X, Kohn J. Preliminary studies exploring a combinatorial approach toward the development of new biomaterials. Annual Meeting of the Society for Biomaterials; 1997; April 1997; New Orleans, LA: Society for Biomaterials; 1997. p. 143. [Google Scholar]
- 39.Weber N, Bolikal D, Bourke SL, Kohn J. Small changes in the polymer structure influence the adsorption behavior of fibrinogen on polymer surfaces: Validation of a new rapid screening technique. J Biomed Mater Res. 2004;68(A):496–503. doi: 10.1002/jbm.a.20086. [DOI] [PubMed] [Google Scholar]
- 40.Lynn DM, Anderson DG, Putnam D, Langer R. Accelerated discovery of synthetic transfection vectors: parallel synthesis and screening of a degradable polymer library. J Am Chem Soc. 2001 Aug 22;123(33):8155–8156. doi: 10.1021/ja016288p. [DOI] [PubMed] [Google Scholar]
- 41.Anderson DG, Levenberg S, Langer R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nature Biotechnol. 2004 July 01;22:863–866. doi: 10.1038/nbt981. [DOI] [PubMed] [Google Scholar]
- 42.Meredith JC, Smith AP, Karim A, Amis EJ. Combinatorial materials science for polymer thin-film dewetting. Macromolecules. 2000;33(26):9747–9756. [Google Scholar]
- 43.Smith AP, Douglas JF, Meredith JC, Amis EJ, Karim A. Combinatorial study of surface pattern formation in thin block copolymer films. Phys Rev Lett. 2001 Jul 2;87(1):015503. doi: 10.1103/PhysRevLett.87.015503. [DOI] [PubMed] [Google Scholar]
- 44.Langer T, Hoffmann RD. Virtual screening: an effective tool for lead structure discovery? Curr Pharm Des. 2001 May;7(7):509–527. doi: 10.2174/1381612013397861. [DOI] [PubMed] [Google Scholar]
- 45.Dobkowski J, Kolos R, Kaminski J, Kowalczynska HM. Cell adhesion to polymeric surfaces: Experimental study and simple theoretical approach. J Biomed Mat Res. 1999;47:234–242. doi: 10.1002/(sici)1097-4636(199911)47:2<234::aid-jbm14>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 46.Smith JR, Kholodovych V, Knight D, Kohn J, Welsh WJ. Predicting fibrinogen adsorption to polymeric surfaces in silico: a combined method approach. Polymer. 2005 MAY 26;46(12):4296–4306. [Google Scholar]
- 47.Kholodovych V, Smith JR, Knight D, Abramson S, Kohn J, Welsh WJ. Accurate Predictions of Cellular Response using QSPR: A Feasibility Test of Rational Design of Polymeric Biomaterials. Polymer. 2004;45:7367–7379. [Google Scholar]