Abstract
The effect of the duration of symptoms on the outcome of lumbar decompression surgery is not known. The aim of our study was to determine the predictors of functional outcome of lumbar decompression surgery for degenerative spinal stenosis with particular emphasis on the duration of symptoms. In this prospective cohort study, we recruited 100 patients with a full data set available at 1-year and 85% at 2-year follow-ups: 49 females and 51 males with an average age of 62 (range 52–82). The pre- and post-operative outcome measures were Oswestry disability index (ODI), low back outcome score (LBOS), pain visual analogue score (VAS), modified somatic perception (MSP) and modified Zung depression (MZD) score. Dural tear occurred in 14%, and there was one post-operative extra-dural heamatoma. Overall, the ODI improved from a pre-operative of 56 (±13) to a 1-year ODI of 40 (±22) and at 2-year ODI of 40 (±21). The VAS improved from an average of 8 to 5.2 at 1 year and 4.9 at 2 years. There was a statistical significant association between symptom duration and the change in ODI (P=0.007 at 1-year follow-up, P=0.001 at 2-year follow-up), LBOS (P=0.001 at 1-year follow-up, P<0.001 at 2-year follow-up) and VAS (P=0.003 at 1-year follow-up, P=0.001 at 2-year follow-up). Subgroup analyses showed that patients with symptom duration of less than 33 months had a more favourable result. In addition, the patients who rated the operation as excellent had a statistically significantly shorter duration of symptoms. We have not found a predictive value for age at operation, MSP or MZD. The number of levels of decompression and the different types of decompression surgery did not influence the surgical results. Our study indicates that the symptom duration of more than 33 months has a less favourable functional outcome.
Keywords: Duration of symptoms, Predictors, Degenerative spinal stenosis, Lumbar decompression surgery, Functional outcome
Introduction
The general incidence of symptomatic lumbar spinal stenosis ranges from 1.7 to 8% [5]. It typically occurs in the fifth to seventh decades of life. However, with the advent of more sophisticated imaging technology, spinal stenosis is likely to be diagnosed more frequently in the increasing aging population. Although the initial treatment should be conservative, the published long-term outcome may be unsatisfactory [2, 3, 15, 25, 27].
The aim of surgery is to relieve disabling leg pain and to restore function by decompressing the stenotic spinal canal. Improved function would be an improved walking distance before the onset of spinal claudication. However, the functional outcome reported in the literature is variable. A meta-analysis conducted by Turner et al. [31] reported a success rate ranging from 26 to 100%. Katz et al. [17] have also shown that up to 40% do not benefit from the surgery. Various predictors for surgical outcomes have been studied in an effort to improve patient selection. A less favourable outcome has been associated with female sex [10] and co-morbidity. Specifically previous back surgery [1, 33], diabetes [1], previous lumbar spine fracture [1], cardiovascular disease [33], coexistence of bone and joint degenerative disease [1, 33], degenerative scoliosis [7], anteroposterior spinal canal diameter of more than 6 mm [14], multi-level decompression [8] and patients associated with compensation or litigation issues [12].
Few studies have investigated the effect of symptom duration on the outcome of surgery. As the symptoms are not life threatening and often progress slowly, there is usually adequate time to pursue conservative management strategies prior to contemplating surgical intervention. However, based on the evidence presented in the literature, the optimum timing of surgical intervention remains unclear. Johnsson et al. [15] concluded a more favourable surgical outcome in patients with symptom duration of less than 4 years. Herron et al. [12] and McGregor et al. [21] did not find any association between symptom duration and surgical outcome.
The objective of this prospective study was to evaluate the outcome and prognostic factors in a cohort of consecutive patients scheduled for lumbar decompression surgery. The specific aim was to ascertain the effect of pre-operative duration of symptoms on the surgical results over a 2-year follow-up.
Patients and methods
The study was carried out at Leicester General Hospital and the patients were recruited from a spine specialist’s surgical cohort. The inclusion and exclusion criteria for this study are shown in Table 1. Written informed consent was obtained from all patients. Patients were prospectively followed-up at 6 weeks, 3 months, 6 months, 1 and 2 years post-operatively.
Table 1.
Inclusion and exclusion criteria for the prospective cohort study
| Inclusion criteria |
| 1. Degenerative central spinal stenosis |
| 2. Degenerative spondylolistheisis |
| 3. Degenerative scoliosis curve less than 20° |
| 4. Neurogenic claudication |
| 5. MRI/CT confirmation of the pathology |
| 6. Failed conservative management of at least 3 months |
| 7. Ability to fill in assessment form |
| Exclusion criteria |
| 1. Previous spinal surgery |
| 2. Vascular claudication |
| 3. Comorbid pathology affecting walking ability |
| 4. Bony metastases |
| 5. Unfit for general anaesthesia |
The recorded pre-operative parameters include gender, age at operation, duration of symptoms (months), modified Zung depression score (MZD) and modified somatic perception score (MSP). The outcomes were assessed according to Oswestry disability index (ODI) score, low back outcome score (LBOS), a 100 mm visual analogue score (VAS) for pain and a subjective evaluation of the surgical results (excellent, good, fair, poor). We recorded both the absolute score and the change in ODI, LBOS and VAS between pre- and post-operative statuses. A reduction of more than 20% of the ODI score was considered clinically significant [29].
All patients underwent posterior lumbar decompression surgery with and without fusion performed by the senior author only. Inter-segmental decompression and undercutting of the facet joint was performed to decompress the traversing and exiting nerve roots at the affected level. In multilevel stenosis midline laminectomy was performed with preservation of the facet joints wherever possible. Non-instrumented fusion was carried out as an inter-transverse fusion using autologous bone graft. Instrumented fusion was performed with pedicle screws and rods (AO USS). Indications for an instrumented fusion were patients with age less than 60, multi-level decompression, degenerative scoliosis, or significant retrolisthesis or spondylolisthesis.
The statistical analyses were performed using SPSS statistical software (version 12.0 for Windows, SPSS Inc, Chicago, IL, USA). The result of the operation was correlated to the pre-operative parameters, which include duration of symptoms, age at operation, MSP score and MZD score. Subgroup analyses were carried out using one-way analysis of variance. The statistical significance was set at P value <0.05.
Between 1994 and 2001, 119 consecutive patients with clinical and radiological signs of degenerative central lumbar spinal stenosis underwent decompression surgery. Nineteen patients were excluded from the study because they had revision decompression surgery. We prospectively followed-up 100 patients with a full data set available at 1-year follow-up and 85% at 2-year follow-up. Ten patients died of old age and five patients were lost to follow-up. Table 2 shows the baseline demographics for all the patients.
Table 2.
Baseline data of patients included in the study [mean + (range) where applicable]
| Gender | 49 females | 51 males |
|---|---|---|
| Age | 62.4 (55–82) | |
| Duration of symptoms (months) | 73 (12–360) | |
| Pre-operative parameters | ODI | 56.6% (28–84) |
| LBOS | 18.34 (3–46) | |
| MZD | 27.13 (0–56) | |
| MSP | 7.12 (0–24) | |
| Types of decompression surgery (No. of cases) | Decompression only | 31 |
| Decompression with non-instrumented fusion | 33 | |
| Decompression with instrumented fusion | 36 | |
| Number of levels of decompression | One level | 29 |
| Two levels | 50 | |
| Three levels | 17 | |
| Four levels | 4 | |
Ninety-four percent of the patients involved in the study presented with back and unilateral or bilateral leg pain. Six patients (6%) had only neurogenic claudication. All the patients had received at least 3 months of conservative management that included physiotherapy and appropriate analgesia. Thirteen patients (13%) had received additional nasal or subcutaneous treatment with calcitonin. Eighteen patients (18%) received peri-radicular infiltration for radicular pain, 14 patients (14%) had epidural injection and 7 patients (7%) had facet joint injection prior to surgical intervention.
We also performed subgroup analysis to examine if the number of levels of decompression surgery and type of decompression method (i.e. decompression only, decompression with and without instrumented fusion) can influence the results of surgery.
Results
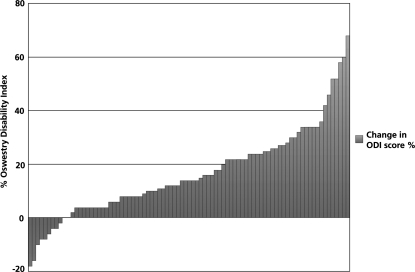
Table 3 shows the overall outcome of the results at 1- and 2-year follow-ups. Figure 1 shows the changes in the ODI score for all the available patients at 2-year follow-up. Thirty-nine percent of the patients had a significant reduction of at least 20% of the ODI score at 2 years. The recorded complications include dural tear (14%), extra-dural haematoma resulting in cauda equina syndrome (1%) and post-operative congestive heart failure (1%).
Table 3.
The functional outcome of lumbar decompression surgery at 1- and 2-year follow-ups (mean ± SD)
| Pre-operative | One-year follow-up | Two-year follow-up | |
|---|---|---|---|
| ODI | 56.6±13.2 | 39.55±22.3 | 31.61±21.6 |
| LBOS | 18.34±9.3 | 29.85±18.8 | 29.84±17.7 |
| VAS | 8.03±1.7 | 5.21±2.7 | 4.86±2.7 |
Fig. 1.
Overview of results of change in ODI score for all available patients at 2-year follow-up
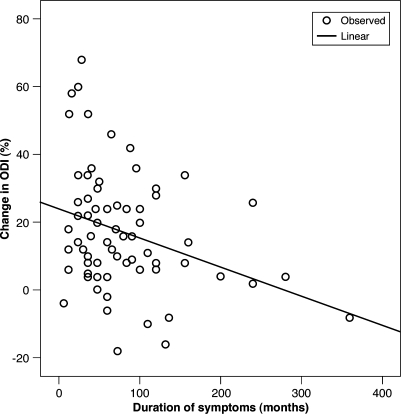
The results of multiple regression analysis using change in ODI as the dependent variable is shown on Table 4. The duration of neurogenic claudication was related to the change in the ODI score (P=0.004 at 1 year, P=0.001 at 2 years), the change in LBOS (P=0.001 at 1 year, P<0.001 at 2 years) and the change in VAS (P=0.003 at 1 year and P=0.001 at 2 years) at both 1- and 2-year follow-ups. The correlations between the change in ODI and pre-operative duration of symptoms can be further illustrated on a scatter plot graph as shown in Fig. 2 (Spearman rank correlation = −0.305, P=0.009)
Table 4.
Association between predictors and change in ODI scores at both 1- and 2-year follow-ups as determined by multiple linear regression analysis
| Predictors | Coefficient b ± SE | t test | P value | |||
|---|---|---|---|---|---|---|
| One-year follow-up | Two-year follow-up | One-year follow-up | Two-year follow-up | One-year follow-up | Two-year follow-up | |
| Duration of symptoms | −0.09±0.03 | −0.10±0.03 | −2.75 | −3.37 | 0.007* | 0.001* |
| Age | −0.11±0.18 | −0.18±0.17 | −0.61 | −1.05 | 0.55 | 0.30 |
| MSP | −0.49±0.5 | 0.16±0.49 | −0.94 | 0.34 | 0.35 | 0.74 |
| MZD | −0.02±0.2 | 0.03±0.20 | −0.10 | 0.16 | 0.92 | 0.88 |
*Statistically significant
Fig. 2.
Scatter plot graph showing a negative correlation between the change in ODI and the duration of symptoms
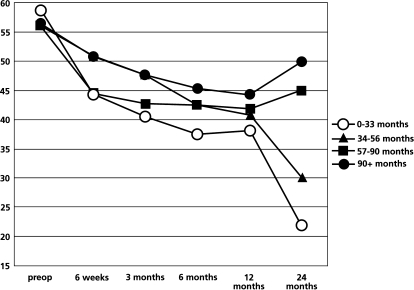
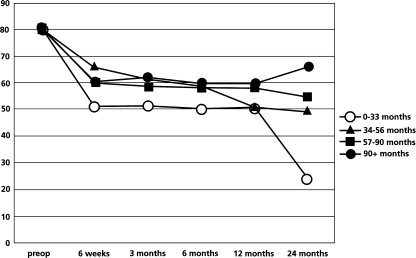
We subdivided the patients into four quartiles to further explore the association between symptom duration and surgical outcome as shown in Table 5. One-way analysis of variance had demonstrated significant statistical difference among groups of patients at 2-year follow-up (P=0.003). The group of patients with symptoms duration of less than 33 months had the greatest reduction in ODI score. The trends f outcomes measures, which include absolute value of ODI, LBOS and VAS over 2 years, follow-up among the subgroups are shown in Figs. 3, 4 and 5. In addition, the subgroup of patients who received surgery before 33 months of pain duration did not differ from the other subgroups regarding age, sex, number of level of decompression and type of surgery.
Table 5.
Mean change in ODI score at 2-year follow-up for subgroup of patients with different duration of symptoms
| Subgroup (months) | Mean change in ODI ± SD |
|---|---|
| Less than 33 | 31.05±13.41 |
| 33–55.9 | 17.64±17.72 |
| 56–89.9 | 13.95±17.57 |
| More than 90 | 11.42±15.48 |
Fig. 3.
The trend of absolute value of post-operative ODI score (%) over 2-year follow-up
Fig. 4.
The trend of absolute value of post-operative LBOS over 2-year follow-up
Fig. 5.
The trend of absolute value of post-operative VAS (100 mm scale) over 2-year follow-up
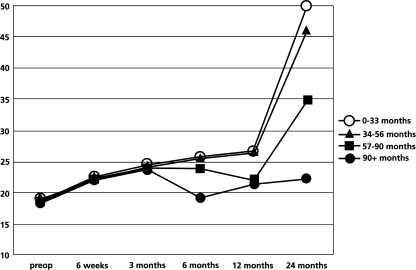
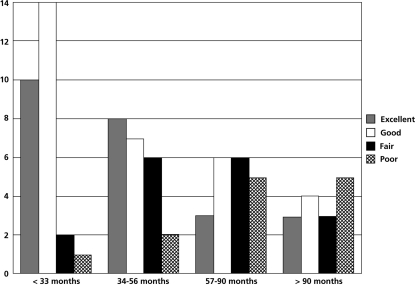
Among the 85 patients available for follow-up at 2 years, 68% of the patients expressed the outcome of the procedure as excellent or good. Figure 6 shows the level of patient satisfaction according to the subgroups of patients with different duration of symptoms.
Fig. 6.
Subgroup analysis of the level of patient satisfaction according to the duration of symptoms
We also subdivided the patients into groups according to the level of patient satisfaction as shown in Table 6. One-way analysis of variance has shown that the duration of symptoms of a subgroup of patients who rated the operation as excellent was statistically significantly different from all the other groups (fair and poor).
Table 6.
Post hoc comparison test results for subgroups of patients with different levels of outcome satisfaction
| Outcome satisfaction | Mean duration of symptoms ± SD | Group comparison | P value |
|---|---|---|---|
| Excellent | 43.85±34 | Fair | 0.06* |
| Poor | 0.03* | ||
| Good | 62.5±55 | Fair | 0.14 |
| Poor | 0.27 | ||
| Fair | 65.13±46 | Excellent | 0.06* |
| Good | 0.14 | ||
| Poor | 98±87 | Excellent | 0.03* |
| Good | 0.27 |
We did not find any statistically significant difference between the subgroups of patients with different levels of lumbar decompression surgery and types of decompression surgery using one-way analysis of variance and change in ODI as outcome measures at 2 years.
Multiple regression analysis did not show any predictive value for pre-operative MSP, MZD and age at operation with all the outcome measures at 2-year follow-up.
Discussion
Despite a heightened awareness of the natural history of degenerative lumbar spinal stenosis [15, 27], the optimum timing of decompression surgery remains unclear. Early operative intervention was recommended in the past for the treatment of symptomatic spinal stenosis based on the view that the disease is always progressive [28]. However, Johnsson et al. [15] noted that 70% of patients’ clinical status remains unchanged over period of 4 years. Although there is adequate time to pursue non-operative options, the duration of a trial conservative management remains unclear. Various long-term studies have also shown that they did not provide sustained improvement [6, 11]. A trial of aggressive non-operative management with therapeutic exercise, analgesics and epidural steroids injection produce little or no improvement in 75% of the non-operated patients over an average period of 33 months [27]. Kornblum et al. [18] recommended surgery for patients who failed 3 months non-operative treatment.
Conflicting results have been found in several studies that have analysed the predictive value of symptom duration to the outcome of surgery [12, 15, 21]. A cohort study by Johnsson et al. [14] with a 5-year follow-up found that patients with symptom duration for more than 4 years had a less favourable result. In contrast, McGregor et al. [21] did not find any association between symptom duration and the outcome of surgery at 1-year follow-up. The difference in the clinical findings may be due to differences in the outcome measures used in the studies. The results are not comparable, as patients with longer follow-up period tend to have less favourable results. Three studies with follow-up between 5 and 10 years have shown that 20–30% of the patients had unsatisfactory results [4, 16, 26].
Our prospective cohort study focused on the predictive value of symptom duration on primary lumbar decompression surgery using standard validated outcome measures that includes ODI, LBOS and VAS. Previous studies included patients who had other spine surgery and revision lumbar decompression surgery [12, 15]. We found a significant negative correlation between symptoms duration and the change in ODI (Table 4), LBOS and VAS at both 1- and 2-year follow-ups. Based on our subgroup analyses using the change in ODI as the outcome measure, the functional outcome was more favourable in those patients with duration of symptoms of less than 33 months. In addition, those patients who rated the outcome of the operation as excellent tend to have much shorter duration of symptoms compared to those who rated the operation as good, fair or poor (Table 6).
Neurogenic claudication may occur as a result of venous congestion, which leads to ischaemia, axonal damage and intra-neural fibrosis [27]. It has been shown that the prolonged compression of the cauda equina in a stenotic spinal canal can lead to nerve root ischaemia and demyelination [32]. An experimental study with porcine cauda equine has also shown that the recovery of nerve root function is dependent on the duration of compression [23]. Chronic pain can lead to permanent pathological changes in nociceptive transmission in the spinal cord [19, 24]. A previous study on the duration of sciatica and the outcome of lumbar discectomy has shown a relationship between duration of symptoms and functional outcome [22].
We routinely use MZD and MSP as part of our assessment of patients’ pre-operative psychosocial status in particular depression, distress and somatization. Three studies have shown that patients who are depressed or highly somatized tend to have a less favourable outcome for lumbar discectomy surgery [13, 30]. We did not find any predictive value of pre-operative MSP and MZD score for the outcome of lumbar decompression surgery for spinal stenosis. The differences in the patient characteristics of these two different pathologies may account for this clinical finding.
Our subgroup analysis has also shown that there was no difference in the functional outcome between patients who had lumbar decompression only, decompression with inter-transverse fusion using autologous bone graft or decompression with instrumental fusion. Various studies support our clinical findings [9, 20]. However, it is obvious that there was a selection bias built into each group in our study. Decompression with instrumented fusion was more likely to be performed in patients with degenerative spondylolisthesis, radiological evidence of instability, decompression of more than two levels or patients aged less than 60. Interpretation that there is no difference between the different types of surgery is therefore not valid on the available evidence in this paper. Our study has shown that the number of levels of decompression did not influence the functional outcome at 2 years. Herron et al. [12], Zheng et al. [33] and Turner’s meta-analysis [31] had similar findings.
Conclusion
This paper contributes to the discussions between surgeons and patients considering lumbar decompression surgery. Although our study has shown that a prolonged duration of symptoms is associated with a less favourable outcome, the balance between the quality of life for the patient and risk assessment of co-morbidity should be carefully considered. Provided that the patient in not limited in the lifestyle or bothered by the symptoms, reassurance and watchful waiting are appropriate. Based on our study population, those patients with duration of symptoms of less than 33 months had a better outcome at 2-year follow-up.
References
- 1.Airaksinen O, Herno A, Turunen V, Saari T, Suolainen O. Surgical outcome of 438 patients treated surgically for lumbar spinal stenosis. Spine. 1997;22:2278–2282. doi: 10.1097/00007632-199710010-00016. [DOI] [PubMed] [Google Scholar]
- 2.Amundsen T, Weber H, Nordal HJ, Magnaes B, Abdelnoor M, Lilleas F. Lumbar spinal stenosis: conservative or surgical management? Spine. 2000;25(11):1424–1436. doi: 10.1097/00007632-200006010-00016. [DOI] [PubMed] [Google Scholar]
- 3.Atlas SJ, Deyo RA, Keller RB, Robson D, Deyo RA, Singer DE. Surgical and non-surgical management of lumbar spinal stenosis: four year outcomes from the Maine lumbar spine study. Spine. 2000;25:556–562. doi: 10.1097/00007632-200003010-00005. [DOI] [PubMed] [Google Scholar]
- 4.Caputy AJ, Luessenhop AJ. Long-term evaluation of decompressive surgery for degenerative lumbar stenosis. J Neurosurg. 1992;77:669–676. doi: 10.3171/jns.1992.77.5.0669. [DOI] [PubMed] [Google Scholar]
- 5.Villiers PD, Booysen EL. Fibrous spinal stenosis: a report on 850 myelograms with a water soluble contrast medium. Clin Orthop. 1976;115:140–144. [PubMed] [Google Scholar]
- 6.Eskola A, Pohjolainen T, Alaranta H, Soini J, Tallroth K, Slatis P. Calcitonin treatment in lumbar spinal stenosis: a randomized, placebo-controlled, double-blind, cross-over study with one year follow-up. Calcif Tissue Int. 1992;50(5):400–403. doi: 10.1007/BF00296769. [DOI] [PubMed] [Google Scholar]
- 7.Frazier DD, Lipson SJ, Fossel AH, Katz JN. Associations between spinal deformity and outcomes after decompression for spinal stenosis. Spine. 1997;22:2025–2029. doi: 10.1097/00007632-199709010-00017. [DOI] [PubMed] [Google Scholar]
- 8.Grabias S. Current concepts review: the treatment of spinal stenosis. J Bone Joint Surg (Am) 1980;62:308–313. [PubMed] [Google Scholar]
- 9.Grob D, Humke T, Dvorak J. Degenerative lumbar spinal stenosis. Decompression with and without arthrodesis. J Bone Joint Surg (AM) 1995;77:1036–1041. doi: 10.2106/00004623-199507000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Herno A, Airaksinen O, Saari T. Long-term results of surgical treatment of lumbar spinal stenosis. Spine. 1993;18:1471–1474. doi: 10.1097/00007632-199318110-00011. [DOI] [PubMed] [Google Scholar]
- 11.Herno A, Airaksinen O, Saari T, Luukkonen M. Lumbar spinal stenosis: a matched-pair study of operated and operated patients. Neurosurgery. 1996;10:461–465. doi: 10.1080/02688699647087. [DOI] [PubMed] [Google Scholar]
- 12.Herron L, Mangelsdorf C. Lumbar spinal stenosis: results of surgical treatment. J Spinal Disord. 1991;4:26–33. [PubMed] [Google Scholar]
- 13.Hurme M, Alaranta H. Factors predicting the results of surgery for lumbar intervertebral disc herniation. Spine. 1987;12:933–938. doi: 10.1097/00007632-198711000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Johnsson B, Annertz M, Sjoberg C, Stromqvist B. A prospective and consecutive study of surgically treated lumbar spinal stenosis: part II: five year follow-up by an independent observer. Spine. 1997;22(24):2938–2944. doi: 10.1097/00007632-199712150-00017. [DOI] [PubMed] [Google Scholar]
- 15.Johnsson KE, Rosen I, Uden A. The natural course of lumber spinal stenosis. Clin Orthop. 1992;279:82–86. [PubMed] [Google Scholar]
- 16.Katz JN, Lipson SJ, Chang LC, Levine SA, Fossel AH, Liang MH. Seven to ten year outcome of decompressive surgery for degenerative lumbar spinal stenosis. Spine. 1994;21:92–98. doi: 10.1097/00007632-199601010-00022. [DOI] [PubMed] [Google Scholar]
- 17.Katz JN, Stucki G, Lipson SJ, Fossel AH, Grobler LJ, Weinstein JN. Predictors of surgical outcome in degenerative lumbar spinal stenosis. Spine. 1999;24:2229–2233. doi: 10.1097/00007632-199911010-00010. [DOI] [PubMed] [Google Scholar]
- 18.Kornblum MB, Fischgrund JS, Herkowitz HN, Abraham DA, Berkower DL, Ditkoff JS. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long term study comparing fusion and pseudoarthrosis. Spine. 2004;29(7):726–733. doi: 10.1097/01.BRS.0000119398.22620.92. [DOI] [PubMed] [Google Scholar]
- 19.Lozier AP, Kendig JJ. Long term potentiation in an isolated peripheral nerve-spinal cord preparation. J Neurophysiol. 1995;74:1001–1009. doi: 10.1152/jn.1995.74.3.1001. [DOI] [PubMed] [Google Scholar]
- 20.Mardjetko SM, Connolly PJ, Shott S. Degenertative lumbar spondylolisthesis. A meta-analysis of literature 1970–1993. Spine. 1994;19:2257S–2265S. doi: 10.1097/00007632-199410151-00002. [DOI] [PubMed] [Google Scholar]
- 21.Mcgregor AH, Hughes SPF. The evaluation of the surgical management of nerve root compression in patients with low back pain: Part 1: the assessment of outcome. Spine. 2002;27(13):1465–1470. doi: 10.1097/00007632-200207010-00018. [DOI] [PubMed] [Google Scholar]
- 22.Ng L, Sell P. The predictive value of duration of radiculopathy for the outcome of lumbar discectomy—a prospective cohort study with one-year follow up. J Bone Joint Surg. 2004;86(B):546–549. [PubMed] [Google Scholar]
- 23.Olmarker K, Lind B, Holm S, et al. Continued compression increases impairment of impulse propagation in experimental compression of the porcine cauda equine. Neuro-orthopedics. 1991;11:75–81. [Google Scholar]
- 24.Pocket S, Figurov A. Long term potentiation and depression in the ventral horn of rat spinal cord in vitro. Neuroreport. 1993;4:97–99. doi: 10.1097/00001756-199301000-00025. [DOI] [PubMed] [Google Scholar]
- 25.Postachinni F. Management of lumbar spinal stenosis. Spine. 1996;78:154–164. [PubMed] [Google Scholar]
- 26.Postacchini F, Cinotti G, Gumina S, Perugia D. Long term results of surgery in lumbar stenosis: 8-year review of 64 patients. Acta Orthop Scand Suppl. 1993;251:78–80. doi: 10.3109/17453679309160127. [DOI] [PubMed] [Google Scholar]
- 27.Simotas A, Dorey F, Hansraj K, Cammisa F. Nonoperative treatment for lumbar spinal stenosis: clinical and outcome results and a 3-year survivorship analysis. Spine. 2000;25(2):197–211. doi: 10.1097/00007632-200001150-00009. [DOI] [PubMed] [Google Scholar]
- 28.Spivak JM. Degenerative lumbar spinal stenosis. J Bone Joint Surg. 1998;80(A):1053–1066. doi: 10.2106/00004623-199807000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Tendon V, Campbell F, Ross ERS. Posterior lumbar interbody fusion: association between disability and psychological disturbance in non-compensation patients. Spine. 1999;24:1833–1833. doi: 10.1097/00007632-199909010-00013. [DOI] [PubMed] [Google Scholar]
- 30.Trief P, Grant W, Fredrickson B. A prospective study of psychological predictors of lumbar surgery outcome. Spine. 2000;25:2616–2621. doi: 10.1097/00007632-200010150-00012. [DOI] [PubMed] [Google Scholar]
- 31.Turner JA, Erse KM, Herron L. Surgery for lumbar spinal stenosis. Attempted meta-analysis of the literature. Spine. 1992;17:1–8. doi: 10.1097/00007632-199201000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe R, Parke WW. Vascular and neural pathology of lumbarsacral spinal stenosis. J Neurosurg. 1986;64:64–70. doi: 10.3171/jns.1986.64.1.0064. [DOI] [PubMed] [Google Scholar]
- 33.Zheng F, Sandhu H, Cammisa F, Girardi F, Khan S. Predictors of functional outcome in elderly patients undergoing posterior lumbar spine surgery. J spinal disord. 2001;14(6):518–521. doi: 10.1097/00002517-200112000-00011. [DOI] [PubMed] [Google Scholar]