Abstract
The sagittal spinopelvic balance is poorly documented in normal pediatric subjects. The purpose of this study is to characterize the sagittal spinopelvic balance in the pediatric population and to evaluate the correlations between spinopelvic parameters. Seven parameters were evaluated from the lateral standing radiographs of 341 normal subjects aged 3–18 years old: thoracic kyphosis (TK), thoracic tilt (TT), lumbar lordosis (LL), lumbar tilt (LT), sacral slope (SS), pelvic tilt (PT) and pelvic incidence (PI). The mean values for the pelvic parameters were 49.1±11.0, 7.7±8.0 and 41.4±8.2° for PI, PT and SS, respectively. The mean values for the spinal parameters were 48.0±11.7, 44.0±10.9, −7.3±5.2 and −3.1±5.2° for LL, TK, LT and TT, respectively. The spinopelvic parameters were different from those reported in normal adults, but the correlations between the parameters were similar. PI was significantly related to SS and PT. Significant correlations were found between the parameters of adjacent anatomical regions. Pelvic morphology (PI) regulates sagittal sacro-pelvic orientation (SS and PT). Sacral orientation (SS) is correlated with the shape (LL) and orientation (LT) of the lumbar spine. Adjacent anatomical regions of the spine and pelvis are interdependent, and their relationships result in a stable and compensated posture, presumably to minimize energy expenditure. Results from this study could be used as an aid for the planning of surgery in pediatric patients with spinal deformity in order to restore a relatively normal sagittal spinopelvic balance.
Keywords: Kyphosis, Lordosis, Pelvic morphology, Pediatric orthopedics, Pelvis, Posture, Sagittal balance, Spine
Introduction
The human standing posture involves a delicate balance between the spine and pelvis. A balanced posture is obtained when these body segments are aligned in order to minimize energy expenditure [2, 5, 7]. Some authors have reported the normal values of sagittal spinal curves in children and adolescents [11, 21, 25, 30–32]. Other studies have also characterized the pelvic orientation and morphology in the sagittal plane of pediatric subjects [5, 11, 21–24]. Recently, Mac-Thiong et al. [21] documented the changes in sagittal spinal and pelvic parameters during growth in 180 normal subjects aged between 4 and 18 years. However, they did not report the correlation between these parameters, although it has been demonstrated that relationships between spinal and pelvic geometries strongly influence spinopelvic balance in adults. Berthonnaud et al. [2] have proposed the concept of a linear chain linking the head to the pelvis, in which shape and orientation of each anatomic segment are closely related and influence the adjacent segment to maintain a stable posture with minimum energy expenditure. Accordingly, a change in the shape or orientation of any anatomic segment will modify the shape and/or orientation of adjacent segments of the spine and pelvis. Up to now, there is no study documenting the correlations between spinal and pelvic sagittal geometries in the normal pediatric population. Knowledge of the normal spinopelvic balance in normal children and adolescents could be particularly helpful in the evaluation and surgical planning of pediatric patients with spinal deformity.
The purpose of this work is to investigate the spinopelvic balance in normal children and adolescents. More specifically, the main objectives are to evaluate the relationships between sagittal spinal and pelvic parameters, and to compare these results with a previous study of normal adults [2].
Materials and methods
A multicenter cohort of 341 normal children and adolescents from the clientele of eight spine surgeons were evaluated in this retrospective study. The first 180 subjects have already been reported in a previous study [21], while the last 161 subjects were recently added to the cohort. These subjects were referred for consultation by their family physician or pediatrician in order to rule out the presence of adolescent idiopathic scoliosis (AIS). All the subjects had standing posteroanterior (PA) and lateral (LAT) radiographs of the spine and pelvis done as part of their clinical evaluation to rule out AIS. All the radiographs were ordered by each spine surgeon and were not done specifically for this study. In all the participating institutions, a 30×90 cm LAT radiograph was obtained for each subject facing forward and standing in a comfortable position with the hips and knees fully extended. For all the subjects, the elbows were fully flexed and the fists were resting on the clavicles, as recommended by Faro et al. [8] and Horton et al. [12], in order to minimize the postural changes in the sagittal plane, while allowing adequate visualization of the spine.
The inclusion criteria for recruitment were: (1) availability of PA and LAT X-rays of the spine and pelvis, (2) no clinical or radiological evidence of spinal deformity, (3) age between 3 and 18 years, and (4) visibility of both femoral heads on the LAT radiographs. All subjects with a history or clinical signs of hip, pelvic or lower limb disorder were excluded from the study. Patients with radiological signs of Legg-Calve-Perthes disease or leg length discrepancy of more than 1 cm were excluded. There were 137 males and 204 females with a mean age of 12.1 years (standard deviation: 3.3). The mean age was similar between males and females.
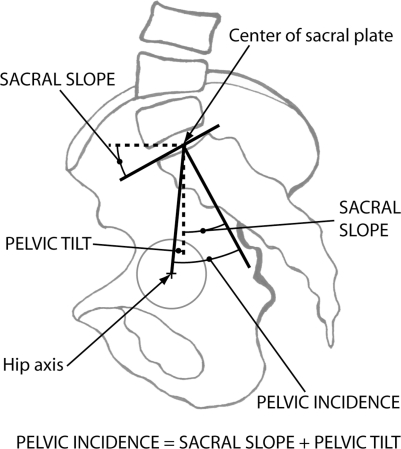
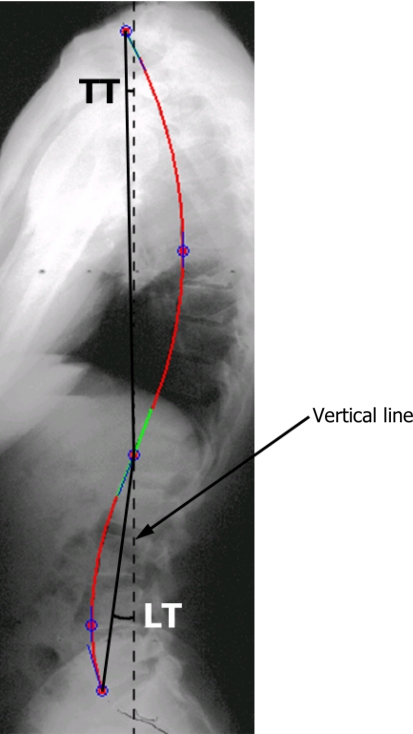
The SagittalSpine software (Optimage, Lyon, France) was used to assess the sagittal alignment of the spine and pelvis of all subjects. All computer measurements were done by the same experienced observer. The technique has been described in previous studies [1, 29] and can be summarized as follows. Pelvic incidence (PI), sacral slope (SS) and pelvic tilt (PT) (Fig. 1) were obtained after identifying eight anatomical landmarks: anterior and posterior corners of S1 vertebra, and any three points on the contour of each femoral head. The software also generates a geometric model of the spine in the sagittal plane (Fig. 2), which is composed of: (1) a thoracic segment, (2) a straight thoracolumbar junction and (3) a lumbar segment. The thoracic kyphosis (TK) and lumbar lordosis (LL) correspond to the angles contained by the two arcs of circle used to model the thoracic and lumbar segments, respectively. The intra- and inter-observer variability of these measurements is ±1° with intraclass correlation coefficients between 0.93 and 0.99, as reported by Berthonnaud et al. [3]. In addition, segmental balance was assessed from the orientation of the thoracic and lumbar segments with respect to a vertical line using the technique described by Berthonnaud et al. [2] (Fig. 3). The thoracic tilt (TT) and the lumbar tilt (LT) are positive when the segment is tilted forward and negative when tilted backward. In total, there were one morphological parameter (PI) that was not influenced by subject positioning, two pelvic orientation parameters (SS and PT), two spinal shape parameters (LL and TK) and two spinal orientation parameters (LT and TT).
Fig. 1.
Sagittal pelvic parameters measured on the standing lateral radiograph. The hip axis is located midway between the center of the femoral heads. Pelvic incidence is defined as the angle between the perpendicular of the upper sacral plate and the line joining the middle of the upper sacral plate and the hip axis. The sacral slope represents the angle between the sacral plate and the horizontal line. Pelvic tilt is measured from the angle between the vertical line and the line joining the middle of the upper sacral plate and the hip axis. It is positive when the hip axis lies in front of the middle of the upper sacral plate. Geometrically, pelvic incidence equals the sum of the sacral slope and the pelvic tilt
Fig. 2.
Geometric model of the spine generated by the software after identification of five anatomical landmarks (P1 to P5) and three tangent lines (T1 to T3). The thoracic and lumbar segments are intercalated by a straight thoracolumbar junction. The thoracic kyphosis (TK) and lumbar lordosis (LL) correspond to the angles contained by the arcs of circle used to model the thoracic (α1 and α2) and lumbar (β1 and β2) segments, respectively
Fig. 3.
Orientation of the thoracic (TT) and lumbar (LT) segments was measured with respect to a vertical line. The tilt is positive when the segment is tilted forward and negative when tilted backward
The data was analyzed using descriptive statistics and correlation studies with the Statistica software (StatSoft, Tulsa, OK, USA). Comparisons were made using two-tailed Student t and the relationships between parameters were assessed using Pearson’s coefficients. The level of significance was set to 0.01 due to the high number of statistical tests performed.
Results
Table 1 provides the measured parameters for all the subjects and separately for males and females. Females tend to have increased absolute values for all the measured parameters except SS. TT is significantly different between males and females, although the mean difference is only 1.5°. TT is tilted backward for 75.1% of subjects. The majority of subjects (93.5%) also stand with a negative LT.
Table 1.
Comparison of sagittal spinopelvic alignment (mean ± standard deviation) between males and females
| Parameter | All subjects (n=341) | Males (n=137) | Females (n=204) | P value |
|---|---|---|---|---|
| Mean age | 12.1±3.3 years (range: 3–18) | 12.3±3.2 years (range: 3–18) | 12.0±3.3 years (range: 3–18) | 0.5 |
| Pelvic incidence | 49.1±11.0° (range: 22.3–86.0) | 49.2±11.2° (range: 24.9–83.9) | 49.7±10.7° (range: 22.3–86.0) | 0.2 |
| Pelvic tilt | 7.7±8.0° (range: −17.2–30.0) | 6.5±7.5° (range: −10.2–30.0) | 8.5±8.3° (range: −17.2–29.7) | 0.02 |
| Sacral slope | 41.4±8.2° (range: 16.1–70.3) | 41.7±8.4° (range: 21.3–67.9) | 41.2±8.0° (range: 16.1–70.3) | 0.6 |
| Thoracic kyphosis | 44.0±10.9° (range: 5.4–76.4) | 43.7±12.3° (range: 5.4–76.4) | 44.1±9.9° (range: 15.2–70.8) | 0.8 |
| Lumbar lordosis | 48.0±11.7° (range: 15.1–101.4) | 46.6±10.8° (range: 15.1–74.1) | 48.8±12.2° (range: 21.2–101.4) | 0.08 |
| Thoracic tilt | −3.1±5.2° (range: −17.7–20.9) | −2.2±5.2° (range: −15.2–11.4) | −3.7±5.1° (range: −17.7–20.9) | 0.009* |
| Lumbar tilt | −7.3±5.2° (range: −22.1–18.7) | −6.7±5.8° (range: −22.1–18.7) | −7.7±4.8° (range: −20.8–7.6) | 0.07 |
*Statistically significant correlation (P<0.01)
Tables 2 and 3 present the values obtained in the current study in comparison to those of Mac-Thiong et al. [21] and Berthonnaud et al. [2], respectively. Results from the current study are similar to those Mac-Thiong et al. [21] reported for normal children and adolescents. All the parameters, except the SS, are significantly different between the current pediatric cohort and the reference adult cohort of Berthonnaud et al. [2].
Table 2.
Comparison of sagittal spinopelvic alignment (mean ± standard deviation) in the current study with historical data of normal children and adolescents
| Parameter | Current study (n=341) | Study of Mac-Thiong et al.a (n=180) | P value |
|---|---|---|---|
| Mean age | 12.1±3.3 years | 12.0±3.1 years | 0.59 |
| Pelvic incidence | 49.1±11.0° | 48.4±11.2° | 0.48 |
| Pelvic tilt | 7.7±8.0° | 7.2±7.9° | 0.52 |
| Sacral slope | 41.4±8.2° | 41.2±8.5° | 0.76 |
| Thoracic kyphosis | 44.0±10.9° | 43.0±10.4° | 0.35 |
| Lumbar lordosis | 48.0±11.7° | 48.5±12.4° | 0.60 |
aData from Mac-Thiong et al. (2004) Spine 29:1642–1647
Table 3.
Comparison of sagittal spinopelvic alignment (mean ± standard deviation) in the current study with historical data of normal adults
| Parameter | Current study (n=341) | Study of Berthonnaud et al.a (n=160) | P value |
|---|---|---|---|
| Mean age | 12.1±3.3 years (range: 3–18) | 25.7±5.5 years (range: 20–70) | <10−3* |
| Pelvic incidence | 49.1±11.0° (range: 22.3–86.0) | 51.8±5.3° (range: 33.7–83.7) | 0.003* |
| Pelvic tilt | 7.7±8.0° (range: −17.2–30.0) | 12.1±3.2° (range: −5.1–30.5) | <10−3* |
| Sacral slope | 41.4±8.2° (range: 16.1–70.3) | 39.7±4.1° (range: 21.2–65.9) | 0.016 |
| Thoracic kyphosis | 44.0±10.9° (range: 5.4–76.4) | 47.5±4.8° (range: 22.5–70.3) | <10−3* |
| Lumbar lordosis | 48.0±11.7° (range: 15.1–101.4) | 42.7±5.4° (range: 16.0–71.9) | <10−3* |
| Thoracic tilt | −3.1±5.2° (range: −17.7–20.9) | 0.7±2.0° (range: −10.8–8.8) | <10−3* |
| Lumbar tilt | −7.3 ± 5.2° (range: −22.1–18.7) | −5.9±2.3° (range: −16.8–10.8) | <10−3* |
aData from Berthonnaud et al. (2005) J Spinal Disord 18:40–47
*Statistically significant correlation (P<0.01)
Results from the correlation analysis between spinopelvic parameters and age are shown in Table 4. Only weak correlations (r<0.3) are observed and statistically significant relationships with age are found only for PI and PT, when all subjects are considered. TK and LT are related to age in males, but not in females. Globally, higher correlation coefficients are observed for pelvic parameters in females and for spinal parameters in males.
Table 4.
Pearson’s correlation coefficient (P value) between sagittal spinopelvic parameters and age
| Correlation analysis | All subjects (n=341) | Males (n=137) | Females (n=204) |
|---|---|---|---|
| Pelvic incidence versus age | 0.21 (P<10−3)* | 0.17 (P=0.05) | 0.24 (P<10−3)* |
| Pelvic tilt versus age | 0.23 (P<10−4)* | 0.19 (P=0.03) | 0.27 (P<10−3)* |
| Sacral slope versus age | 0.05 (P=0.3) | 0.05 (P=0.5) | 0.05 (P=0.5) |
| Thoracic kyphosis versus age | 0.19 (P=0.0102) | 0.24 (P=0.004)* | 0.06 (P=0.4) |
| Lumbar lordosis versus age | 0.14 (P=0.0106) | 0.15 (P=0.08) | 0.14 (P=0.05) |
| Thoracic tilt versus age | −0.08 (P=0.1) | −0.21 (P=0.013) | −0.01 (P=0.9) |
| Lumbar tilt versus age | −0.10 (P=0.06) | −0.29 (P<10−3)* | 0.04 (P=0.6) |
*Statistically significant correlation (P<0.01)
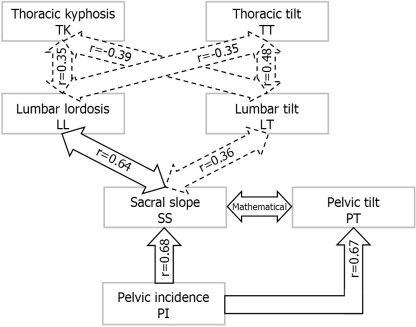
Correlations between all spinopelvic parameters are provided in Table 5. Pelvic incidence, which is the only morphological parameter, is significantly related to pelvic (PT and SS) and lumbar (LL and LT) parameters. SS is strongly related to LL and moderately related to LT. PT is only weakly related to LL and is not correlated with LT. Pelvic morphological and orientation parameters are not related to thoracic parameters. No statistical correlation is found between PT and SS in this study, although they are mathematically related (Fig. 1). Significant relationships are found between spinal shape parameters (LL vs TK) and between spinal orientation parameters (TT vs LT). Spinal shape and orientation parameters are weakly correlated only in the thoracic region (TK vs TT), while they are unrelated in the lumbar (LL vs LT) regions. Figure 4 provides an overview of the correlations observed between parameters of adjacent anatomical regions. Parameters are separated in three conceptual groups: morphology (PI), shape (LL and TK) and orientation (SS, PT, LT and TT). Figure 5 is a graph illustrating the relationship between PI and LL and the associated logistic regression equation.
Table 5.
Correlation analysis between sagittal spinopelvic parameters using Pearson’s coefficient (P value)
| Parameter | Pelvic tilt | Sacral slope | Thoracic kyphosis | Lumbar lordosis | Thoracic tilt | Lumbar tilt |
|---|---|---|---|---|---|---|
| Pelvic incidence | 0.67 (P<10−15)* | 0.68 (P<10−15)* | 0.08 (P=0.1) | 0.58 (P<10−15)* | −0.05 (P=0.3) | 0.34 (P<10−9)* |
| Pelvic tilt | −0.08 (P=0.1) | 0.10 (P=0.08) | 0.14 (P=0.0099)* | 0.05 (P=0.3) | 0.09 (P=0.08) | |
| Sacral slope | 0.01 (P=0.8) | 0.64 (P<10−15)* | −0.12 (P=0.02) | 0.36 (P<10−11)* | ||
| Thoracic kyphosis | 0.35 (P<10−10)* | 0.15 (P=0.005)* | −0.39 (P<10−13)* | |||
| Lumbar lordosis | −0.35 (P<10−10)* | 0.05 (P=0.4) | ||||
| Thoracic tilt | 0.48 (P<10−20)* |
*Statistically significant correlation (P<0.01)
Fig. 4.
Overview of statistical significant correlations between parameters of adjacent anatomical regions. Moderate (0.3≤r<0.5) and strong (r≥0.5) correlations are shown in dotted and full arrows, respectively. The mathematical relationship between pelvic tilt and sacral slope is also shown. The relationship between pelvic incidence and lumbar lordosis and between pelvic incidence and lumbar tilt, as well as weak correlations (0.1≤r<0.3), are not included in the figure
Fig. 5.
Logistic regression illustrating the relationship between lumbar lordosis and pelvic incidence
Discussion
It has been previously shown that pelvic morphology significantly influences the sagittal spinal geometry, especially LL [2, 10, 14, 19, 28, 29]. Some studies also suggest that there is an abnormal pelvic morphology and spinopelvic balance in patients with spondylolisthesis [4, 6, 11, 13, 15, 18, 24, 28] and AIS [20]. It is therefore important to understand thoroughly spinopelvic balance in normal individuals in order to evaluate the influence of pelvic morphology on the progression and treatment of spinal deformities. The cohort reported in the current study is the largest reported so far in the literature on pelvic morphology and spinopelvic balance in the pediatric population. In addition, this study is the first to suggest a postural model in the normal pediatric population based on the relationships between spinal and pelvic geometries.
This study confirms the findings of our preliminary work [21], with similar values for all measured parameters (less than 1° in mean difference) (Table 2). In addition, we report the global orientation of the kyphotic and lordotic segments of the spine in the thoracic and lumbar regions, respectively. TT and LT are negative for most patients, indicating a tendency for the backward tilt of both the thoracic and lumbar segments. This backward tilt of the spine may be required to counterbalance the upper body weight, which is mainly anterior to the spine, in order to maintain a stable posture.
As observed in our previous study [21], all statistically significant correlations between spinopelvic parameters and age are clinically weak (0.1≤r<0.3). However, in contrast to this study [21], only the age-related changes in PI and PT reach statistical significance, although the cohorts are similar in age (mean and standard deviation). Accordingly, PI and PT are significantly higher in normal adults (Table 3). The similar SS in pediatric and adult subjects is in agreement with the observed lack of relationship between SS and age. All spinal parameters are significantly different between children and adults, although they are not correlated with age (Table 4). It is possible that the relationship between spinal parameters and age during growth is not linear and, therefore, could not be quantified appropriately using Pearson’s coefficient. Another possible explanation for this finding is that the changes in spinal geometry during growth may not be associated with chronological age, but instead with other factors such as weight, height or bone age. In addition, since the adult reference cohort used for comparison was composed of individuals aged between 25 and 70 years, degenerative changes in the spine may have also intervened in some patients, as suggested in previous studies [9, 16, 17].
The results from the correlation study between all spinopelvic parameters are similar to those already reported in adults [2], except for PT and LT. Differences observed between adult and pediatric subjects in the strength of correlation for some parameters could be partly explained by the presence of ‘‘immature’’ control mechanisms of the sagittal balance in growing individuals. We have modified the postural model proposed by Berthonnaud et al. [2] to represent better the interdependence between morphology, shape and orientation of the spine and pelvis (Fig. 4). In this modified scheme of correlations, we suggest that PI (a morphological parameter) is of prime importance in determining the orientation of the pelvis (PT and SS). As indicated by the strong correlation coefficients, the morphology of the pelvis (PI) regulates both the orientation of the sacrum (SS) and of the pelvis (PT). Sacrum orientation (SS) is correlated with the shape (LL) and orientation (LT) of the lumbar spine. There was no statistical relationship between both the pelvic orientation parameters (SS vs PT), although they are mathematically related because for each individual PI is the sum of SS and PT (Fig. 1). Since each individual has a constant pelvic morphology (PI), the correlation between SS and PT is theoretically perfect (r = −1.0). The current investigation is a cross-sectional study, so there is a wide variation in PI when all the subjects are considered for statistical analysis. Consequently, we could not find a statistical relationship between SS and PT. When limiting the statistical analysis to subgroups of subjects with similar PI (results not shown), the correlation coefficient (r) reached statistical significance and was closer to the expected relationship (r = −1).
As previously described by Berthonnaud et al. [2], the correlation scheme (Fig. 4) also supports the concept by which parameters of adjacent anatomical regions are interdependent, and their relationships result in a stable and compensated posture, presumably to minimize energy expenditure. This concept does not imply a causal relationship, but suggests that a modification in the shape or orientation parameter in a given anatomical region will affect the adjacent anatomical regions. Correlations between the pelvic and lumbar regions were particularly strong, as opposed to the moderate correlations between the lumbar and thoracic regions. This last finding suggests that the thoracic parameters could also be significantly influenced by the cervical region, so as to provide adequate balance of the head over the pelvis. The failure to assess the cervical sagittal balance is a recognized limitation of this study and was due to the inadequate visualization of the cervical spine on the radiographs for most of the subjects.
Shape and orientation parameters of the thoracic and lumbar regions are not necessarily correlated, although a weak relationship (r=0.15) was found in the thoracic region between TK and TT (see Table 5, Fig. 4). This implies that subjects with highly variable TK or LL tend to stand with similar TT and LT. In addition, PT was not related to the shape and orientation of the spine, except for a weak correlation with the LL (r=0.14). These findings, added to the fact that the tilt parameters (TT, LT and PT) present the smallest range and standard deviation, as also observed in adults [2], suggest that the appropriate tilt of the pelvic, lumbar and thoracic regions may be the most important determinants to achieve a balanced posture. In particular, maintaining PT and LT within a strict range would be particularly important, since the pelvic and lumbar regions carry a great percentage of body weight and abnormal orientation of these spinal segments could result in undue energy expenditure to maintain a balanced posture.
Results from this study could be used as an aid in the planning of the surgical treatment of pediatric patients with spinal deformity, in order to provide an estimation of the sagittal spinal curves that need to be restored by the instrumentation. For example, the PI could be used to estimate the LL that needs to be set intraoperatively, because PI is a true morphological parameter (not affected by the positioning of pelvis or by surgery limited to the spine) and is significantly related to the LL. As shown in Fig. 5, the regression line between PI and LL can be used as a guide to estimate the amount of total LL that should be expected by the surgeon with respect to a specific PI value. Alternatively, the logistic regression equation provided can also be used. However, it has to be remembered that other parameters such as the remaining growth, the spinal flexibility and the vertebral anatomy should also be considered when estimating the optimal sagittal curves to be restored.
Conclusion
When evaluating and treating patients with spinal disorders, significant knowledge of the normal spinopelvic balance is of primary importance. This study documents the spinopelvic balance in normal children and adolescents, and describes a scheme of correlations between morphological, shape and orientation parameters of the spine and pelvis. It is found that the PI regulates the SS and PT. In addition, shape and orientation parameters of adjacent anatomical regions are interdependent, and their relationships result in a stable and compensated posture, presumably to minimize energy expenditure. Results from this study could be used as an aid in the planning of the surgical treatment of pediatric patients with spinal deformity, in order to provide an estimation of the sagittal spinal curves that need to be restored by the instrumentation.
Acknowledgments
The authors sincerely thank the following members of the Spinal Deformity Study Group for contributing cases to this study: John R. Dimar II (Kenton D. Leatherman Spine Center, Louisville, KY, USA), Peter O. Newton (Children’s Hospital and Health Center, San Diego, CA, USA), Charles E. Johnston II (Texas Scottish Rite Hospital for Children, Dallas, TX, USA), Keith H. Bridwell (Barnes-Jewish Hospital, St. Louis, MO, USA), Ensor E. Transfeldt (Twin Cities Spine Center, Minneapolis, MN, USA), and Michael F. O’Brien (Woodbridge Orthopaedic and Spine Center, Denver, CO, USA). This research was assisted by support from the Spinal Deformity Group. This research was funded by an educational/research grant from Medtronic Sofamor Danek, by the Canadian Institute of Health Research and by the Fonds de Recherche en Santé du Québec.
References
- 1.Berthonnaud É, Roussouly P, Dimnet J. The parameters describing the shape and the equilibrium of the set back pelvis and femurs in sagittal view. Innov Techn Biol Med. 1998;19:411–426. [Google Scholar]
- 2.Berthonnaud É, Dimnet J, Roussouly P, Labelle H. Analysis of the sagittal balance of the spine and pelvis using shape and orientation parameters. J Spinal Disord. 2005;18:40–47. doi: 10.1097/01.bsd.0000117542.88865.77. [DOI] [PubMed] [Google Scholar]
- 3.Berthonnaud É, Labelle H, Roussouly P, Grimard G, Vaz G, Dimnet J. A variability study of computerized sagittal spinopelvic radiological measurements of trunk balance. J Spinal Disord. 2005;18:66–71. doi: 10.1097/01.bsd.0000128345.32521.43. [DOI] [PubMed] [Google Scholar]
- 4.Curylo LJ, Edwards C, DeWald RW. Radiographic markers in spondyloptosis. Implications for spondylolisthesis progression. Spine. 2002;27:2021–2025. doi: 10.1097/00007632-200209150-00010. [DOI] [PubMed] [Google Scholar]
- 5.Descamps H, Commare-Nordmann MC, Marty C, Hecquet J, Duval-Beaupère G. Modification of pelvic angle during the human growth (in French) Biom Hum Anthropol. 1999;17:59–63. [Google Scholar]
- 6.During J, Goudfrooij H, Keessen W, Beekr TW, Crowe A. Toward standards for posture. Postural characteristics of the lower back system in normal and pathologic conditions. Spine. 1985;10:83–87. doi: 10.1097/00007632-198501000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Duval-Beaupère G, Schimdt C, Cosson P. A barycentremetric Study of the sagittal shape of spine and pelvis: the conditions required for an economic standing position. Ann Biomed Eng. 1992;20:451–462. doi: 10.1007/BF02368136. [DOI] [PubMed] [Google Scholar]
- 8.Faro FD, Marks MC, Pawelek J, Newton PO. Evaluation of a functional position for lateral radiograph acquisition in adolescent idiopathic scoliosis. Spine. 2004;29:2284–2289. doi: 10.1097/01.brs.0000142224.46796.a7. [DOI] [PubMed] [Google Scholar]
- 9.Gelb DE, Lenke LG, Bridwell KH, Blanke K, McEnery KW. An analysis of sagittal alignment in 100 asymptomatic middle and older aged volunteers. Spine. 1995;20:1351–1358. doi: 10.1097/00007632-199506000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Guigui P, Levassor N, Rillardon L, Wodecki P, Cardinne L. Physiological value of pelvic and spinal parameters of sagittal balance: analysis of 250 healthy volunteers (in French) Rev Chir Orthop Reparatrice Appar Mot. 2003;89:496–506. [PubMed] [Google Scholar]
- 11.Hanson DS, Bridwell KH, Rhee JM, Lenke LG. Correlation of pelvic incidence with low- and high-grade isthmic spondylolisthesis. Spine. 2002;27:2026–2029. doi: 10.1097/00007632-200209150-00011. [DOI] [PubMed] [Google Scholar]
- 12.Horton WC, Brown CW, Bridwell KH, Glassman SD, Suk S-I, Cha CW. Is there an optimal patient stance for obtaining a lateral 36′′ radiograph? A critical comparison of three techniques. Spine. 2005;30:427–433. doi: 10.1097/01.brs.0000153698.94091.f8. [DOI] [PubMed] [Google Scholar]
- 13.Inoue H, Ohmori K, Miyasaka K. Radiographic classification of L5 isthmic spondylolisthesis as adolescent or adult vertebral slip. Spine. 2002;27:831–838. doi: 10.1097/00007632-200204150-00010. [DOI] [PubMed] [Google Scholar]
- 14.Jackson RP, Hales C. Congruent spinopelvic alignment on standing lateral radiographs of adult volunteers. Spine. 2000;25:2808–2815. doi: 10.1097/00007632-200011010-00014. [DOI] [PubMed] [Google Scholar]
- 15.Jackson RP, Phipps T, Hales C, Surber J. Pelvic lordosis and alignment in spondylolisthesis. Spine. 2003;28:151–160. doi: 10.1097/00007632-200301150-00011. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi T, Atsuta Y, Matsuno T, Takeda N. A longitudinal study of congruent sagittal spinal alignement in an adult cohort. Spine. 2004;29:671–676. doi: 10.1097/01.BRS.0000115127.51758.A2. [DOI] [PubMed] [Google Scholar]
- 17.Korovessis PG, Stamatakis MV, Baikousis AG. Reciprocal angulation of vertebral bodies in the sagittal plane in an asymptomatic Greek population. Spine. 1998;23:700–704. doi: 10.1097/00007632-199803150-00010. [DOI] [PubMed] [Google Scholar]
- 18.Labelle H, Roussouly P, Berthonnaud É, Transfeldt E, O’Brien M, Chopin D, Hresko T, Dimnet J. Spondylolisthesis, pelvic incidence, and spinopelvic balance. A correlation study. Spine. 2004;29:2049–2054. doi: 10.1097/01.brs.0000138279.53439.cc. [DOI] [PubMed] [Google Scholar]
- 19.Legaye J, Duval-Beaupère G, Hecquet J, Marty C. Pelvic incidence: a fundamental pelvic parameter for three-dimensional regulation of spinal sagittal curves. Eur Spine J. 1998;7:99–103. doi: 10.1007/s005860050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mac-Thiong J-M, Labelle H, Charlebois M, Huot M-P, Guise JA. Sagittal plane analysis of the spine and pelvis in adolescent idiopathic scoliosis according to the coronal curve type. Spine. 2003;28:1404–1409. doi: 10.1097/00007632-200307010-00010. [DOI] [PubMed] [Google Scholar]
- 21.Mac-Thiong J-M, Berthonnaud É, Dimar JR, II, Betz RR, Labelle H. Sagittal alignment of the spine and pelvis during growth. Spine. 2004;29:1642–1647. doi: 10.1097/01.BRS.0000132312.78469.7B. [DOI] [PubMed] [Google Scholar]
- 22.Mangione P, Sénégas J. Normal and pathologic sagittal balance of the spine and pelvis (in French) Rev Chir Orthop Reparatrice Appar Mot. 1997;83:22–32. [PubMed] [Google Scholar]
- 23.Mangione P, Gomez D, Senegas J. Study of the course of the incidence angle during growth. Eur Spine J. 1997;6:163–167. doi: 10.1007/BF01301430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marty C, Boisaubert B, Descamps H, Montigny JP, Hecquet J, Legaye J, Duval-Beaupère G. The sagittal anatomy of the sacrum among young adults, infants, and spondylolisthesis patients. Eur Spine J. 2002;11:119–125. doi: 10.1007/s00586-001-0349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Öhlen G, Aaro S, Bylund P. The sagittal configuration and mobility of the spine in idiopathic scoliosis. Spine. 1988;13:413–416. doi: 10.1097/00007632-198804000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Poussa M, Härkönen H, Mellin G. Spinal mobility in adolescent girls with idiopathic scoliosis and in structurally normal controls. Spine. 1989;14:217–219. doi: 10.1097/00007632-198902000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Propst-Proctor SL, Bleck EE. Radiographic determination of lordosis and kyphosis in normal and scoliotic children. J Pediatr Orthop. 1983;3:344–346. doi: 10.1097/01241398-198307000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Rajnics P, Templier A, Skalli W, Lavaste F, Illés T. The association of sagittal spinal and pelvic parameters in asymptomatic persons and patients with isthmic spondylolisthesis. J Spinal Disord. 2002;15:24–30. doi: 10.1097/00024720-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Vaz G, Roussouly P, Berthonnaud E, Dimnet J. Sagittal morphology and equilibrium of pelvis and spine. Eur Spine J. 2002;11:80–87. doi: 10.1007/s005860000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vedantam R, Lenke LG, Keeney JA, Bridwell KH. Comparison of standing sagittal spinal alignment in asymptomatic adolescents and adults. Spine. 1998;23:211–215. doi: 10.1097/00007632-199801150-00012. [DOI] [PubMed] [Google Scholar]
- 31.Voutsinas SA, MacEwen GD. Sagittal profiles of the spine. Clin Orthop. 1986;210:235–242. [PubMed] [Google Scholar]
- 32.Wright JG, Bell D. Lumbosacral joint angles in children. J Pediatr Orthop. 1991;11:748–751. doi: 10.1097/01241398-199111000-00009. [DOI] [PubMed] [Google Scholar]