Abstract
Botulinum toxin (BTX) is a potent neurotoxin produced by Clostridium botulinum and has wide usage in different areas. The current study aimed to analyze the effects of C. botulinum toxin on the central nerve system in chick embryos. Forty fertile Hubbard Broil eggs, all at Stage 8 of development, were divided into four equal groups: Group 1 embryos (n=10), the control group, were explanted and grown for 18 h in a nutrient medium (thin albumin). Group 2 embryos (n=10) were grown in medium containing 5 U BTX, Group 3 embryos (n=10) in a medium containing 10 U BTX and Group 4 embryos (n=10) in medium containing 20 U BTX. After the incubation period, 80% of Group 1 and 2 embryos and 90% of Group 3 and 4 embryos had intact neural tubes (P>0.05). The results of this study suggest that BTX had no additional effect on neural tube development in early chick embryos.
Keywords: Neural tube defect, Botulinum toxin, Early chick embryo
Introduction
Neural tube defects (NTDs) are among the most prevalent of congenital malformations. In new borns, 65% of congenital malformations occur in the central nervous system [1]. NTDs, which encompass a broad spectrum of phenotypes ranging from spina bifida to anencephaly, affect approximately 89 in 1,000 neonates in Turkey. Three distinct phases of caudal neural tube development are described in the literature, namely caudal neuropore closure, secondary neurulation, and retrogressive differentiation [3, 6, 7].
Botulinum toxin (BTX) is a potent neurotoxin produced by Clostridium botulinum. Eight serotypes of botulinum toxin are known. Of these, type A is available for clinical use [11]. BTX has been widely used in cosmetic dermatology in recent years for skin rejuvenation by localized injection. To our knowledge there are not enough studies about the effects of BTX on the fetal system and organs. The current study is based on histopathological examination of the effects of BTX on neural tube development defects in chick embryos by light microscopy.
Materials and methods
In this study forty fertile Hubbard Broil eggs were incubated at 37.5°C until the embryos reached Stage 8 of development [8]. The mean egg weight was 65 g. The eggs were then divided into four equal groups, and the embryos were explanted using New’s technique [10]. The embryos in Group 1 (n=10) were grown for 18 h in a BTX-free medium (thin albumin); Group 2 embryos (n=10) in a medium containing 5 U BTX; Group 3 embryos (n=10), in a medium containing 10 U BTX; and the embryos in Group 4 (n=10) in a medium containing 20 U BTX. The BTX solution (100 U) was prepared by diluting with 2 cc saline and 0.1 cc of the solution contained 5 U BTX. After the incubation period, the chicks’ neural tube development was examined under a light microscope. The embryos were assigned to one of the following three categories based on gross morphology: (1) no development; (2) abnormal development; or (3) normal development.
Embryos were embedded in paraffin, serially sectioned at 7 μm, stained with Delafield’s hematoxylin and eosin, and examined under light microscopy.
Results
Incubation allowed all of the embryos to advance to Stage 8 of development. At the time of explantation, each embryo had four somite pairs, and the neural folds in the future midbrain and a portion of the hindbrain had already made contact. After the incubation period, the chicks’ neural tube development was examined under a light microscope.
After a further 18 h of postexplantation incubation, eight embryos each (80%) in Groups 1 and 2; and nine embryos each (90%) in Groups 3 and 4 exhibited characteristics of Stage 12 development. Some of the features defining this stage are: head turning to left side; anterior neuropore closed; telencephalon identifiable; primary optic vesicles and optic stalk well established; auditory pit deep but wide open; heart slightly S-shaped; and headfold amnion covering the entire region. The neural tube was closed in these embryos (Figs. 1, 2). The two remaining embryos each from Groups 1 and 2, and one each from Groups 3 and 4 showed NTDs. The defects were such that the neural folds showed no signs of contact through the neuroepithelium. In these embryos, microcephaly, neural tube closure defect, and significant developmental retardation were noticed.
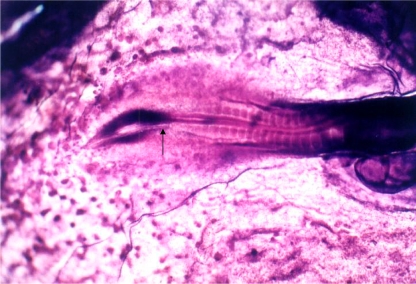
Fig. 1.
View of a normal chick embryo explanted at Stage 8 of development and cultured for 18 h in a nutrient medium (thin albumin). Neural tube (arrow)
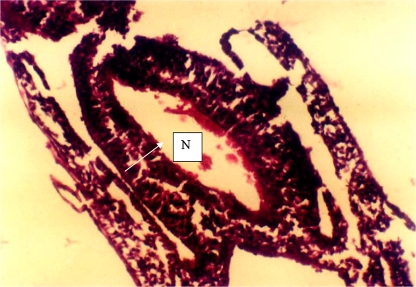
Fig. 2.
Transverse section through the spinal cord region of a control embryo (Group 1). The neural tube is closed. The neural tube is closed. N neural tube
Statistically, these results were analyzed using the χ2 test. When the incidents of neural tube development defects were compared, there was no statistically significant difference among the four groups (P>0.05).
Discussion
In the present study, we took the early chick embryo as a model that corresponds to the first month of embryonal development in mammals and is well suited to the investigation of the effects of chemicals on the development of embryos. In this model we showed that BTX had no extra effect on chick embryo for causing NTDs.
Birth defects are the leading cause of death in babies under 1 year of age. NTDs, with a birth incidence of approximately 1/1,000 in American Caucasians, are the second most common type of birth defect after congenital heart defects. The most common presentations of NTDs are spina bifida and anencephaly. The etiologies of NTDs are complex, with both genetic and environmental factors implicated [5].
Clostridium botulinum was first discovered in the late 1800s as a key agent involved in food poisoning [2]. Its exotoxin was soon hailed as one of the most toxic naturally occurring substances, with lethal doses at around 10−9 g/kg5. To date, there have been eight serotypes identified (A, B, C alpha, C beta, D, E, F, and G), producing seven exotoxins. These serotypes, although structurally and functionally similar, vary in biosynthesis, size, dose requirement, and mechanism of action. Types A, B, and F are the types that have been studied the most. Botulinus toxin type A was the first type to be purified. It is the strongest of the known serotypes and the one traditionally used in cosmetic dermatology on humans, by localized injections only, to maximize efficacy and minimize potential side effects. The purified Botox complex is a sterile, vacuum-dried form of the type A toxin that requires normal saline to reach solution. One unit is defined as the amount of the Botox complex that is lethal in 50% of female Swiss-Webster mice [2]. The different serotypes require different concentrations to reach their effects, which typically is obtained with doses much lower than toxic, administered through local drug delivery. This neurotoxin blocks neuromuscular conduction by binding and entering the presynaptic nerve termini and interfering with exocytosis of the acetylcholine, thus inhibiting its release and muscular contraction [9].
The esthetic applications of BTX are well known to medical and nonmedical communities. This nonsurgical rejuvenation modality continues to be high demand in part because of the immediacy of its effects, its high safety profile, and no downtime in the recovery period [12].
In an animal study, when pregnant mice and rats were injected with BTX intramuscularly during the period of organogenesis, the maximum safe dosage was 4 U/kg. Higher doses (8 or 16 U/kg) were associated with reductions in fetal body weights and/or delayed ossification which may be reversible [4].
In a range finding study in rabbits, daily injection of 0.125 U/kg/day (days 6–18 of gestation) and 2 U/kg/day (days 6 and 13 of gestation) produced severe maternal toxicity, abortions and/or fetal malformations. Higher doses resulted in death of the dams. The rabbits appeared to be a very sensitive species to BTX [4].
Our study showed that exposure to increasing dosage of BTX had no extra effect on the incidence of NTDs in explanted early chick embryos. However, animal reproductive studies are not always predictive of human response, so BTX should be administered during pregnancy only if the potential benefit justifies the potential risk to the fetus. There is a dearth of well-controlled studies of the effects of BTX in pregnant women. Therefore, if this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential risks, including abortion or fetal malformations which have been observed in animal studies.
In conclusion, although our studies did not indicate any additional side-effect of BTX on NTD in chick embryo, latent risks on fetal development exists and should be considered before using the drug.
References
- 1.Caglayan S, Kayhan B, Mentesoglu S, Aksit S. Changing incidence of neural tube defects in Aegean Turkey. Paediatr Perinat Epidemol. 1989;3(1):62–65. doi: 10.1111/j.1365-3016.1989.tb00370.x. [DOI] [PubMed] [Google Scholar]
- 2.Carruthers A, Carruthers J. Botulinum toxin type A: history and current cosmetic use in the upper face. Semin Cutan Med Surg. 2001;20:71–84. doi: 10.1053/sder.2001.25138. [DOI] [PubMed] [Google Scholar]
- 3.Catala M, Teillet MA, Robertis EM, Le Douarin ML. A spinal cord fate map in the avian embryo: while regressing, Henesen’s nade lays down the notochord and floor plate thus joining the spinal cord lateral walls. Development. 1996;122(9):2599–2610. doi: 10.1242/dev.122.9.2599. [DOI] [PubMed] [Google Scholar]
- 4.Data on file, Allergan, Inc. 1999
- 5.Detrait ER, George TM, Etchevers HC, Gilbert JR, Vekemans M, Speer MC. Human neural tube defects: developmental biology, epidemiology, and genetics. Neurotoxicol Teratol. 2005;27(3):515–524. doi: 10.1016/j.ntt.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Güney O, Selcuki M, Ünlü A. The effect of diazepam on the development of the chick embryos. Turk Neurosurg. 1999;9:44–47. [Google Scholar]
- 7.Güney O, Canbilen A, Konak A, Acar O. The effects of folic acid in the prevention of neural tube defects caused by phenytoin in early chick embryos. Spine. 2003;28(5):442–445. doi: 10.1097/00007632-200303010-00006. [DOI] [PubMed] [Google Scholar]
- 8.Hamburger V, Hamilton HL. A series of normal development of the chick embryo. J Morphol. 1951;88:49–92. doi: 10.1002/jmor.1050880104. [DOI] [PubMed] [Google Scholar]
- 9.Klein AW. Complications and adverse reactions with the use of botulinum toxin. Semin Cutan Med Surg. 2001;20:109–120. doi: 10.1053/sder.2001.25964. [DOI] [PubMed] [Google Scholar]
- 10.New DAT. A new technique for the cultivation of the chick embryos in vitro. J Embryol Exp Morphol. 1955;3:326–331. [Google Scholar]
- 11.Robinson LR, Hillel AD. Botulinum toxin treatment for motor control disorders. Phys Med Rehabil Clin N Am. 1993;4:731–744. [Google Scholar]
- 12.Vartanian AJ, Dayan SH. Facial rejuvenation using botulinum toxin: a review and updates. Facial Plast Surg. 2004;20:11–19. doi: 10.1055/s-2004-822954. [DOI] [PubMed] [Google Scholar]