Abstract
In the operative treatment of spinal injuries, the reconstruction of the anterior column of the thoracolumbar spine is still controversial. We conducted a prospective clinical study to investigate the clinical and radiological outcome of 50 patients treated with a vertebral body replacement of adjustable height (Synex™). Fifty consecutive patients were evaluated during in-patient treatment and at 12 and 20 months post-operatively in clinical notes and radiographs. 38/50 patients were operated for traumatic fractures. Out of 50 patients 45 attended the follow-up clinic 1 year post-operatively and 39 of these patients were examined after 20 months. Twenty-five patients returned to pre-injury activities within 1 year. This number increased to 29/39 patients at 20 months. Seventy-three percent of the patients returned to their job. After 1 year 25/45 patients complained of little or no back pain and 6 months later six patients were limited in their back function. At 1 year only three patients complained of surgical site pain which was improved at their final follow-up at 20 months. Individual satisfaction was determined using a score on a visual analog scale containing 19 questions on back pain, and functional limitation of the spine that has to be filled in by the patients at three different points of time. The score decreased from 87/100 pre-operatively to 65/100 at 1 year follow-up (P<0.001). The average permanent correction of the injured vertebra was 16.8° (88%) including 2.3° (12%) loss of correction at 12 months after operation. Bony integration was obtained in 83%. Early and intermediate outcome with the Synex™ vertebral replacement device for reconstruction of the anterior column appears promising. The loss of correction or reduction was only minimal. On the basis of our results we recommend the Synex™ implant as an alternative for the fixation and stabilisation of thoracolumbar fractures. However, long-term results and a clinically random control study are still required.
Keywords: Thoracolumbar spine, Spinal injuries, Vertebral body replacement, Spinal fixation, Stability
Introduction
Operative treatment is generally practised as a treatment for unstable fractures of the thoracic and lumbar spine, and for injuries with neurological deficit. Posterior stabilisation with an internal fixator is seen as a standard by most authors [1–3]. Many authors also consider reconstruction of the anterior spinal column to be necessary [4–6] for an immediate and permanent stability and posture. The technique of transpedicular cancellous bone grafting [7, 8] has not always been successful. Several authors have reported that anterior bony fusion of the inserted spongiosa was only achieved in some of the patients; in some cases with considerable post-operative loss of correction [9–12]. Another option for providing anterior support is the use of one or two autogenous corticospongiosal bone grafts. Morbidity at the donor site on the iliac crest remains a problem [13–16]. In addition there have been reports of problems with graft healing and, in cases of bisegmental stabilisation, fractures of the bone graft [14, 17, 18]. The loss of correction was limited but still significant by using cancellous bone grafting [1]. With transplant of mainly femoral allografts filled in with autologeous cancellous bone, bony integration was obtained in many patients [19–21]. The risk of an infection by using allografts should be almost zero now according to up-to-date recommendation. Using xenogenic bone the bony integration is not yet satisfactory [22].
Various implants are available for disc and vertebral body replacement in the operative treatment of degenerative changes in the spine, and in the correction of malalignment and the stabilisation of pathological fractures. These implants are made from titanium, ceramic material and carbon [23–25]. They are cut into the required size and fitted into the spinal defect. Tightening of the previously inserted posterior internal fixator can provide a “press fit in situ”, as can additional anterior instrumentation [26].
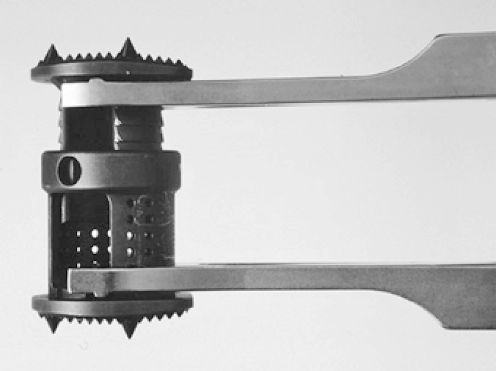
The Synex™ (Stratec Medical) is a distractable vertebral body replacement made from titanium which is extended in situ to the required size (Fig. 1). In biomechanical trials in vitro the anterior support was demonstrated to be at least as good as that provided by the established MOSS™ (DePuy Spine) [27]. The aim of the present study was to record and analyse the course of treatment, and to evaluate the clinical and radiological outcome of the patients first treated with the Synex™. Until now the Synex™ has performed well in terms of handling during operations [28–30]. Long-term results are, however, still required.
Fig. 1.
Synex™ expanded with distraction forceps (Stratec medical, Oberdorf, Switzerland)
Patients and methods
This study was a prospective investigation of the first 50 patients to receive the Synex™ vertebral body replacement in operations performed at our department.
Special recording forms (the current recording form of the “spine” working group of the German Trauma Society (Deutsche Gesellschaft für Unfallchirurgie, DGU) [2]) were completed for each of these 50 consecutive patients during their initial in-patient stay and during the course of treatment. The authors themselves received the data on medical reports and patients’ comments concerning their lifetime activities and back-related problems. Documentation was practised with printed forms and transferred to a database (Microsoft ACCESS). The following data were recorded and evaluated:
Patient’s personal data; dates of accident (in case of a traumatic fracture) and operation.
Reason for operation and diagnosis: accident details, type and severity of the injury, classification [31], additional vertebral body fractures, neurological status at admission and discharge according to the modified Frankel/ASIA classification [32, 33].
Operative treatment: type of operation, duration of posterior and anterior operation, posterior and anterior X-ray times, intraoperative blood loss, anterior approach, implant used, number of segments bridged by anterior and posterior instrumentation, number of segments fused anterior and posterior, posterior and anterior bone transplantation, details of operation (decompression and fusion techniques and technical details).
Intra- and post-operative complications, details of revisions.
Post-operative course: duration of stay in hospital, duration of inpatient rehabilitation, duration of outpatient physiotherapy, duration of inability to work.
Results of examination after 12 and 18 months or after successful removal of implant: leisure activities, job before fracture, reintegration into work, neurological status, finger-to-floor distance, back function, morbidity at the surgical site, morbidity at the donor site (Table 1).
Radiological results: pre- and post-operative measurement of conventional X-ray pictures, course over 3–6 months, follow-up 12 and 18 months after the operation and (if performed) after successful removal of the implant: segmental kyphosis angle (SKA) measured bisegmentally for the most severely injured vertebral body, recording of bony integration of the Synex™, narrowing of the spinal canal (according to computer tomography, if available).
In addition to the collection of these objective data, the patient’s subjective state and back function in daily life were also ascertained using a visual analogue scale (VAS) [34]. The scale contains 19 questions on back pain and functional limitation of the spine or back. It is completed without help from the investigator by means of a cross, placed on a 10 cm line with no scale markings. The total score is calculated from the average of all answered questions and is between 0 and 100. The questionnaire was to be completed for the period before the fracture/first operation, at follow-up after 12 months, and after 18 months or after successful removal of the implant. To get the individual score before the accident/first operation the patients were asked to remember as good as possible for this period when staying in hospital for the first operation. So this is a retrospective score for this point of time, but of course there is no chance to ask the patients before they sustained their spinal injuries. As additional information the individual score loss (difference between the score before the accident and at follow-up) was calculated. This value can be between 0 and 100. The questionnaire is specific to the spine and has been validated [34, 35]. Since a certain level of understanding of the language is required to answer the questions, the VAS questionnaire was completed only by patients who had adequate knowledge of German.
Table 1.
Follow-up examination (individual grading in percent)
| Leisure activities | 0 as before (100%) |
| 1 same activities with limitations (75%) | |
| 2 distinct limitations, change of activities (50%) | |
| 3 everyday domestic tasks, no extra activities (25%) | |
| 4 needs care or assistance (0%) | |
| Job before accident | 0 retired receiving pension, unable to work or unemployed |
| 1 no physical work/sedentary | |
| 2 light physical work/standing | |
| 3 physical work | |
| Reintegration into work | 0 as before (100%) |
| 1 same job, minor limitation (75%) | |
| 2 change of job (50%) | |
| 3 change of job, distinct limitation (25%) | |
| 4 unable to work or retired receiving pension (0%) | |
| Neurological status | A complete paraplegia |
| B incomplete paraplegia: sensitivity, no strength | |
| C incomplete paraplegia: strength <3 | |
| D incomplete paraplegia: strength ≥3 | |
| E no neurological deficits | |
| Finger-to-floor distance | In cm |
| Back function | 0 no symptoms (100%) |
| 1 occasional/minor symptoms (75%) | |
| 2 frequent/distinct symptoms or minor limitation (50%) | |
| 3 frequent/severe symptoms or distinct limitation (25%) | |
| 4 constant/very severe or disabling symptoms(0%) | |
| Morbidity at surgical approach | 0 no symptoms (100%) |
| 1 occasional/minor symptoms (75%) | |
| 2 frequent/distinct symptoms without functional limitation (50%) | |
| 3 frequent/severe symptoms with functional limitation (25%) | |
| 4 disabling symptoms (0%) | |
| Morbidity at donor site | 0 no symptoms (100%) |
| 1 occasional/minor symptoms (75%) | |
| 2 frequent/distinct symptoms without functional limitation (50%) | |
| 3 frequent/severe symptoms with functional limitation (25%) | |
| 4 disabling symptoms(0%) |
The programme SPSS for Windows (SPSS Inc., Chicago, IL, USA), version 10.0, was used for statistical evaluation of the results. Mean and standard deviation were calculated for all measured and calculated values. Significance was calculated using the Wilcoxon and Mann–Whitney tests. The significance level was taken as P<0.05 for all values.
Results
In all, 50 stabilisations of the thoracic and lumbar spine were carried out with the Synex™ titanium vertebral body replacement between 10.02.1999 and 03.05.2000. The patient group consisted of 21 women (42%) and 29 men (58%) with an average age of 43.1 (20–77) years at the time of the operation.
Diagnosis
We treated 36 patients with fresh fractures (≤3 weeks) and 2 patients with older fractures (>3 weeks). The most common causes of injury were: falls from a great height (16/38) and car accidents (10/38). Other causes of injury were: insignificant falls (2/38), motorcycle accidents (3/38), being hit by an object (3/38), suicidal jumps (2/38) and other causes (2/38). In eight patients a corrective operation was performed for post-traumatic deformity following primary conservative treatment or for residual instability following prior operative treatment. Operations on three female patients were performed because of vertebral body tumours and on a fourth because of spondylodiscitis.
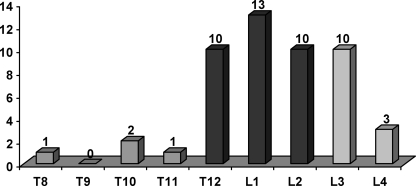
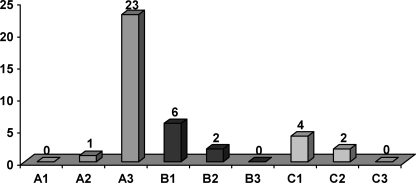
In 46 of 50 patients there was an injury in one to two segments, in two patients more than two segments were affected and two patients had multi-level injuries. In 37 patients only one vertebral body was affected. In eight patients we found a lesion in one further thoracic or lumbar vertebral body and, in five, we found damage to two additional vertebral bodies. The vertebrae most frequently affected were L1 (13/50), T12, L2 and L3 (10/50 each) (Fig. 2). About two-thirds of all fractures were compression injuries (type A), followed by flexion-distraction injuries (type B) and rotation injuries (type C) (Fig. 3).
Fig. 2.
Localisation of spinal injuries
Fig. 3.
Classification (n=38)
At the time of admission one patient exhibited complete paraplegia Frankel/ASIA A following a fall from a great height. The paraplegia did not improve with time. At the start 13 patients exhibited incomplete neurological deficits (Frankel/ASIA B–D). The correlation between the severity of injury and neurological deficits can be seen in Table 2. Six patients improved post-operatively by one Frankel/ASIA grade (three patients changed from Frankel/ASIA C to D and three from Frankel/ASIA D to E). We did not observe worsening of neurological symptoms post-operatively in any of the patients. The proportion of neurological deficits was correlated with the severity of the injury: 2/24 patients with type A fractures, 3/8 with type B fractures and 4/6 with type C fractures showed neurological deficits.
Table 2.
Severity of injury and neurological status
| Severity/Cause of injury | Number of patients with neurological deficit |
|---|---|
| Type A fracture | 2/24 |
| Type B fracture | 3/8 |
| Type C fracture | 4/6 |
| Post-traumatic deformity | 2/8 |
| Tumour | 2/3 |
| Spondylodiscitis | 1/1 |
| Total | 14/50 |
Operative treatment
In 47 of the 50 patients (94%) we performed anterior and posterior stabilisation. In seven of these patients both approaches were performed in a single operation. Only one of these seven patients had a fresh fracture. In five of these seven patients a correction was performed for an injury that had healed in malalignment. Further one of these seven patients, who had been transferred from elsewhere for operative treatment, had a primary missed injury of the eighth thoracic vertebra. Three patients were treated exclusively from the anterior side. Two of these cases involved spinal tumours and the third involved a pincer fracture (A 2.3.).
Anterior approach was performed by means of thoracotomy in 22 patients, by thoracoscopy in 16 patients and by lumbotomy in 16 patients.
The duration of the operation was 386 (260–600) min for the patients treated combined in a single operation. This was significantly longer than that of the two-stage procedures [280 (160–530) min, P=0.006]. However, in the one-stage operation, the time taken to change position between the posterior and anterior steps of the operation (about 30 min) was also included. The corrective spinal fusion of a primary-missed thoracic fracture in an extremely obese patient lasted about 600 min. Five of the eight corrective spinal fusions were also included in the one-stage group, some of which were carried out in three operating steps (anterior–posterior–anterior). In four of these cases anterior thoracoscopy was used.
In cases with isolated anterior treatment (n=3) the operation took about 145 (115–180) min. For the two-stage combined procedures (n=40) the duration of the anterior operation [144 (75–275) min] was comparable to that of isolated anterior procedures. The intraoperative X-ray time was comparable for these two procedures at an average of 37 s for the isolated anterior operation and 26 s for the anterior part of the two-stage combined procedure. The total intraoperative X-ray time in the two-stage operations (120±60 s) was, likewise, comparable with the X-ray time in the combined one-stage operation procedure (88±44 s). The intraoperative loss of blood was comparable in the one stage and two stage procedures, with average values of 1,550 and 1,450 ml, respectively. A comparison with the patients exclusively undergoing anterior operations does not seem appropriate because of the small number of cases and the markedly different causes (two tumours out of a total of three patients by contrast with the fresh fractures predominating in the other groups).
Out of 50 patients 47 patients were given a combined operation receiving a dorsal internal fixator (USS™) with at least one additional crosslink. In remaining 3/50 patients operated upon exclusively from the anterior side, bisegmental instrumentation was performed using one or two Wolter plate fixators. In 30/50 patients the Synex™ was employed bisegmentally, while in 20/50 anterior spinal fusion was carried out monosegmentally.
Posterior spinal fusion of the small vertebral joints was performed in 45/47 patients using cancellous bone from the iliac crest. Bone for anterior spinal fusion was taken from the destroyed vertebral body and additional cancellous bone was taken from the iliac crest (18 times) and a rib (once). Decompression was carried out four times from the posterior and five times from the anterior side.
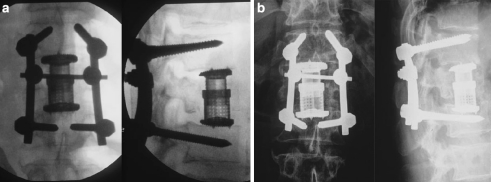
The following intraoperative complications were seen in the anterior operation: one case of bleeding following injury to the opposing segment artery without need for a transfusion; three cases of intraoperative loss of blood ≥2,000 ml including two cases of B1 injury (3,000 ml/4 erythrocyte concentrate, and 2,000 ml/5 erythrocyte concentrate in one patient with initial complete paraplegia) and one case of hypernephromal metastasis of the 12th thoracic vertebra with nephrectomy during the same anaesthesia. In one case we performed a pedicle screw replacement. One patient developed a deep wound infection which required two revision operations but subsequently healed without further consequences. In one patient with a clear osteoporotic bone, the Synex™ subsided into the neighbouring vertebral body following intraoperative injury to the endplate (Fig. 4). With time bony consolidation took place without further operative revision and with no change of position. In one case there was a failure of the lower pedicle screws. Following bony integration the internal fixator was removed and the patient became almost free of symptoms.
Fig. 4.
“Subsidence” of the Synex™ in osteoporotic bone (62-year-old-male patient, L1 fracture, A 3.3.3.): a post-operative (image intensifier): Synex™ in the correct position (b). X-ray pictures following mobilisation: subsidence of Synex™
Follow-up
Out of the 50 patients, 45 received follow-up examinations on an average 11.4 (9–15) months after the operation. One patient with a pathological fracture died 3 months after the operation due to the consequences of breast cancer. One patient lived abroad and three others moved away to unknown addresses. For 39 patients a final result was available 19.5 (14–31) months after the operation. Of these, 18 patients had the posterior implant removed after 15 (9–31) months. For seven further patients no final decision has yet been made on removal of the implant.
The hospital stay for the primary operation lasted 7–83 (22.1±11.5) days. More than half of the patients (25/45) completed a period of residential rehabilitation lasting for an average of 6.9 (2–24) weeks. Almost all patients (41/45) received ambulatory physiotherapy for 3 (1–8) months after discharge.
Out of the 45 patients 33 were employed before the first operation (16 in physical jobs, 8 in light physical jobs or standing and 9 in sedentary jobs). Twelve patients had previously retired and receiving pension, unemployed or unable to work. After 1.5 years 18/28 patients had returned to their former job (11 of them without limitations). The number of those in physical jobs was reduced from 16 to 6 patients at the follow-up examination. Patients were unable to work between two and nine months (5.3±2.3 months). Before the operation 12 patients were not in work (retired, unemployed or unable to work); 12 months later this group had more than doubled in size (28/45). By the follow-up examination after 19.5 months, 24/28 (86%) of those previously working were once again in work (six had changed their job).
Over half of the patients (25/45) had resumed their previous leisure activities after 1 year, 19 of them with limitations. Seventeen patients reported distinct limitations which had made it necessary for them to change their activities. Two patients could only carry out daily domestic tasks. The patient with post-traumatic complete paraplegia required long-term care. After 19.5 months, 29/39 patients had returned to their former leisure activities.
In five patients the traumatic neurological deficit remaining post-operatively had improved with time. One patient improved from Frankel/ASIA C to Frankel/ASIA D. Two others with post-traumatic incomplete paraplegia Frankel/ASIA D had no neurological abnormalities at follow up. One patient improved two steps from Frankel/ASIA B to Frankel/ASIA D. One other patient with initial incomplete paraplegia Frankel/ASIA C and post-operative improvement to Frankel/ASIA D had no neurological deficit on removal of the implant. In all, the pre-operative neurological deficit improved in 10/14 patients by at least one Frankel/ASIA grade within 12 months (Table 3).
Table 3.
Neurological status at admission, post-operatively and at follow-up examination (n=14)
| Localisation | Classification | Neurological Status pre-/post-op/follow-up |
|---|---|---|
| L 4 | A 3.1.3. | C → D → E |
| L 2 | A 3.2.1. | C → D → D |
| T 10 | B 1.2.1. | D → E → E |
| T 10 | A 3.1.3. | A → A → A |
| T 8 | A 3.2.1. | D → D → E |
| L 2 | B 1.2.1. | D → D → D |
| L 3 | B 1.2.1. | D → E → E |
| T 12 | A 3.1.3. | C → C → D |
| L 2 | A 3.2.1. | D → E → E |
| L 2 | Correction | C → D → D |
| T 11 | Correction | D → D → D |
| L 3 | Tumour | D → D → E |
| L 3 | Tumour | D → D → death |
| T 12 | Spondylodiscitis | B → B → D |
| Total | 14 |
The finger-to-floor distance as an indicator of back function was about 16 (0–45) cm after 12 months and was significantly improved (P=0.002) to 11 (0–40) cm 8 months later. After 1 year 25/45 patients had no or occasional back pain. Nineteen out of 45 patients reported distinct back pain and one patient complained of severe symptoms. After 19.5 months only 6/39 patients reported limitations in their back function. The surgical approach gave no or just slight trouble in 42/45 patients while 3/45 patients reported significant symptoms but without limitation of function. After 19.5 months none of the patients experienced more than occasional symptoms from the surgical approach. In 42 of the 45 patients cancellous bone for the anterior spinal fusion was taken from the iliac crest. Thirty nine of the 42 were almost or completely free of symptoms at the donor site while 3/42 complained of frequent and significant pain there. After 19.5 months one of these three patients was completely free of symptoms and another almost free of symptoms at the donor site. Thus all but one of the patients reported no or only occasional minor symptoms from the surgical approach or donor site.
Visual analogue scale (VAS)
For measuring the individual satisfaction the patients filled in a questionnaire in the form of a visual analogue scale (VAS) at three different points of time: before the operation, this was done retrospectively at the patients’ stay in hospital for the first operation, and 12 and 20 months later. For 29 patients VAS scores are available for all the three points in time. The following observations apply exclusively to 23 patients with fresh fractures to take score values with homogeneous clinical diagnosis into account.
Prior to the fracture the patients obtained a mean VAS score of 86.8±15.6 (36–100) out of 100. Eighty percent had almost unlimited back function and achieved a score of >80 prior to the fracture. Twelve months later the VAS score was significantly lower at 56.3±19.9 (27–97) (P<0.001). Up to 20 months, the average VAS score improved significantly to 64.7±19.5 (25–99) (P<0.001). These values account to an average loss in the VAS score by about 21.1±21.3. These changes in scoring represent a significant change in patients’ individual satisfaction concerning their back function. There were no significant differences in VAS score comparing patients obtaining implant removal with those who retained the dorsal internal fixator (Table 4).
Table 4.
VAS scores before the accident and after 12 and 20 months (fracture patients, n=23)
| VAS | Accident | 12 months | 20 months | Score reduction |
|---|---|---|---|---|
| All cases (23) | 86.8±15.6 (36–100) | 56.3±19.9 (27–97), P<0.001 | 64.7±19.5 (25–99), P<0.001 | 21.1±21.3, P<0.001 |
| Implant removed (11) | 90±7.8 (74–100) | 55.6±17.5 (30–76), P=0.003 | 68.0±14.3 (40–84), P=0.003 | 22.0±14.5, P=0.003 |
| Implant not removed (12) | 82.0±20.0 (36–100) | 57.0±22.7 (27–97), P=0.012 | 61.6±23.5 (25–99), P=0.050 | 20.3±26.6, P=0.034 |
Radiological results
The following radiological results concern the fresh and older fractures and the corrective operations. The tumours and spondylodiscitis are not considered here any further as these groups are very small. Because of the physiological differing in each vertebral body’s angle, the absolute value for each level is documented. Further calculations are only performed for the levels T12–L3 because the other groups included less than three patients per segment (Table 5).
Table 5.
Segmental kyphosis angle (SKA, in degrees) measured bisegmentally: correction, loss and gain, distinction between mono- and bisegmental anterior spinal fusion
| Segmental kyphosis angle (Synex bisegmental) | Segmental kyphosis angle (Synex monosegmental) | |
|---|---|---|
| Correction (°) | 20.3±9.9 (P=0.005) | 18.4±10.5 (P=0.043) |
| Loss (°) up to 12 months | −3.9±3.2 (P=0.018) | −2.8±1.9 (NS) |
| Gain (°) up to 12 months | 16.4±8.9 (P=0.005) | 15.6±12.0 (P=0.043) |
| Loss (°) between12 months and follow-up | −1.0±1.5 (NS) | −1.8±0.8 (P=0.043) |
| Gain (°) up to follow-up | 19.4±7.9 (P=0.005) | 13.8±11.7 (NS) |
| Number | 10 | 5 |
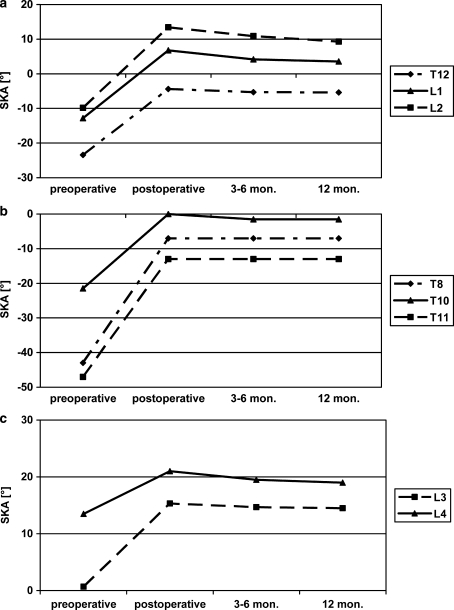
The operation led to a significant correction of 4–38° (19.1±10.1) being achieved at all levels, with more correction in the thoracic than in the lumbar spine. Up to the follow-up after 1 year there was an average loss of correction of 2.3±3.0 (0–12) degrees (P<0.001) corresponding to a significant correction gain of 16.8 (−3 to 36) degrees. This value includes the patient with subsidence of the Synex™ after infraction of the endplate of the adjacent vertebra with a 2° increase in kyphosis by comparison with the time of the accident. In the thoracolumbar spine (T12–L3), corrections between 14.7° at L3 and 23.1° at L2 were achieved (P=0.012). At follow-up after 12 months, a significant loss of correction between 1.0° and 4.1° occurred at T12, L1 and L2, but not at L3. Comparing the X-rays from 3 to 6 months and those from the follow-up examination, a further significant loss of correction occurred at L2. At the follow-up examination a significant corrective gain by comparison with the pre-operative state was evident in all segments investigated. This gain was between 13.8° at L3 (P=0.027) and 19.0° at L2 (P=0.012), Fig. 5.
Fig. 5.
Segmental kyphosis angle at different points in time (values in degree, minus kyphosis, plus lordosis): a thoracolumbar junction, b thoracic spine, c lumbar spine
Posterior removal of the implant was performed in 18 patients. X-ray pictures taken 4.2 (1–10) months after implant removal are available for 11 of these patients (ten monosegmental and one bisegmental).
In mono- and bisegmental anterior spinal fusion the operation led to a comparable correction in the segmental kyphosis angle (monosegmental: 18.4°; bisegmental: 20.3°). At the examination after 12 months a significantly greater loss of correction had occurred in the monosegmental-anterior group (monosegmental: 1.8°; bisegmental: 1.0°). There was no significant change in the segmental kyphosis angle later on (Table 5).
In 25 of 30 patients, a ventral fusion was demonstrated in X-rays. In four patients fusion was not definite and in one patient no fusion was evident on conventional X-rays. In CT scans we confirmed lateral and posterior but not anterior bony bridges (Figs. 6, 7).
Fig. 6.
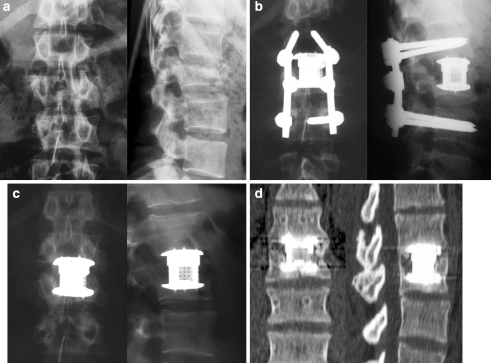
Twenty-three-year-old male patient with L2 fracture (A 3.2.1.) with incomplete paraplegia (Frankel C): bisegmental instrumentation L1-3 with the USS™ internal fixator, monosegmental anterior fusion with the Synex™ a. accident, b post-operative, c after implant removal, d CT after implant removal (14 months after accident): bony integration of the Synex™
Fig. 7.
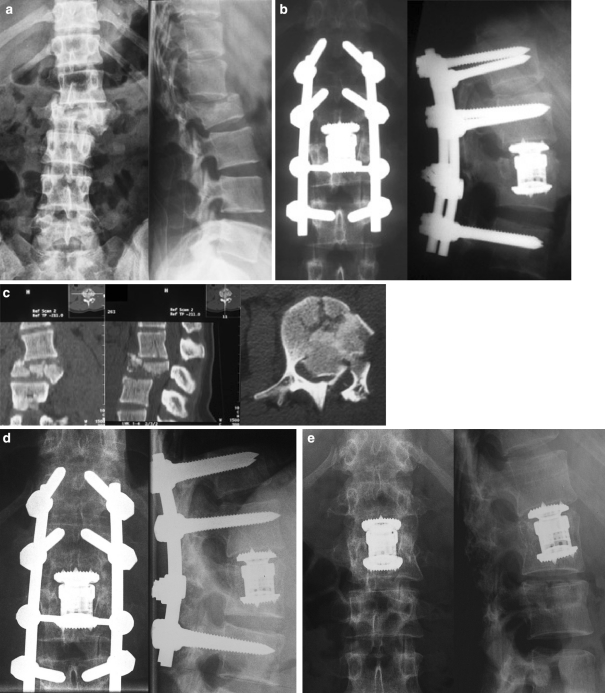
Nineteen-year-old female patient with L2 rotational burst fracture and incomplete paraplegia (Frankel D): a accident, b post-operative, c CT accident, d 12 months post-operative, e 1 month after implant removal
Discussion
The average age (43.1 years) and the distribution of gender (58% men) are well in agreement with the descriptions in other studies [6, 10, 36–38]. Falls from a great height are the most frequent cause of injury, followed by road accidents [10, 36–39]. The patient group of the present study includes post-traumatic malalignments and spinal tumours as well as fresh fractures, in contrast to most other studies. In all the studies most injuries occurred around the thoracolumbar junction with L1 fractured most frequently [10, 36, 40]. The frequency of neurological deficits increased considerably with increasing severity of injury [31, 38]. In 10/13 of our patients with initial incomplete paraplegia the neurological status improved within the first year by at least one Frankel/ASIA grade. Improvement rates of between 17 and 100% are reported in the literature. Almost all patients received combined antero-posterior stabilisation in two separate operations. Immediate posterior stabilisation of unstable fractures is a common treatment in many hospitals. Simultaneous decompression can be performed in case of a neurological deficit and relevant narrowing of the spinal canal. The anterior approach is then performed a few days later. A two-stage procedure for combined antero-posterior treatment is also preferred by the members of the spine working group of the DGU (German Trauma Society) [1]. Posterior instrumentation was, as a rule, performed with a USS internal fixator with an additional cross-link. An earlier biomechanical study showed that a cross-link significantly improved stabilisation [41].
Within 1.5 years 2/3 of patients previously in work returned to their former job. Knop et al. [17] reported that about 1/3 of patients experienced definite long-term consequences from their injury. Patients in the present study were unable to work as a result of their accident for 5.3 (2–9) months. This was similar to the patient population of the DGU’s multicentre spine study [17]. Eighty-five percent (23/27) of patients who were in work before the first operation had returned to work by the follow-up examination. One year after the operation 20/45 patients still had significant or severe back problems. This group decreased to 6/37 by the final examination. Using a vertebral body replacement it was no longer necessary to use bone chips from the iliac crest. Only cancellous bone was harvested for spinal fusion. After 12 months 42/45 patients, therefore, had no or only occasional symptoms at the donor site. In the literature a morbidity from 19 to 49% is given for bone harvest from the iliac crest [13, 17, 42, 43]. Knop et al. [17] demonstrated a correlation between the extent of symptoms and the extent of bone harvest. Obviously there was a real decrease in donor site morbidity while using the Synex™.
As an indicator of back function we measured the finger-to-floor distance after 12 months and at the final examination. We found a significant improvement from an average of 16 (0–45) cm to 11 (0–40) cm. Finger-to-floor distances between 11.6 and 17 cm are also reported in other studies after spinal operations [10, 17, 44].
The operation led to a significant vertebral correction of between 14.7° and 23.1°. The loss of correction was lower than in other studies (0.9°–4.1°). One reason for this is the rigidity of the construction when distracting the Synex™ in situ so that there is little subsidence later. In in-vitro compression tests significantly higher compression forces were required to make the Synex™ subside 1–2 mm into the endplate of the neighbouring vertebral body than were required for the Harmscage (MOSS™) [27]. When bony integration was documented in X-rays a gain of between 13.8° and 19.0° was found. Combined treatment with an internal fixator and anterior vertebral body replacement with the Synex™ thus yielded a markedly better radiological result than that was achieved in comparable studies using bone chips. In these studies a lower initial correction (13.7°), a considerably greater loss (7.4°) and significantly lower gain (6.3°) were achieved [17]. A cross-link was regularly used in these patients. A significant increase of stability when using a cross-link was demonstrated in a previous biomechanical study [41].
Bhat et al. [26] had good short-term results by reconstruction of the anterior column with the MOSS™. Sufficient compression with an additional implant is necessary for a press fit of the MOSS™ to obtain a bony integration. Defino and Rodriguez-Fuentes [45] reported 43 patients with a dorso-ventral stabilization. For ventral reconstruction autologeous bone from iliac crest was used in 41 patients while a fibular transplant was used in the remaining two cases. The clinical outcome was promising but there was a distinct loss of correction in 11/39 patients, by about 5–7° in nine and ≥10° in two patients. The use of bovine bone for anterior reconstruction was disappointing and let to bony integration in just 2/11 patients compared to 8/11 patients sustaining autologeous bone grafts [22].
At the final examination patients achieved mean VAS scores of 64.7 (out of 100). This represents a significant reduction by comparison with the pre-operative score (86.8 out of 100). There was nevertheless a significant improvement by comparison with the examination after 12 months. We did not detect any significant difference in scores between the patients whose implants were removed and those whose implants were not removed. In the present study the reduction in VAS score was 21.1 which accounts for 1/5 of attainable score value. This value is just little lower than the score reduction of 24.1 reported by Knop et al. [34] for patients treated with an internal fixator and bone chips. The score reduction obtained is similar to those found by previous [10, 17, 38] studies using other questionnaires. Briem et al. [44] reported about a relevant long-term impairment in patients’ quality of life after dorso ventral stabilization of thoracolumbar fractures and concluded pain as the most compromising factor being related as well to the severity of the injury and as to the operation itself.
In contrast to using autologeous bone grafting there are additional costs for the implant that have to be taken into account. The positioning of the Synex™ has to be done accurately to avoid a laceration of adjacent structures from sharp tines of the Synex™ endplates.
Conclusion
The procedure described leads to a significant correction. In most cases X-rays showed bony bridging of the fused segments. Further investigations are required using thin layer CT scans for more precise evaluation of intercorporal fusion. However, a critical factor for lasting stable anterior fusion seems to be the firm anchorage of the Synex™ endplates in the vertebral body. The morbidity at the donor site on the iliac crest is decreased compared with bone chip harvesting. Implant-related complications were not found. In 10/14 patients with post-traumatic peripheral neurological deficits, the neurological status had improved by the follow-up examination. Most patients attained a good or very good clinical outcome with further improvement between 12 and 20 months and a relatively high rate of returning to work. Nevertheless long-term follow-up as well as a clinically random control study are still required.
References
- 1.Knop C, Blauth M., Bühren V, Hax P-M, et al. Operative Behandlung von Verletzungen des thorakolumbalen Übergangs. Teil 2: Operation und röntgenologische Befunde. Unfallchirurg. 2000;103:1032–1047. doi: 10.1007/s001130050667. [DOI] [PubMed] [Google Scholar]
- 2.Knop C, Blauth M, Bühren V, Hax P-MKL, et al. Operative Behandlung von Verletzungen des thorakolumbalen Übergangs. Teil 1: Epidemiologie. Unfallchirurg. 1999;102(12):924–935. doi: 10.1007/s001130050507. [DOI] [PubMed] [Google Scholar]
- 3.Stambough JL. Posterior instrumentation for thoracolumbar trauma. Clin Orthop. 1997;335:73–88. [PubMed] [Google Scholar]
- 4.Beisse R, Potulski M, Temme C, Bühren V. Das endoskopisch kontrolierte Zwerchfellsplitting. Ein minimal-invasiver Zugang zur ventralen Versorgung thorakolumbaler Frakturen der Wirbelsäule. Unfallchirurg. 1998;101(8):619–627. doi: 10.1007/s001130050315. [DOI] [PubMed] [Google Scholar]
- 5.Haas N, Blauth M, Tscherne H. Anterior plating in thoracolumbar spine injuries. Indication, technique, and results. Spine. 1991;16(3 Suppl):S100–S111. doi: 10.1097/00007632-199103001-00015. [DOI] [PubMed] [Google Scholar]
- 6.Kaneda K, Taneichi H, Abumi K, Hashimoto T, Satoh S, Fujiya M. Anterior decompression and stabilization with the Kaneda device for thoracolumbar burst fractures associated with neurological deficits. J Bone Joint Surg Am. 1997;79(1):69–83. doi: 10.2106/00004623-199701000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Daniaux H, Seykora P, Genelin A, Lang T, Kathrein A. Application of posterior plating an modifications in thoracolumbar spine injuries. Indication, techniques and results. Spine. 1991;16:S125–S133. doi: 10.1097/00007632-199103001-00018. [DOI] [PubMed] [Google Scholar]
- 8.Liljenqvist U, Mommsen U. Die operative Behandlug thorakolumbaler Wirbelsäulenverletzungen mit dem Fixateur interne und transpedikulärer Spongiosaplastik. Unfallchirurgie. 1995;21(1):30–39. doi: 10.1007/BF02588348. [DOI] [PubMed] [Google Scholar]
- 9.Blattert TR, Delling G, Weckbach A. Pedikuloskopisch assistierte transpedikuläre Spongiosaplastik zur interkorporellen Fusion an der lumbalen Wirbelsäule. Unfallchirurg. 2002;105(8):680–687. doi: 10.1007/s00113-001-0404-1. [DOI] [PubMed] [Google Scholar]
- 10.Knop C, Fabian HF, Bastian L, Blauth M. Late results of thoracolumbar fractures after posterior intrumentation and transpedicular bone grafting. Spine. 2001;26(1):88–99. doi: 10.1097/00007632-200101010-00016. [DOI] [PubMed] [Google Scholar]
- 11.Weckbach A, Vogel S. Einfluß der transpedikulären intercorporellen Spongiosaplastik auf den Korrekturverlust nach alleiniger dorsaler Instrumentierung thoracolumbaler Wirbelsäulenverletzungen. Unfallchirurg, Hefte zu. 1997;268:205–208. [Google Scholar]
- 12.Winkler H, Fischer M, Kessler T, Fernandez F, Köpke J. Korrekturverlust und Einheilungsverhalten transpedikulärer Spongiosaplastiken bei der Behandlung thorakolumbaler Wirbelfrakturen. Trauma Berufskrankh. 1999;1:294–301. doi: 10.1007/s100390050052. [DOI] [Google Scholar]
- 13.Goulet JA, Senunas LE, Silva GL, Greenfield ML. Autogenous iliac crest bone graft. Complications and functional assessment. Clin Orthop. 1997; (339):76–81. doi: 10.1097/00003086-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Kossmann T, Ertel W, Platz A, Trentz O. Die kombinierte Operation von Frakturen des thorakolumbalen Übergangs mit der Inlay-Span-Technik. Orthopade. 1999;28(5):432–440. doi: 10.1007/s001320050368. [DOI] [PubMed] [Google Scholar]
- 15.Kurz LT, Garfin SR, Booth RE., Jr Harvesting autogenous iliac bone grafts. A review of complications and techniques. Spine. 1989;14(12):1324–1331. doi: 10.1097/00007632-198912000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Wippermann Komplikationen der Spongiosaentnahme am Beckenkamm. Eine retrospektive Analyse von 1191 Fällen. Chirurg. 1997;68(12):1286–1291. doi: 10.1007/s001040050361. [DOI] [PubMed] [Google Scholar]
- 17.Knop C, Blauth M., Bühren V, Arand M, et al. Operative Behandlung von Verletzungen des thorakolumbalen Übergangs - Teil 3: Nachuntersuchung. Unfallchirurg. 2001;104(7):583–600. doi: 10.1007/s001130170089. [DOI] [PubMed] [Google Scholar]
- 18.Kostuik JP. Anterior fixation for burst fractures of the thoracic and lumbar spine with or without neurological involvement. Spine. 1988;13(3):286–293. doi: 10.1097/00007632-198803000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Liljenqvist U, O’Brien J, Renton P. Simultaneous combined anterior and posterior lumbar fusion with femoral cortical allograft. Eur Spine J. 1998;7:125–131. doi: 10.1007/s005860050042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molinari RW, Bridwell KH, Klepps SJ, Baldus C. Minimum 5-year follow-up of anterior column structural allografts in the thoracic and lumbar spine. Spine. 1999;24(10):967–972. doi: 10.1097/00007632-199905150-00007. [DOI] [PubMed] [Google Scholar]
- 21.Vanderschot P, Caluwe G, Lateur L, Broos P. The use of “hybrid” allografts in the treatment of fractures of the thoracolumbar spine: first experience. Eur Spine J. 2001;10:64–68. doi: 10.1007/s005860000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schultheiss M, Sarkar M, Arand M, Kramer M, et al. Solvent-preserved, bovine cancellous bone blocks used for reconstruction of thoracolumbar fractures in minimally invasive spinal fracture surgery—first clinical results. Eur Spine J. 2005;14:192–196. doi: 10.1007/s00586-004-0764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oda I, Cunningham BW, Abumi K, Kaneda K, McAfee PC. The stability of reconstruction methods after thoracolumbar total spondylectomy. An in vitro investigation. Spine. 1999;24(16):1634–1638. doi: 10.1097/00007632-199908150-00003. [DOI] [PubMed] [Google Scholar]
- 24.Stoltze D, Harms J. Korrekturen posttraumatischer Fehlstellungen. Prinzipien und Techniken. Orthopade. 1999;28(8):731–745. doi: 10.1007/s001320050403. [DOI] [PubMed] [Google Scholar]
- 25.Vahldiek MJ, Panjabi MM. Stability potential of spinal instrumentations in tumor vertebral body replacement surgery. Spine. 1998;23(5):543–550. doi: 10.1097/00007632-199803010-00006. [DOI] [PubMed] [Google Scholar]
- 26.Bhat AL, Lowery GL, Sei A. The use of titanium surgical mesh-bone graft composite in the anterior thoracic or lumbar spine after complete or partial corporectomy. Eur Spine J. 1999;8:304–309. doi: 10.1007/s005860050178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knop C, Lange U, Bastian L, Oeser M, Blauth M. Biomechanical compression tests with a new implant for thoracolumbar vertebral body replacement. Eur Spine J. 2001;10(1):30–37. doi: 10.1007/s005860000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kossmann T, Rancan M, Jacobi D, Trentz O. Minimally invasive vertebral replacement with cages in thoracic and lumbar spine. Eur J Trauma. 2001;27(6):292–300. [Google Scholar]
- 29.Lange U, Knop C, Bastian L, Blauth M. Prospective multicenter study with a new implant for thoracolumbar vertebral body replacement. Arch Orthop Trauma Surg. 2003;123(5):203–208. doi: 10.1007/s00402-003-0486-z. [DOI] [PubMed] [Google Scholar]
- 30.Vieweg U, Sölch O, Kalff R. Wirbelkörperersatzsystem Synex bei instabilen Berstungsfrakturen der Brust- und Lendenwirbelsäule - eine retraospektive Studie bei 30 Patienten. Zentralbl Neurochir. 2003;64:58–64. doi: 10.1055/s-2003-40373. [DOI] [PubMed] [Google Scholar]
- 31.Magerl F, Aebi M, Gertzbein SD, Harms J, Nazarian S. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J. 1994;3(4):184–201. doi: 10.1007/BF02221591. [DOI] [PubMed] [Google Scholar]
- 32.American Spinal Injuries Association (1992) ASIA classification. Standards for neurological and functional classification of spinal cord injury. Chicago, Illinois
- 33.Frankel HL, Hancock DO, Hyslop G, Melzak J. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Paraplegia. 1969;7:179–192. doi: 10.1038/sc.1969.30. [DOI] [PubMed] [Google Scholar]
- 34.Knop C, Oeser M, Bastian L, Lange U, Zdichavsky M, Blauth M. Entwicklung und Validierung des VAS-Wirbelsäulenscores. Unfallchirurg. 2001;104(6):488–497. doi: 10.1007/s001130170111. [DOI] [PubMed] [Google Scholar]
- 35.Oeser M. Bewertung von Behandlungsergebnissen nach Verletzungen der thorakolumbalen Wirbelsäule: Entwicklung und Validierung eines subjektiven Bewertungsmaßstabes. Medizinische Hochschule Hannover: Unfallchirurgische Klinik; 2000. [Google Scholar]
- 36.Bertram R, Bessem H, Diedrich O, Wagner U, Schmitt O. Vergleich von dorsolateralen mit dorsoventralen Stabilisierungsverfahren in der Behandlung von Wirbelfrakturen. Z Orthop. 2003;141:573–577. doi: 10.1055/s-2003-42841. [DOI] [PubMed] [Google Scholar]
- 37.Briem D, Rueger JM, Linhart W. Einheilung autologer Transplantate nach dorsoventraler Instrumentierung instabiler Frakturen der thorakolumbalen Wirbelsäule. Unfallchirurg. 2003;106(3):195–203. doi: 10.1007/s00113-002-0508-2. [DOI] [PubMed] [Google Scholar]
- 38.Knop C, Fabian HF, Bastian L, Rosenthal H, et al. Fate of the transpedicular intervertebral bone graft after posterior stabilisation of thoracolumbar fractures. Eur Spine J. 2002;11(3):251–257. doi: 10.1007/s00586-001-0360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu R, Mustard CA, Burns C. Epidemilogy of incident spinal fracture in a complete population. Spine. 1996;21:492–499. doi: 10.1097/00007632-199602150-00016. [DOI] [PubMed] [Google Scholar]
- 40.Magerl F, Engelhardt P (1994) 3. Brust- und Lendenwirbelsäule - Verlaufsformen. In: Witt AN, Rettig H, Schlegel HF (Hrsg.) Orthopädie in Klinik und Praxis. Spezielle Orthopädie (Wirbelsäule - Thorax - Becken), 1. Aufl., Thieme, Stuttgart, New York, pp 3.82–3.132
- 41.Bastian L, Knop C, Kulhawy T, Lange U, Blauth M (1999) Biomechanische Untersuchung zum Stabilitätsverhalten kombinierter knöcherner und diskoligamentärer Verletzungen der thorakolumbalen Wirbelsäule. Therapeutische Konsequenzen und Wertigkeit der AO-Klassifikation. in: Wilke HJ, Claes L (Hrsg.) Die traumatische und degenerative Bandscheibe., vol 271, edition 1. Aufl., Springer, Berlin Heidelberg New York, pp 52–61
- 42.Sawin PD, Traynelis VC, Menezes AH. A comparative analysis of fusion rates and donor-site morbidity for autogeneic rib and iliac crest bone grafts in posterior cervical fusions. J Neurosurg. 1998;88(2):255–265. doi: 10.3171/jns.1998.88.2.0255. [DOI] [PubMed] [Google Scholar]
- 43.Summers BN, Eisenstein SM. Donor site pain from the ilium. a complication of lumbar spine fusion. J Bone Joint Surg Br. 1989;71:677–680. doi: 10.1302/0301-620X.71B4.2768321. [DOI] [PubMed] [Google Scholar]
- 44.Briem D, Linhart W, Lehmann W, Bullinger M, et al. Untersuchung der Lebensqualität nach dorsoventraler Stabilisierung von Wirbelkörperfrakturen des thorakolumbalen Übergangs. Unfallchirurg. 2003;106(8):625–632. doi: 10.1007/s00113-003-0627-4. [DOI] [PubMed] [Google Scholar]
- 45.Defino Treatment of fractures of the thoracolumbar spine by combined anteriorposterior fixation using the Harms method. Eur Spine J. 1998;7:187–194. doi: 10.1007/s005860050054. [DOI] [PMC free article] [PubMed] [Google Scholar]