Abstract
Anterior cervical discectomy and fusion (ACDF) may be considered to be the gold standard for treatment of symptomatic degenerative disc disease within the cervical spine. However, fusion of the segment may result in progressive degeneration of the adjacent segments. Therefore, dynamic stabilization procedures have been introduced. Among these, artificial disc replacement by disc prosthesis seems to be promising. However, to be so, segmental motion must be preserved. This, again, is very difficult to judge and has not yet been proven. The aim of the current study was to first analyse the segmental motion following artificial disc replacement using a disc prosthesis. A second aim was to compare both segmental motion as well as clinical result to the current gold standard (ACDF). This is a prospective controlled study. Twenty-five patients with cervical disc herniation were enrolled and assigned to either study group (receiving a disc prosthesis) or control group (receiving ACDF, using a cage with bone graft and an anterior plate.) Radiostereometric analysis was used to quantify intervertebral motion immediately as well as 3, 6, 12 and 24 weeks postoperatively. Further, clinical results were judged using visual analogue scale and neuro-examination. Cervical spine segmental motion decreased over time in the presence of disc prosthesis or ACDF. However, the loss of segmental motion is significantly higher in the ACDF group, when looked at 3, 6, 12 and 24 weeks after surgery. We observed significant pain reduction in neck and arm postoperatively, without significant difference between both groups (P > 0.05). Cervical spine disc prosthesis preserves cervical spine segmental motion within the first 6 months after surgery. The clinical results are the same when compared to the early results following ACDF.
Keywords: Spine, Bone, Fusion, Implants, Disc replacement, Clinical study
Introduction
To date, anterior cervical discectomy and fusion (ACDF) may be considered to be the standard procedure for treatment of degenerative disc disease of the cervical spine [4–6, 39, 42]. However, there are clues that ACDF may result in progressive degeneration of the adjacent segments [15–17, 24, 33, 38, 44]. To avoid this, there have been numerous attempts to develop, test and use artificial cervical discs [1, 9, 10, 13, 14, 25, 31, 40]. Recently, first clinical experiences with cervical spine disc prosthesis are available, and most of them seem to be encouraging [1, 3, 7, 9, 10, 12–14, 23, 29, 35]. However, it is to early to judge if the clinical long-term results are favourable [3, 7, 12–14, 28]. These will be influenced by the function of such an implant that has been designed to preserve segmental motion. If this is true, the implant should preserve segmental motion for a long time. However, there is, to the authors’ knowledge, no study to show that such an implant works as it has been considered to work. One of the reasons for the lack of such a study is the difficulty to measure segmental motion precisely. Therefore we performed this radiostereometric analysis (RSA) to get information concerning long-term results of segmental motion.
Radiostereometric analysis has been developed by Selvik et al. [36, 37]. It is a radiographic technique, used to measure micro-motion in spine and its principles are explained elsewhere especially in orthopaedic fields [18–21, 32, 36, 37]. Pape et al. [26] showed that RSA is a useful tool to exclude segmental motion and to document a sold fusion in the lumbar spine. RSA is also used by Zoegea et al. [45] to measure the additional effect of plate fixation in cervical spine fusion. In our study, RSA has been used to measure motion in patients who received cervical disc prosthesis or ACDF.
The objective of the current study was to investigate segmental motion following implantation of a cervical spine disc prosthesis (Synthesis Spine, Im Kirchenhürstle 4–6, 79224 Umkirch, Germany) versus segmental motion of a segment having received ACDF using a cage polyether–ether–ketone “PEEK” (Stryker Howmedica GmbH, Gewerbeallee 18, 45478 Mülheim, Germany) with bone graft and an anterior titanium alloy plate (Aesculap AG + CoKG, Am Aesculap-Platz, Tuttlingen, Germany), 6 months after surgery.
Methods
Study design
This is a prospective randomised and controlled study, approved by the local ethical committee of Saarland (Germany), n 119/04.
Thirty-three patients [19 male, 14 female, mean age 45 years, standard deviation (SD) 11 years] suffering from symptomatic soft disc herniation, not responding to a trial of conservative treatment and or progressive radicular deficits were included between April and December 2004. Inclusion as well as exclusion criteria are listed in Table 2. All patients had confirmatory imaging studies consistent with clinical findings. All patients were informed about the purpose of the study giving a written informed consent that was signed at least 24 h before surgery.
Table 2.
Criteria for patients with Pro-Disc C and RSA study
| Inclusion criterIa | Exclusion criteria |
|---|---|
| Mono-segmental cervical disc disease between C3 and C7 | Marked cervical instability on resting or flexion/extension radiographs |
| Greater than 11 of angulations | |
| Translation greater than 3 mm | |
| Unresponsive to conservative treatment or presence of signs of nerve root compression with paresis | More than one level pathology |
| Soft disc herniation | Myelopathy |
| Age between 20 and 60 years | Radiographic confirmation of severe facet joint degeneration |
| Signed informed consent | Hard disc disease |
| Osteoporosis, infection, rheumatoid arthritis | |
| Spondylodiscitis and active infection | |
| Malignant disease | |
| System disease, e.g. Hepatitis, HIV, AIDS | |
| Known allergy to cobalt, chromium, molybdenum, titanium, or polyethlene | |
| Traumatic injury of spine | |
| Pregnant or possible pregnancy in the next 3 years |
Next, patients were assigned to the study group (prosthesis) or control group (ACDF) by randomisation. Sixteen patients received disc replacement, whereas 17 patients were treated with ACDF. Eight patients were excluded during first RSA measurement due to some markers being obscured (three patients with prosthesis and five with ACDF).
Surgery was performed by two surgeons from the author board (A. N., T. P.). A standard left-sided anterior approach was performed, the symptomatic disc was removed, the lateral parts of the annulus were preserved; however, the posterior longitudinal ligament (PLL) was removed. The disc or cage and plate were inserted according to the manufacturer’s recommendation.
Finally, 7–9 tantalum markers of 0.8 mm diameter (RSA Biomedical, Box 7972, S-907 19 Umea, Stockholm, Sweden) were placed into the adjacent vertebrae.
Clinical symptoms as neck and arm pain were investigated preoperatively and immediately, 3, 6, 12 and 24 weeks postoperatively. Visual analogue scale (VAS) was used for grading of neck and arm pain [11, 22, 30].
Implants used in the study
The cage used for ACDF named Solis consists of polyether–ether–ketone (PEEK). It has one large central perforation to allow bony ingrowths.
The plate used here is a nonconstrained plate which we used in combination with mono-cortical screws, both made from titanium alloy. The screw for monocortical fixation is a titanium alloy, self-tapping, conical screw of 14, 15, 16, 17 or 19 mm length with an outer diameter of 4.0 mm and an inner diameter of 2.2 mm at the tip, increasing to 2.7 mm at its head. This is a nonconstrained plate–screw system for anterior cervical fixation.
The prosthesis implant is a metal polyethylene ball-in-socket design with two metal fins; the material used is an interface ultra-high-molecular-weight polyethylene inlay; the superior and inferior plate are made of cobalt-chrome alloy with titanium surface facing the bony side.
Radiostereometric analysis (RSA)
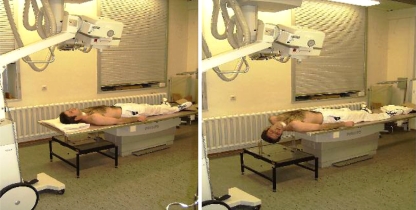
Patients were followed by RSA postoperatively. The insertion of 4–6 radio-opaque tantalum markers in each vertebra is required to determine the geometric characteristics of the vertebral anatomy. Radiostereometric examinations were carried out uniformly in all patients in the supine position using a uniplanar technique. All patients were positioned above the combined reference plate and calibration device with 0.8 mm tantalum indicators, placed at known positions in front of the film plane. The cage markers defined the laboratory coordinate system and were used to calculate the position of the X-ray foci. RSA radiographs were taken directly after surgery and 3, 6, 12 and 24 weeks after the procedure in two positions to induce intervertebral mobility. Mobility provocation was obtained by examining the patient in two different positions of the head versus cervical spine (neutral position of the head and extension with rotation of the head to the right side, Fig. 1a, b). The patient’s head was passively moved to the different end points by the examiner. At subsequent evaluations, the three-dimensional coordinates of the patient marker were determined at each examination. Intervertebral motion occurring between neutral and extended with right-rotated head was computed. For mathematical evaluation of the radiographs, we used a software package (UmRSA, RSA Biomedical Innovations, Umea, Sweden), mainly based on RSA measurement techniques according to Selvik [36, 37]. The calculated intervertebral motion allowed visualisation of persisting translation and rotation between the vertebrae. The most distal cervical vertebrae were used as a fixed reference segment. All motion were related to the laboratory co-ordinate system defined by the cage. Raw data were expressed as translation (Table 4). The relative translation of the centre of gravity of the markers in the most proximal vertebra was recorded in all cases. Translations were measured as:
Medial–lateral translations (left–right, x-axis)
Proximal–distal translations (distraction–compression, y-axis)
Anterior–posterior translations (sagittal direction, z-axis)
The data were used to evaluate motion. Rotations were calculated in the order:
Extension (deg) (rotations along the transverse axis)
Right-sided axial rotation (deg) (rotation along the longitudinal axis)
Right-sided lateral bending (deg)(rotation along the sagittal axis)
The precision (= reproducibility) of the measurement was calculated by double examinations on the same day in six patients at different times during the follow-up period. We calculated the 99% confidence limits for significant translations and rotations as the absolute mean value ± 2.8 SD based on a normal distribution (Table 1). The precision of our RSA set-up was determined similar to the method used by Zoegea et al. [45], who published comparable values for the three axis of motion. (Tables 2, 3, 4)
Fig. 1.
Positioning of the patient and X-ray projection in neutral position (a) as well as extension combined with right-sided lateral bending (b) for RSA examination
Table 4.
Translation in (mm) at postop, 3, 6, 12, and 24 weeks
| Translation (mm) | Postop | 3 weeks | 6 weeks | 12 weeks | 24 weeks | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prosthesis | ACDF | Prosthesis | ACDF | Prosthesis | ACDF | Prosthesis | ACDF | Prosthesis | ACDF | |
| Medio-lateral | 0.69 (0.9) | 0.25 (0.30) | 0.39 (0.16) | 0.11 (0.06) | 0.29 (0.16) | 0.07 (0.06) | 0.39 (0.18) | 0.06 (0.05) | 0.33 (0.17) | 0.06(0.09) |
| Cranio-caudal | 0.48 (0.27) | 0.28 (0.4) | 0.21 (0.10) | 0.14 (0.07) | 0.22 (0.12) | 0.11(0.07) | 0.17 (0.1) | 0.06(0.06) | 0.23 (0.13) | 0.05(0.06) |
| Anterior–posterior | 1.67 (0.93) | 0.42 (0.5) | 1.08 (0.4) | 0.16 (0.05) | 0.71 (0,38) | 0.18 (0.07) | 0.53 (0.3) | 0.13 (0.09) | 0.67 (0.42) | 0.05(0.05) |
Mean value and standard deviation (SD) are given for each time
Table 1.
The 99% confidence limits of radiostereometry in the cervical spine in six double examinations
| Translations (mm) | |
|---|---|
| Anterior–posterior (X-axis) | 0.45 |
| Proximal–distal (Y-axis) | 0.30 |
| Medial–lateral (Z-axis) | 0.65 |
| Rotations (deg) | |
| Transverse axis | 4.2 |
| Longitudinal axis | 3.9 |
| Sagittal axis | 1.5 |
Table 3.
Visual analogue scale (VAS) for neck and arm pain
| Preoperatively | 1 week | 3 weeks | 6 weeks | 12 weeks | 24 weeks | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prosthesis | ACDF | Prosthesis | ACDF | Prosthesis | ACDF | Prosthesis | ACDF | Prosthesis | ACDF | Prosthesis | ACDF | |
| Neck pain | 6.2 (1.2) | 6.4 (0.9) | 3.3 (0.6) | 2.9 (0.7) | 3.4 (0.6) | 2.2 (0.7) | 2.4 (0.5) | 1.8 (0.5) | 2.5 (0.6) | 1.9 (0.5) | 2.8 (0.4) | 2.0 (0.5) |
| Arm pain | 7.6 (1.4) | 7.2 (1.7) | 1.6 (0.4) | 1.5 (0.4) | 1.5 (0.4) | 1.7 (0.4) | 1.3 (0.3) | 1.5 (0.3) | 1.6 (0.3) | 1.7 (0.3) | 1.4 (0.2) | 1.7 (0.3) |
Mean value and standard deviation (SD) are given for each time
The accuracy of the RSA method depends most importantly on stability, spacing and number of tantalum markers inserted into the bony region of interest [19]. The degree of marker instability is expressed in millimetres as the mean error of rigid body fitting. In this study only values below 0.4 mm were accepted.
Statistics
Mann–Whitney test for unpaired values were used to determine a statistical difference of residual intervertebral translations before and after prosthesis as well as ACDF in the three axis of motion (P < 0.05). The segmental motion was additionally calculated using a vector of X, Y, and Z axes.
Results
Patients excluded from study
Out of 33 patients 8 were excluded during first RSA measurement due to some markers being obscured.
Results from radiographic study
Study group (prosthesis)
Segmental rotation between 3 weeks and immediately postoperative significantly decreased in extension (P = 0.024), right-sided axial rotation (P = 0.005) and right-sided bending (P < 0.01).
Six weeks postoperative there was no further decreased segmental motion in extension (P = 0.32), right-sided axial rotation (P = 0.20) and right-sided bending (P = 0.42) (Table 5; Figs. 2, 3, 4, 5, 6).
Table 5.
Rotations in (deg) postoperatively and 3, 6, 12, and 24 weeks
| Rotation (deg) | Postop | 3 weeks | 6 weeks | 12 weeks | 24 weeks | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prosthesis | ACDF | Prosthesis | ACDF | Prosthesis | ACDF | Prosthesis | ACDF | Prosthesis | ACDF | |
| Extension (deg) | 5.77 (1.6) | 2.1 (1.73) | 3.79 (2,0) | 2.06 (0.89) | 2.67 (1.4) | 0.92 (0.83) | 2.79 (1.7) | 0.94 (0.90) | 2.36 (1.0) | 0.95 (0.80) |
| Right-sided motion (deg) | 2.5 (1,5) | 2.20 (1.80) | 4.83 (0.9) | 1.53 (1.20) | 2.13 (1.2) | 1.35 (0.81) | 3.07 (1.5) | 0.82 (0.72) | 2.56 (0.7) | 0.79 (0.64) |
| Right-sided bending (deg) | 3.15 (2.1) | 1.22 (1.14) | 3.24 (1.0) | 0.91 (0.72) | 2.06 (0.6) | 0.76 (0.57) | 2.24 (1.0) | 0.72 (0.39) | 2.24 (1.3) | 0.74 (0.44) |
Mean value and standard deviation (SD) are given for each time
Fig. 2.
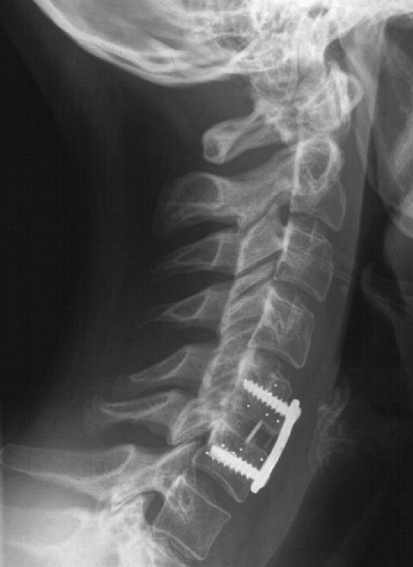
Lateral X-ray of a cervical spine. ACDF was performed within C5/6. Please note the tantal markers of C5 and C6, some of the markers are obscured by the screws
Fig. 3.
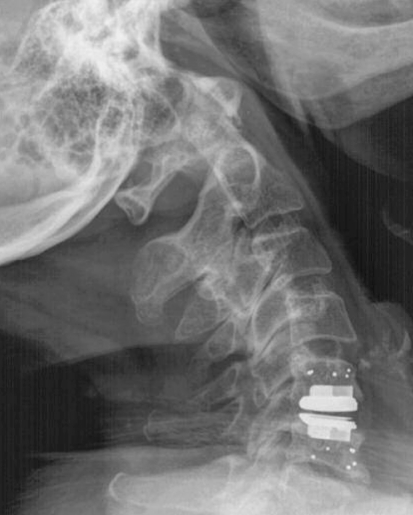
Lateral X-ray of a cervical spine. Disc prosthesis was implanted in C5/6. Please note the tantal markers in the adjacent vertebra
Fig. 4.
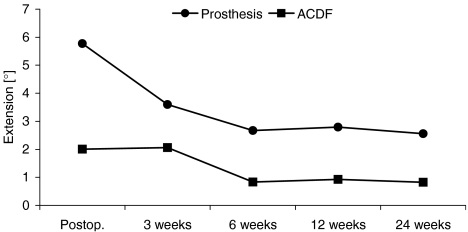
Graph illustrating segmental motion in extension (deg) (mean value) for each follow-up examination. For each follow-up examination, the micro-motion of a segment receiving prosthesis is more pronounced when compared to fusion group. However, segmental motion decreases in both groups over time
Fig. 5.
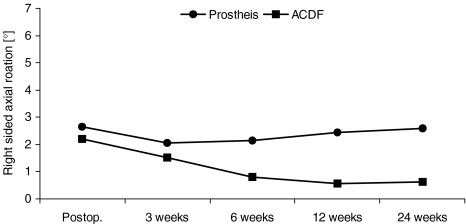
Graph illustrating segmental motion in right-sided axial rotation (deg) (mean value) for each follow-up examination. For each follow-up examination, the micro-motion of a segment receiving prosthesis is more pronounced when compared to fusion group. However, segmental motion decreases in both groups over the time
Fig. 6.
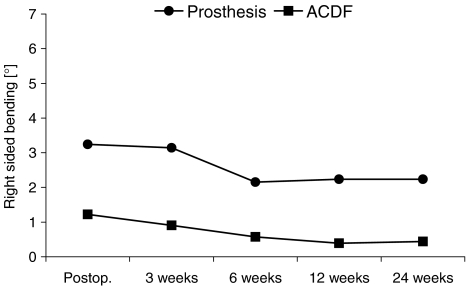
Graph illustrating segmental motion in right sided lateral bending (°) (mean value) for each follow-up examination. For each follow-up examination, the micro-motion of a segment receiving prosthesis is more pronounced when compared to fusion group. However, segmental motion decreases in both groups over the time
Data for each motion plane (extension, right-sided axial rotation and right-sided bending) are shown in Table 5 and Figs. 4, 5, and 6.
Control group (ACDF)
Segmental rotation between 3 weeks and immediately postoperative significantly decreased in right-sided axial rotation (P = 0.016). However, there was no significant decrease in motion in extension (P = 0.06) and right-sided bending (P = 0.48). Segmental rotation between 24 and 3 weeks shows further decreased motion significantly only in extension (P = 0.001). Six weeks postoperative there was no further decreased segmental motion in extension (P = 0.32), right-sided axial rotation (P = 0.20) and right-sided bending (P = 0.42) (Table 5, Figs. 4, 5, 6).
Six weeks after surgery there was no significant decreased motion compared to 24 weeks (P > 0.05). Data for each axis (extension, right-sided axial rotation and right-sided bending) are shown in Table 5 and Figs. 4, 5, and 6.
Study group versus control group
Segmental motion was significantly different between both groups for extension (deg): (P = 0.0001 immediately postoperative, P = 0.025 after 3 weeks, P = 0.001 after 6 weeks, P < 0.01 after 12 weeks and P = 0.0001 after 24 weeks).
Segmental motion was not significantly different between both groups for right-sided axial rotation (deg): (P = 0.0001 immediately postoperative, P = 0.61 after 3 weeks, P = 0.23 after 6 weeks, whereas there was significant difference 6 weeks after surgery, P = 0.012, 12 weeks after surgery P = 0.01 and after 24 weeks, P > 0.01).
Segmental motion was significantly different between both groups for right-sided bending (deg): (P = 0.0001 immediately postoperative, P = 0.015 after 3 weeks, P = 0.01 after 6 weeks, P < 0.01 after 12 weeks, P < 0.01 and P = 0.001 after 24 weeks).
Results from clinical study
Study group (prosthesis)
Mean value and standard deviation for neck pain measured using VAS decreased from 6.2 (1.2) preoperatively to 2.8 (0.4) after 24 weeks postoperatively. Mean value and standard deviation for arm pain measured using VAS decreased from 7.6 (1.4) preoperatively to 1.4 (0.2) after 24 weeks postoperatively.
Control group (ACDF)
Mean value and standard deviation for neck pain measured using VAS decreased from 6.4 (0.9) preoperatively to 2.0 (0.5) after 24 weeks postoperatively. Mean value and standard deviation for arm pain measured using VAS decreased from 7.2 (1.7) preoperatively to 1.7 (0.3) after 24 weeks postoperatively.
Study group versus control group
Statistical comparison between the two groups showed nonsignificant differences in pain relief (P > 0.05; Table 3).
Discussion
Results of the current study
Within the current study, we found, that cervical spine segmental motion decreases over time in the presence of disc prosthesis or ACDF. However, the loss of segmental motion is significantly higher in the ACDF group.
Advantages and disadvantages of the study
To the authors’ knowledge, the current study is unique in its design. First, a group of patients having received an prosthesis (Pro-Disc C) were compared to a control group with ACDF. Next, the design of the study is randomised. Finally, RSA has been used to document persisting motion of a cervical spine segment treated with an artificial disc device or ACDF. Persisting motion is a desired goal in cervical spine surgery that also has been transformed into an industrial philosophy. However, up to now there is no evidence that this goal has been achieved over a long time period. The current data, however, given by an accepted technique for both the lumbar and cervical spine region, give some evidence that this goal may be achieved by this implant [8–10, 12, 17, 34, 41]. This, however, is due to our data, just true for the observed postoperative follow-up period. Furthermore, it should be considered that our patient population is small and results may change if more data from more patients will be available. Nevertheless, our data give insight into the motion of a surgically treated cervical spine segment under physiological loads. Finally, it should be mentioned that these results are just true for both, the time period given here, and the implants used here.
Former experiences with artificial disc replacement
The results of the current study support the results of some former in vitro investigations. Puttlitz et al. [31] found that cervical spine disc replacement replicates both segmental motion in the treated and the adjacent levels of a human cervical spine. The value of this finding supports the increased clinical application of disc prosthesis. This again has been found in some clinical studies [3, 7, 12, 14, 34, 43]. Again, it must be pointed out that (especially facing these former results) the value of our study lies in the information of a measured motion under physiological loads within the treated segment. Nevertheless, there is still the possibility of reduced segmental motion within the further follow-up period by calcification or scar formation as observed by Parkinson and Sekhon [27] and Bartels and Donk [2].
Clinical results
Within this study, both treatment concepts resulted in [3, 7, 13, 14, 34] significant reduction of neck and arm pain without statistical difference between groups. This is not surprising, especially not if arm pain is illuminated: this result is much more if it is not only an effect of decompression. Decrease in neck pain may be due to stabilisation, which is given by disc replacement, being considered to be a dynamic stabilisation. Moreover, treatment of neck pain may be effect of discectomy. Finally, long-term results will show, if cervical arthroplasty could prevent or reduce the incidence of adjacent segment disease.
Conclusion
Cervical spine segmental motion decreases over time in the presence of both disc replacement with Pro-Disc C or ACDF. However, the loss of segmental motion is significantly higher in the ACDF group, when checked 3, 6, 12, and 24 weeks after surgery.
References
- 1.Anderson PA, Sasso RC, Rouleau JP, Carlson CS, Goffin J. The Bryan Cervical disc: wear properties and early clinical results. Spine J. 2004;4:303–309. doi: 10.1016/j.spinee.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 2.Bartels RH, Donk R. Fusion around cervical disc prosthesis: case report. Neurosurgery. 2005;57(1):146–151. doi: 10.1227/01.NEU.0000163419.59635.78. [DOI] [PubMed] [Google Scholar]
- 3.Bertagnoli R, Yue JJ, Pfeiffer F, Fenk-Mayer A, Lawrence JP, Kershaw T, et al. Early results after prodisc-c cervical disc replacement. J Neurosurg Spine. 2005;2:403–410. doi: 10.3171/spi.2005.2.4.0403. [DOI] [PubMed] [Google Scholar]
- 4.Bohlman HH, Emery SE, Goodfellow DB, Jones PK. Robinson anterior cervical diskectomy and arthrodesis cervical for cervical radiculopathy. J Bone Joint Surg Am. 1993;75:1298–1307. doi: 10.2106/00004623-199309000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Caspar W, Geisler FH, Pitzen T, Johnson TA. Anterior cervical plate stabilisation in one- and two level degenerative disease: overtreatment or benefit? J Spinal Disord. 1998;11:1–11. doi: 10.1097/00002517-199802000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Cloward RD. Treatment of acute fractures and fracture dislocation of cervical spine by vertebral body fusion: A report of 11 cases. J Neurosurg. 1961;18:205–209. doi: 10.3171/jns.1961.18.2.0201. [DOI] [PubMed] [Google Scholar]
- 7.Cummins BH, Robertson JT, Gill SS. Surgical experience with an implanted artificial cervical joint. J Neurosurg. 1998;88:943–948. doi: 10.3171/jns.1998.88.6.0943. [DOI] [PubMed] [Google Scholar]
- 8.Delamarter RB, Fribourg DM, Kanim LEA, Bae H. ProDisc artificial total lumbar disc replacement: introduction and early results from the United States. Spine. 2003;28:167–175. doi: 10.1097/01.BRS.0000092220.66650.2B. [DOI] [PubMed] [Google Scholar]
- 9.Di Angelo DJ, Foley KT. An improved biomechanical testing protocol for evaluating spinal arthroplasty and motion preservation devices in a multilevel human cadaveric model. Neurosurg Focus. 2004;17:E7. [PubMed] [Google Scholar]
- 10.Di Angelo DJ, Foley KT, Morrow BR, Schwab JS, Song J, German JW, et al. In vitro biomechanics of cervical disc arthroplasty with the ProDisc-C total disc replacement. Neurosurg Focus. 2004;17:E7. [PubMed] [Google Scholar]
- 11.Downie WW, Leatham PA, Rhind VM, Wright V, Branco JA, Anderson JA. Studies with pain rating scales. Ann Rheum Dis. 1978;37:378–381. doi: 10.1136/ard.37.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duggl N, Pickett GE, Mitsis DK, Keller JL. Early clinical and biomechanical results following cervica arthoplatsy. Neurosurg Focus. 2004;17:E9. doi: 10.3171/foc.2004.17.1.2. [DOI] [PubMed] [Google Scholar]
- 13.Goffin J, Casey A, Kehr P, Liebig K, Lind B, Logroscino C, et al. Preliminary clinical experience with the Bryan Cervical Disc Prosthesis. Neurosurgery. 2003;53:785–786. doi: 10.1227/00006123-200209000-00048. [DOI] [PubMed] [Google Scholar]
- 14.Goffin J, Calenbergh F, Loon J, Casey A, Kehr P, Liebig K, et al. Intermediate follow-up after treatment of degenerative disc disease with the Bryan Cervical Disc Prothesis: single-level and bi-level. Spine. 2003;28:2673–2678. doi: 10.1097/01.BRS.0000099392.90849.AA. [DOI] [PubMed] [Google Scholar]
- 15.Gore DR, Sepic SB. Anterior diskectomy and fusion for painful cervical disc disease: a report of 50 patients with an average follow-up of 21 years. Spine. 1998;23:2047–2051. doi: 10.1097/00007632-199810010-00002. [DOI] [PubMed] [Google Scholar]
- 16.Hilibrand AS, Carlson GD, Palumbo MA, Jones PK, Bohlman HH. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999;81:519–528. doi: 10.2106/00004623-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J. 2004;4:190S–194S. doi: 10.1016/j.spinee.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Johnsson R, Selvik G, Stromqvist B, Sunden G. Mobility of the lower lumbar spine after posterolateral fusion determined by roentgen stereophotogrammetric analysis. Spine. 1990;15:347–350. doi: 10.1097/00007632-199005000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Karrholm J. Roentgen stereophotogrammetry: review of orthopedic applications. Acta Orthop Scand. 1989;60:491–503. doi: 10.3109/17453678909149328. [DOI] [PubMed] [Google Scholar]
- 20.Karrholm J, Hansson LI, Selvik G. Mobility of the lateral malleolus: A roentgen stereophotogrammetric analysis. Acta Orthop Scand. 1985;56:479–483. doi: 10.3109/17453678508993039. [DOI] [PubMed] [Google Scholar]
- 21.Karrholm J, Jonsson H, Nilsson KG, Soderqvist I. Kinematics of successful knee prostheses during weight-bearing: three- dimensional movements and positions of screw axes in the Tricon-M and Miller-Galante designs. Knee Surg Sports Traumatol Arthrosc. 1994;2:50–59. doi: 10.1007/BF01552655. [DOI] [PubMed] [Google Scholar]
- 22.Langley GB, Sheppeard H. The visual analogue scale: its use in pain measurement. Rheumatol Int. 1985;5:145–148. doi: 10.1007/BF00541514. [DOI] [PubMed] [Google Scholar]
- 23.Link HD, Afee MC PC, Pimenta L. Choosing a cervical disc replacement. Spine J. 2004;4:294–302. doi: 10.1016/j.spinee.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 24.Matsunaga S, Kabayama S, Yamamoto T, Yone K, Sakou T, Nakanishi K. Strain on intervertebral discs after anterior cervical decompression and fusion. Spine. 1999;24:670–675. doi: 10.1097/00007632-199904010-00011. [DOI] [PubMed] [Google Scholar]
- 25.Oskouian RJ, Whitehill R, Samii A, Shaffrey ME, Johnson JP, Shaffrey CI. The future of spinal arthroplasty: a biomaterial perspective. Neurosurg Focus. 2004;17:10–14. doi: 10.3171/foc.2004.17.3.2. [DOI] [PubMed] [Google Scholar]
- 26.Pape D, Adam F, Fritsch E, Muller K, Kohn D. Primary lumbosacral stability after open posterior and endoscopic anterior fusion with interbody implants: a Roentgen stereophotogrammetric analysis. Spine. 2000;25:2514–2518. doi: 10.1097/00007632-200010010-00014. [DOI] [PubMed] [Google Scholar]
- 27.Parkinson JF, Sekhon LH. Cervical arthroplasty complicated by delayed spontaneous fusion, case report. J Neurosurg Spine. 2005;2:377–380. doi: 10.3171/spi.2005.2.3.0377. [DOI] [PubMed] [Google Scholar]
- 28.Pickett GE, Mitsis DK, Sekhon LH, Sears WR, Duggal N. Effect of cervical disc prosthesis on segmental and cervical spine alignment. Neurosurg Focus. 2004;17:30–35. doi: 10.3171/foc.2004.17.3.5. [DOI] [PubMed] [Google Scholar]
- 29.Pimenta L, McAfee PC, Cappuccino A, Bellera FP, Link HD. Clinical experience with the new artificial cervical PCM (Cervitech) disc. Spine J. 2004;4:315–321. doi: 10.1016/j.spinee.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 30.Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement characteristics of mechanical visual and simple numerical rating scales. Pain. 1994;56:217–226. doi: 10.1016/0304-3959(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 31.Puttlitz CM, Rousseau MA, Xu ZBS, Hu S, Tay Bk-B, Lotz JC. Intervertebral disc replacement maintains cervical spine kinetics. Spine. 2004;29:2809–2814. doi: 10.1097/01.brs.0000147739.42354.a9. [DOI] [PubMed] [Google Scholar]
- 32.Ragnarsson JI, Eliasson P, Karrholm J, Lundstrom B. The accuracy of measurements of femoral neck fractures. Conventional radiography versus roentgen stereophotogrammetric analysis. Acta Orthop Scand. 1992;63:152–156. doi: 10.3109/17453679209154812. [DOI] [PubMed] [Google Scholar]
- 33.Reitman CA, Hipp JA, Nguyen L, Essen SI. Changes in segmental intervertebral motion adjacent to cervical arthrodesis: a prospective study. Spine. 2004;29:E221–E226. doi: 10.1097/00007632-200406010-00022. [DOI] [PubMed] [Google Scholar]
- 34.Robertson JT, Newton HM. Long-term outcome after implantation of the prestige I disc in an end-stage indication: 4-years results from a pilot study. Neurosurg Focus. 2004;17:69–71. doi: 10.3171/foc.2004.17.3.10. [DOI] [PubMed] [Google Scholar]
- 35.Sekhon LH. Cervical arthroplasty in the managment of spondylotic myelopathy: 18-month results. Neurosurg Focus. 2004;15:E8. doi: 10.3171/foc.2004.17.3.8. [DOI] [PubMed] [Google Scholar]
- 36.Selvik G, Alberius P, Aronson AS. A roentgen stereophotogrammetric system, construction, calibration and technical accuracy. Acta Radiol Diagn (Stockh) 1983;24:343–352. doi: 10.1177/028418518302400412. [DOI] [PubMed] [Google Scholar]
- 37.Selvik G. Roentgen stereophotogrammetric analysis. Acta Radiol. 1990;31:113–126. doi: 10.3109/02841859009177472. [DOI] [PubMed] [Google Scholar]
- 38.Shinomiya K, Okamoto A, Kamikozuru M, Furuya K, Yamaura Il An analysis of failures in primary cervical anterior spinal cord decompression and fusion. J Spinal Disord. 1993;6:277–288. doi: 10.1097/00002517-199306040-00001. [DOI] [PubMed] [Google Scholar]
- 39.Simmons E, Bhalla S. Anterior cervical discectomy and fusion. J Bone Joint Surg Br. 1969;51:225–237. [PubMed] [Google Scholar]
- 40.Smith HE, Wimberley DW, Vaccaro AR. Cervical arthroplasty: material properties. Neurosurg Focus. 2004;17:15–21. doi: 10.3171/foc.2004.17.3.3. [DOI] [PubMed] [Google Scholar]
- 41.Tropiano P, Huang RC, Girardi FP, Marnay T. Lumbar disc replacement, preliminary results with ProDisc II after a minimum follow-up period of 1 year. J Spinal Disord. 2003;16:362–368. doi: 10.1097/00024720-200308000-00008. [DOI] [PubMed] [Google Scholar]
- 42.Whitecloud TS, LaRoocca SH. Fibular strut graft in reconstructive surgery of the cervical spine. Spine. 1976;1:33–43. doi: 10.1097/00007632-197603000-00005. [DOI] [Google Scholar]
- 43.Wigfield CC, Gill SS, Nelson RJ, Metcalf NH, Robertson JT. The new Frenchay artificial cervical joint: results from a two-year pilot study. Spine. 2002;27:2446–2452. doi: 10.1097/00007632-200211150-00006. [DOI] [PubMed] [Google Scholar]
- 44.Wu W, Thuomas KA, Hedlund R, Leszniewski W, Vavruch L. Degenerative changes following anterior cervical discectomy and fusion evaluated by fast spin-echo MR imaging. Acta Radiol. 1996;37:614–617. doi: 10.1177/02841851960373P239. [DOI] [PubMed] [Google Scholar]
- 45.Zoegea B, Kärrholm J, Lind B. Plate fixation adds stability to two-level anterior fusion in the cervical spine: a randomised study using radiostereometry. Eur Spine J. 1998;7:302–307. doi: 10.1007/s005860050079. [DOI] [PMC free article] [PubMed] [Google Scholar]