Abstract
In this study, we evaluated the efficacy of transcranial motor-evoked potentials (tc-MEPs), compared with segmental spinal cord-evoked potentials (SCEPs), for detecting spinal cord ischemia (SCI) and assessed the relationship between neurological outcome and tc-MEPs or SCEPs in the rat aortic occlusion model. In the rats, SCI was induced by aortic occlusion for 10 min with a balloon catheter. At first, tc-MEPs (Group A: n = 6) or segmental SCEPs (Group B: n = 6) was recorded during SCI. Second, in using the quantal bioassay for the relationship between an interval of aortic occlusion and the probability of positive response in tc-MEPs or segmental SCEPs, the P50MEP and P50SCEP which represent the interval of aortic occlusion associated with 50% probability of assessment of ischemic spinal cord dysfunction by tc-MEP and SCEP were analyzed. The amplitude of tc-MEPs decreased significantly at 30 s and disappeared completely at 2 min after aortic occlusion. In Group B, it took about 6 min after aortic occlusion to diminish SCEP signal amplitude by approximately 50%. P50MEP obtained in the quantal analysis was 0.3 ± 0.1 min. P50SCEP was calculated as 6.2 ± 0.5 min that was significantly (P < 0.01) longer than P50MEP. Our data indicated that tc-MEP monitoring could detect the onset of SCI so rapidly in comparison with segmental SCEP monitoring, which could provide therapeutic windows in a surgical approach that includes spinal cord protection.
Keywords: Spinal cord ischemia, Spinal cord monitoring, Motor-evoked monitoring, Sensory-evoked monitoring
Introduction
Spinal cord ischemic (SCI) injury remains the most devastating complication after descending thoracic or thoracoabdominal aneurysm repair. The risk of SCI and neurological deficit has not changed much since it was first reported [1] in 1956 by Adams and Van Geertruyde, which varies between 4.4 and 16% in large series of patients undergoing thoracic or thoracoabdominal aortic aneurysm repair [4, 16, 23]. During the past two decades, several interventions have tried to preserve spinal cord blood flow during and after aortic aneurysm surgery for the purpose of reducing the incidence of postoperative paraplegia. However, efforts to further decrease the rate of paraplegia have been hampered by the inability to assess the adequacy of spinal cord blood supply during surgery [11]. One of the main limitations of protective strategies is the inability to assess the adequacy of spinal cord perfusion and spinal cord function intraoperatively.
In the orthopedic field, somatosensory evoked- (SSEPs) or spinal cord-evoked potentials (SCEPs) recorded from the cerebral sensory area or spinal cord by stimulating the peripheral nerve or skin have been used since the early 1970s. In the cardiovascular surgery field, Coles et al. [3] and Cunningham et al. [5] demonstrated an earlier detection of SCI by using SSEPs that were generated by intraoperative stimulation of peripheral nerves. In addition, it was reported that both of lumbar descending SCEP and segmental SCEP were the most sensitive for SCI [26]. As the blood flow in the motor neuronal system located in the anterolateral part of the spinal cord is supplied form anterior spinal artery, it is questioned whether either SSEPs or SCEPs can be reliable monitoring for spinal cord function in aortic surgery. On the other hand, myogenic transcranial motor-evoked potentials (tc-MEPs) monitor the descending motor system located in the anterior and lateral corticospinal tracts and the anterior horn motor neuronal system, including the function of the ischemia-sensitive α-motor neurons. Therefore, tc-MEP monitoring can rapidly reflect the changes of spinal cord blood flow during thoracic or thoracoabdominal aneurysm repair surgery. Indeed, our recent paper demonstrated that tc-MEP provided the rapid assessment of spinal cord blood flow during aortic occlusion [14].
To our knowledge, there are no studies systematically comparing tc-MEP with segmental SCEP for rapid detection of SCI. In this study, we evaluated the efficacy of tc-MEPs, compared with SCEPs, for detecting SCI and the relationship between neurological outcome and tc-MEPs or SCEPs during reperfusion period in the rat aortic occlusion model.
Material and methods
General preparation
Studies described in this report were performed according to a protocol approved by the Animal Subjects Committee of the University of the Ryukyus. Animals were allowed free access to food and water until they were anesthetized. Male Sprague–Dawley rats (350–400 g; n = 65) were used in the present study. Animals were anesthetized in a plexiglas box with 4% isoflurane in room air. Following induction, rats were maintained with 1–2% isoflurane delivered by an inhalation mask. An intravenous catheter (PE-10) was inserted into the right external jugular vein for continuous infusion of ketamine (2–5 mg/kg/h). After tissue infiltration with 1% lidocaine, the trachea was intubated via a tracheostomy and the lungs were ventilated with 100% oxygen using a small-animal respirator (Harvard Apparatus, Edenbridge, UK) to maintain arterial carbon dioxide tension between 35 and 45 mmHg. After achievement of stable ventilation, infusion of ketamine was started following stopping of isoflurane. Anesthetic depth was determined by noting the withdrawal reflex when pinching the tail and was supplemented with intravenous doses of ketamine (usually 1 mg/kg per dose) as necessary. After the end of surgery, ketamine infusion was ceased. The tracheal tube was removed followed by closure of the tracheostomy with 5-0 nylon after confirming adequacy of spontaneous breathing.
Recording of evoked potentials
Myogenic motor-evoked potentials [14]
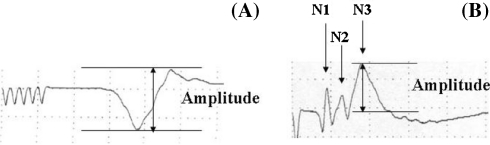
Transcranial motor-evoked potential stimuli were applied with a transcranial electrical stimulator (SEN 3301; Nihon Kohden, JAPAN) through two needle electrodes attached to the scalp. A train of five square wave pulses (voltage 80–100 V), with a duration of 50 μs and an interstimulus interval of 2 ms, was distributed over the motor area. Compound muscle action potentials (CMAP) were recorded from the right soleus muscle using needle electrodes. A grounding electrode was placed over the tail. The signals were amplified 5,000–20,000 times and filtered between 30 and 1,500 Hz using a Neuropack II™ (Nihon Kohden, Tokyo). Stimulus intensity was adjusted to acquire maximal amplitude (Supramaximal stimulation), and recording was performed at a setting 10% above the stimulus level that obtained maximal amplitude. Amplitude of the CMAP was defined as the peak-to-peak distance in milivolts (Fig. 1a). After preischemic tc-MEP recording, SCI was induced.
Fig. 1.
Typical waveforms of tc-MEP (a) and segmental SCEP (b) before aortic occlusion. The amplitudes were measured from peak to peak in tc-MEP (a) and from baseline to peak in segmental SCEP (b)
Spinal cord-evoked potentials
To monitor SCEP, the right sciatic nerve was exposed and bipolar electrodes were placed around the nerve. The stimuli used were square wave pulses of 0.1 ms duration and 0.6 mA intensity delivered at 3 Hz. Two silver needle electrodes were inserted into the midline interspinous ligament so that they were in contact with the lamina at the L4-5 and L5-6 levels. The SCEPs were recorded in a bipolar fashion from the silver electrodes using Neuropack II™ (Model MEB-5304; Nihon Kohden). Sixty-four repetitions were averaged. Yamamoto et al. [25] demonstrated that the typical recording of SCEP from L5 consisted of four positive waves (P1, P2, P3, P4) and three negative waves (N1, N2, N3). The amplitude of N3, representing the activity of spinal interneruons in the posterior horn, was measured from baseline to peak [10] (Fig. 1b).
Induction of SCI
Details of the aortic occlusion model have been reported previously [24]. In brief, a polyethylene catheter (PE-50) was inserted into the tail artery for monitoring of distal arterial pressure and for injection of heparin. For the induction of spinal ischemia, the left femoral artery was isolated and a 2 F Fogarty catheter was placed into the descending thoracic aorta so that the tip of the catheter reached the level of the left subclavian artery. To control the proximal arterial blood pressure at 40 mmHg during the period of aortic occlusion, a 20 G Teflon catheter connected to an external blood reservoir (37.5°C) was inserted into the left carotid artery. To control and maintain the degree of spinal cord normothermia during aortic occlusion, water (38.0–38.3°C) was perfused through the heat exchanger at 100 ml/min. At the completion of all cannulations, heparin (200 U) was injected into the tail artery. To induce spinal ischemia, a balloon catheter was inflated with 0.05 ml of saline and the blood was allowed to flow into an external reservoir. The efficiency of the occlusion was evidenced by an immediate and sustained loss of any detectable pulse pressure and drop of distal arterial pressure. After ischemia, the balloon was deflated and the blood was reinfused over a period of 60 s. In the sham operated rats, all surgical procedures were performed as described; however, the balloon catheter was not inflated. Protamine sulfate (4 mg) was then administered subcutaneously. All arterial lines were then removed, incisions were closed and the animals were allowed to recover.
Assessment of neurological function
At 3 days of reperfusion, recovery of motor function was assessed by the grading system described as follows. Motor function was quantified by the assessment of ambulation and placing and stepping responses [24]. For statistical purposes, ambulation (walking with lower extremities) was graded as follows: 0-normal; 1-toes flat under the body when walking, but ataxia present; 2-knuckle walking; 3-movement in lower extremities but unable to knuckle walk; or 4-no movement, drags lower extremities. Placing/stepping reflex was assessed by dragging the dorsum of the hind paw over the edge of a surface. This normally evokes a coordinating lifting and placing response (e.g., stepping) that was graded as follows: 0-normal, 1-weak or 2-no stepping. A motor deficit index (MDI) was calculated for each rat at each time interval. The final MDI was the sum of the scores (walking with lower extremities plus placing and stepping reflex). The MDI was calculated by observers without the knowledge of the treatment group (M.K.).
Experimental groups and design
Study 1: comparison between tc-MEP and segmental SCEP in detecting the SCI
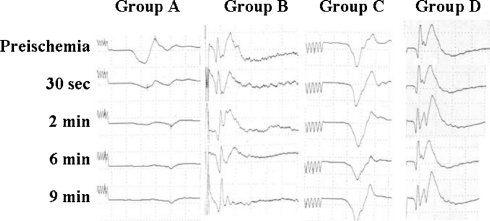
Rats (n = 24) were assigned to one of the following four groups (n = 6/group): Group A, tc-MEP recorded with a 10 min of aortic occlusion, and Group B, segmental SCEP recorded with a 10 min of aortic occlusion, Group C, tc-MEP recorded with a sham operation and Group D, SCEP recorded with a sham operation. tc-MEP and SCEP were recorded preischemia, at 30 s, 2, 6, 9 min after the start of SCI.
Study 2: relationship between an interval of aortic occlusion and the probability of neurological dysfunction, positive assessment of tc-MEP or SCEP after SCI for using quantal bioassay
For quantal bioassay [27] for the relationship between an interval of aortic occlusion and the probability of neurological dysfunction after SCI, the duration of aortic occlusion was selected to span all grades of neurological function ranging from “walk” (MDI: 0–4) to “paraplegia or paraparesis” (MDI: 5–6). Taira and Marsala [10] showed previously that there was a good relationship between the duration of aortic occlusion and the extent of spinal cord damage. The duration of aortic occlusion for individual rats (n = 14) was varied from 6 min up to 12 min. tc-MEP (n = 12) were recorded at preischemic, 15, 30, 60 s, 2, 6, 10 and 12 min after aortic occlusion. SCEP (n = 15) were also recorded at preischemic, 2, 4, 6, 8 10 and 12 min after aortic occlusion. An indication of ischemic spinal cord dysfunction was considered by the reductions of tc-MEP and SCEP amplitude monitored to less than 25 and 50% of the baseline value, respectively.
For quantal bioassay for the relationship between an interval of aortic occlusion and neurological function at 7 days after SCI, the P50N, which represents the interval of aortic occlusion associated with 50% probability of resultant paraplegia, was analyzed and graphically demonstrated by computer construction of a dose-response curve [12]. In using the same methods, the P50MEP and P50SCEP, which represent the interval of aortic occlusion associated with 50% probability of assessment of ischemic spinal cord dysfunction by MEP and SCEP, were analyzed. The computer calculated a P50N, P50MEP and P50SCEP. The quantal dose-response analysis method used in the present study has been published previously [13].
Statistical analysis
Statistical analyses of physiologic data were performed by the unpaired Student’s t test. In the analysis of neurological function, testing of overall neurological function was performed with the Kruskal–Wallis test. Significance (P < 0.05) of results was probed further using the Mann–Whitney test for comparisons. In the study of tc-MEPs, comparisons between groups were performed by two-factor ANOVA for repeated measures. When overall differences were detected, individual comparisons between groups after each time period that indicated either ischemia or reperfusion were performed by the unpaired Student’s t test. In addition, comparisons between baseline values and values at each time point were made within each group by one-factor ANOVA for repeated measures, and post hoc comparisons were made by using the Dunnett test with the baseline value. Changes were considered significant at a value of P < 0.05.
Results
Study 1
Pre- and intraischemic observations
During the pre- and intraischemic periods, body temperature ranged between 37.4–38.2°C. Baseline distal arterial pressure was 71 ± 15 mmHg and decreased to 6 ± 3 mmHg at the end of aortic occlusion. No significant differences among experimental groups were detected.
Time course changes of evoked potentials (Figs 2, 3)
Fig. 2.
Myogenic tc-MEPs recorded from the right soleus muscle and segmental SCEP. tc-MEPs in Group A disappeared at 2 min after aortic occlusion. In Group B, it took about 6 min after aortic occlusion to diminish SCEP signal amplitude by approximately 50%. Neither tc-MEP (Group C) nor segmental SCEP (Group D) changed throughout this experiment
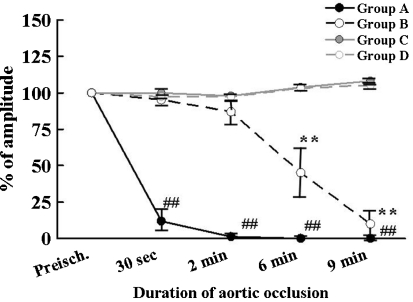
Fig. 3.
Time course changes in the amplitude of myogenic tc-MEPs and segmental SCEPs. Data were expressed as mean ± standard deviation. The amplitudes of tc-MEPs decreased significantly and disappeared within a minute after spinal cord ischemia. (** ## P < 0.01, ## and ** compared with baseline in Group A and in Group B, respectively
Reproducible tc-MEPs and SCEPs were recorded in all animals. In Group A, the amplitude of tc-MEPs decreased significantly at 30 s and disappeared completely at 2 min after aortic occlusion (P < 0.0001 compared with baseline). On the other hand, it took about 6 min after aortic occlusion to diminish SCEP signal amplitude by approximately 50% in Group B. There were significant differences between tc-MEPs (Group A) and SCEPs (Group B) in the amplitude of evoked potentials at 30 s, 2 min and 6 min of reperfusion (P < 0.01) (Fig. 2).
Study 2
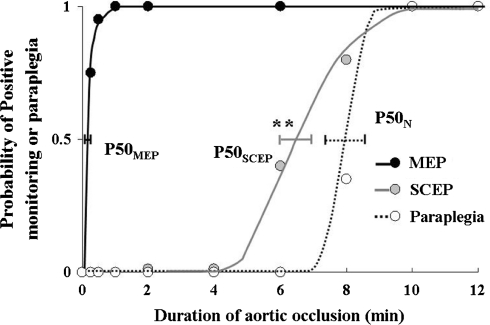
In behavioral analysis assessed 3 days after SCI (Table 1, Fig. 3), the P50N was 7.5 ± 0.6 min. Of 12 animals in recording MEPs, amplitude less than 25% of baseline at 15 s after aortic occlusion was observed in 9 rats (Table 2). P50MEP obtained in the quantal analysis was 0.3 ± 0.1 min (Fig. 3). Of 15 animals in recording segmental SCEPs, amplitude less than 50% of baseline at 6 and 8 min after aortic occlusion was observed in 7 and 13 animals, respectively (Table 2). P50SCEP was calculated as 6.2 ± 0.5 min that was significantly (P < 0.01) longer than P50MEP (Figs. 3, 4).
Table 1.
Effects of interval of aortic occlusion on experimental paraplegia in rat spinal cord ischemia model
| Interval of SCI (min) | Neurological assessment | |
|---|---|---|
| Normal | Paraplegia | |
| 6 | 2 | 0 |
| 7 | 2 | 1 |
| 8 | 1 | 0 |
| 9 | 0 | 3 |
| 10 | 0 | 3 |
| 11 | 0 | 1 |
| 12 | 0 | 1 |
Table 2.
Effects of interval of aortic occlusion on a positive response of spinal cord functional monitoring (tc-MEP or segmemntal SCEP) in rat spinal cord ischemia model
| Duration after aortic occlusion | MEPs (n = 12) | Segmental SCEP (n = 15) | ||
|---|---|---|---|---|
| Negative | Positiveb | Negative | Positiveb | |
| 0 sa | 12 | 0 | 15 | 0 |
| 15 s | 3 | 9 | ||
| 30 s | 0 | 12 | ||
| 60 s | 0 | 12 | ||
| 2 min | 0 | 12 | 15 | 0 |
| 4 min | 15 | 0 | ||
| 6 min | 0 | 12 | 8 | 7 |
| 8 min | 2 | 13 | ||
| 10 min | 0 | 12 | 0 | 15 |
| 12 min | 0 | 12 | 0 | 15 |
aPreischemia
bThe reductions of tc-MEP and SCEP amplitude monitored to less than 25 and 50% of the baseline value, respectively
Fig. 4.
Relation of duration of ischemia to probability of paraplegia, probability of amplitude less than 25% of baseline in tc-MEPs and less than 50% of baseline in segmental SCEP. Horizontal bars represent standard errors at the P50 required to produce paraplegia, amplitude less than 25% of baseline in tc-MEPs and less than 50% of baseline in segmental SCEP. ** Significant differences (P < 0.01) in P50SCEP from P50MEP
Comment
The present study showed that the amplitude of tc-MEP decreased much more rapidly than that of segmental SCEP, and that P50MEP was much shorter than P50SCEP. These data indicated that tc-MEP monitoring could detect the onset of SCI so rapidly in comparison with segmental SCEP monitoring, which could provide therapeutic windows in a surgical approach that includes spinal cord protection.
Segmental SCEPs basically consists of three components, the afferent compound action potential of the dorsal root fibers (N1), postsynaptic potentials (N2) generated in the dorsal horn neurons and presynaptic inhibitory process (N3) generated by inhibitory spinal interneurons [10]. Perioperative monitoring of myogenic MEPs in response to transcranial stimulation of the motor cortex provides a method to monitor the functional integrity of descending motor pathways. In rats, MEPs, elicited by bipolar electrical stimulation to the motor cortex, arise from activation of the spinal pyramidal pathway [8, 9, 21]. Based on previous study [17], SCEPs have monitored functions in the posterior columns of the spinal cord, while tc-MEPs have assessed functions in the anterior and anterolateral columns. The previous studies showed that the spinal interneuronal population localized in laminae IV–VII was the most affected by SCI in canine [18], rabbit [19] and rat [25]. Indeed, Yamamoto et al. [25] emphasized the importance to monitor the compound action potentials representing the activity of interneuons in segmental SCEP from the lumbar spinal cord during ischemia. Therefore, we observed the changes of amplitude of the N3 wave representing presynaptic inhibitory process generated by inhibitory interneurons in segmental SCEP during SCI.
Although, in general, the criterion for SCI during sensory-evoked potentials monitoring is a decrease of amplitude to less than 50% of baseline [2], the rationale for the 25% tc-MEP amplitude decrease criterion that is used clinically to detect SCI is based on theoretical considerations and has been confirmed empirically [20]. The experimental data, using the porcine SCI model, supported the validity of the empirical criterion (25% tc-MEP amplitude reduction) for initiating interventions to maintain spinal cord perfusion pressure during surgical procedures on the thoracoabdominal aneurysm [15]. Therefore, these criteria applied to the detection of SCI in this experiment.
Meylaerts et al. [20] suggested that SSEPs showed delayed ischemia detection and a high rate of false-negative results in comparison with tc-MEP because SSEPs reflect conduction in dorsal columns. In contrast, according to results in a clinical study, Yamamoto et al. [26] mentioned that both lumbar descending SCEP and segmental SCEP were the most sensitive monitoring for SCI. As for MEPs in aortic surgery, Jacobs and his colleagues [7, 12, 20] demonstrated that myogenic MEPs could response to SCI more rapidly than SSEP and no false-negative monitoring results were obtained in myogenic MEPs. Our results in this experiments showed that the P50MEP (0.3 min) was much shorter than the P50SCEP (6.2 min), indicating that myogenic MEPs are much more sensitive to SCI than segmental SCEPs.
With respect to blood supply in the spinal cord, one-third of the posterior spinal cord is supplied by a pair of continuous posterior spinal arteries and the anterior two-thirds are supplied by the discontinuous anterior spinal artery [10]. The anterior spinal artery receives its blood supply from radicular branches. The major radicular supply to the anterior spinal artery in the thoracic and upper abdominal region is from three to five anterior radicular arteries. Since these arteries originate from T9 to L3 in most patients, occlusion of lower thoracic and/or upper abdominal aorta is subject to induce ischemia of the anterior spinal cord motor tracts, resulting in paraplegia. In general, it is believed that SSEP or SCEP fails to monitor directly the function of spinal motor tracts because these are the anterior of spinal cord supplied by the anterior spinal artery. In this animal model, withdrawal of arterial blood from the left carotid artery controlled systemic blood pressure proximal to the aortic occlusion, and this could effectively decrease collateral perfusion [24], thus producing a near total elimination of collateral flow to the spinal cord. Therefore, the delayed detection of SCI by segmental SCEPs in the present study might be associated not only with a blood distribution of the spinal cord, but also with other mechanisms. The study for metabolic activity in the lumbar spinal cord in rats demonstrated that metabolic rate in the anterior/anterolateral columns (Laminae V–IX) was higher by about 25–45% than the posterior column (Laminae I–IV) [22]. This regional difference in metabolic rate may, in part, explain the different sensitivity between tc-MEPs and segmental SCEPs to SCI in this study.
As shown in Fig. 3, segmental SCEPs monitoring could detect only 50% subjects of SCI 6.2 min after aortic occlusion, meaning that others were included falsely negative as an index of SCI. Hence, from Fig. 3, we should be aware of the fact that the curves of PN overlapped that of PSCEP about 8 min after aortic occlusion. This overlap means a possibility that segmental SCEPs produce false negative as an index of spinal cord dysfunction. In contrast, SCI can be detected by tc-MEPs monitoring within a minute, and the curve of PMEP cannot be overlapped by that of PN. This rapid assessment of the adequacy of spinal cord blood flow with MEPs offers several advantages in a surgical approach that includes spinal cord protection; for example, the identification of critical segmental arteries during aortic cross-clamp, assessment of adequate blood supply via reattachment segmental arteries and so on [6]. The present results showed that segmental SCEPs were unable to afford a short interval between the onset and detection of the SCI. This delayed response to spinal ischemia could provide much less time for any surgical intervention in comparison with tc-MEPs. Therefore, we concluded that myogenic tc-MEP monitoring could provide useful information to clinicians for making decisions with regard to timely interventions aimed at correcting ischemic conditions and preserving spinal cord blood flow.
Acknowledgments
This study was performed under the support of the Grant-in-aid for Scientific Research from the Ministry of Education of Japan (No. 16591551, 17591479).
References
- 1.Adams DH, Geertruyden HH. Neurologic complications of aortic surgery. Ann Surg. 1956;144:574–610. doi: 10.1097/00000658-195610000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown RH, Nash CL, Berilla JA, Amaddio MD. Cortical evoked potential monitoring: a system for intraoperative monitoring of spinal cord function. Spine. 1984;9:256–261. doi: 10.1097/00007632-198404000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Coles JG, Wilson GJ, Sima AF, Klement P, Tait GA. Intraoperative detection of spinal cord ischemia using somatosensory cortical evoked potentials during thoracic aortic occlusion. Ann Thorac Surg. 1982;34:299–306. doi: 10.1016/S0003-4975(10)62499-X. [DOI] [PubMed] [Google Scholar]
- 4.Crawford ES, Crawford JL, Safi HJ, Coselli JS, Hess KR, Brooks B, Norton HJ, Glaeser DH. Thoracoabdominal aortic aneurysms: preoperative and intraoperative factors determining immediate- and long-term results of operations in 605 patients. J Vasc Surg. 1986;3:389–404. doi: 10.1067/mva.1986.avs0030389. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham JN, Jr, Laschinger JC, Merkin HA, Nathan IM, Colvin S, Ransohoff J, Spencer FC. Measurement of spinalc cord ischemia during operations upon the thoracic aorta. Initial clinical experience. Ann Surg. 1982;196:285–296. doi: 10.1097/00000658-198209000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haan P, Kalkman CJ. Spinal cord monitoring: somatosensory- and motor-evoked potentials. Anesth Clin North Am. 2001;19:923–945. doi: 10.1016/S0889-8537(01)80017-1. [DOI] [PubMed] [Google Scholar]
- 7.Haan P, Kalkman CJ, Mol BD, Ubags LH, Veldman DJ, Jacobs MJ. Efficacy of transcranial motor-evoked myogenic potentials to detect spinal cord ischemia during operations for thoracoabdominal aneurysms. J Thorac Cardiovasc Surg. 1997;113:87–100. doi: 10.1016/S0022-5223(97)70403-3. [DOI] [PubMed] [Google Scholar]
- 8.Elger CE, Speckmann EJ, Caspers H, Janzen RW. Corticospinal connections in the rat. I. Monosynaptic and polysynaptic responses of cervical motorneurons to epicortical stimulation. Exp Brain Res. 1977;28:385–404. doi: 10.1007/BF00235718. [DOI] [PubMed] [Google Scholar]
- 9.Fehlings MG, Tator CH, Linden RD, Piper IR. Motor evoked potentials recorded from normal and spinalo cord-injured rats. Neurosurgery. 1987;20:125–130. doi: 10.1097/00006123-198701000-00027. [DOI] [PubMed] [Google Scholar]
- 10.Gharagozloo F, Neville RFJ, Cox JL. Spinal cord protection during surgical procedures on the descending thoracic and thoracoabdominal aorta: a critical overview. Semin Thorac Cardiovasc Surg. 1998;10:73–86. doi: 10.1016/s1043-0679(98)70022-x. [DOI] [PubMed] [Google Scholar]
- 11.Griepp RB, Ergin MA, Galla JD, Galla JD, Klein JJ, Spielvogel D, Griepp EB. Mininizing spinal cord injury during repair of descending thoracic and thoracoabdominal aneurysms: the Mount Sinai Approach. Semin Thorac Cardiovasc Surg. 1998;10:25–28. doi: 10.1016/s1043-0679(98)70013-9. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs M, Meylaerts SA, Haan P, Mol BA, Kalkman CJ. Strategies to prevent neurologic deficit based on motor-evoked potentials in type I and II thoracoabdominal aortic aneurysm repair. J Vasc Surg. 1999;29:48–57. doi: 10.1016/S0741-5214(99)70349-6. [DOI] [PubMed] [Google Scholar]
- 13.Kakinohana M, Fuchigami T, Nakamura S, Sasara T, Kawabata T, Sugahara K. Intrathecal administration of morphine, but not small dose, induced spastic paraparesis after a noninjurious interval of aortic occlusion in rats. Anesth Analg. 2003;96:769–775. doi: 10.1213/01.ANE.0000048855.24190.5F. [DOI] [PubMed] [Google Scholar]
- 14.Kakinohana M, Kawabata T, Miyata Y, Sugahara K. Myogenic transcranial motor evoked potentials monitoring cannot always predict neurological outcome after spinal cord ischemia in rats. J Thorac Cardiovasc Surg. 2005;129:46–52. doi: 10.1016/j.jtcvs.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Lips J, Haan P, Jager SW, Vanicky I, Jacobs MJ, Kalkman CJ. The role of transcranial motor evoked potentials in predicting neurologic and histopathologic outcome after experimental spinal cord ischemia. Anesthesiology. 2002;97:183–191. doi: 10.1097/00000542-200207000-00026. [DOI] [PubMed] [Google Scholar]
- 16.Livesay JJ, Cooley DA, Ventemiglia RA, Ventemiglia RA, Montero CG, Warrian RK, Brown DM, Duncan JM. Surgical experience in descending thoracic aneurysmectomy with and without adjuncts to avoid ischemia. Ann Thorac Surg. 1985;39:37–46. doi: 10.1016/S0003-4975(10)62520-9. [DOI] [PubMed] [Google Scholar]
- 17.Machida M, Weinstein SL, Yamada T, Kimura J, Toriyama S. Dissociation of muscle action potentials and spinal somatosensory evoked potentials after ischemic damage of spinal cord. Spine. 1988;13:1119–1124. doi: 10.1097/00007632-198810000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Marsala J, Sulla I, Santa M, Marsala M, Mechirova E, Jalc P. Early neurohistopathological changes of canine lumbosacral spinal cord segments in ischemia-reperfusion-induced paraplegia. Neurosci Lett. 1989;106:83–88. doi: 10.1016/0304-3940(89)90206-1. [DOI] [PubMed] [Google Scholar]
- 19.Marsala M, Danielisova V, Chavko M, Hornakova A, Marsala J. Improvement of energy state and basic modification of neuropathological damage in rabbits as a result of graded postischemic spinal cord reoxygenation. Exp Neurol. 1989;105:93–103. doi: 10.1016/0014-4886(89)90176-3. [DOI] [PubMed] [Google Scholar]
- 20.Meylaerts SA, Jacobs MJ, Iterson V, Haan P, Kalkman CJ. Comparison of transcranial motor evoked potentials and somatosensory evoked potentials during thoracoabdominal aortic aneurysm repair. Ann Surg. 1999;230:742–749. doi: 10.1097/00000658-199912000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryder J, Zappulla R, Nieves J. Motor evoked potentials elicited from pyramidal stimulation and recorded from the spinal cord in the rat. Neurosurgery. 1991;28:550–558. doi: 10.1097/00006123-199104000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Schadrack J, Neto FL, Ableitner A, Castro-Lopes JM, Willoch F, Bartenstein P, Zieglgansberger W, Tolle TR. Metabolic activity changes in the rat spinal cord during adjuvant monoarthritis. Neuroscience. 1999;94:595–605. doi: 10.1016/S0306-4522(99)00186-4. [DOI] [PubMed] [Google Scholar]
- 23.Svensson LG, Crawford ES, Hess KR, Coselli JS, Safi HJ. Experience with 1509 patients undergoing thoracoabdominal aortic operations. J Vasc Surg. 1993;17:357–368. doi: 10.1067/mva.1993.42297. [DOI] [PubMed] [Google Scholar]
- 24.Taira Y, Marsala M. Effect of proximal arterial perfusion pressure on function, spinal cord blood flow, and histopathologic changes after increasing intervals of aortic occlusion in the rat. Stroke. 1996;27:1850–1858. doi: 10.1161/01.str.27.10.1850. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto N, Takano H, Kitagawa H, Kawaguchi Y, Tsuji H. Changes of evoked action potentials and histopathology of the spinal cord, and hind limb dysfunction in spinal cord ischemia of cats. J Spinal Disord. 1994;7:285–295. doi: 10.1097/00002517-199408000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto N, Takano H, Kitagawa H, Kawaguchi Y, Tsuji H, Uozaki Y. Monitoring for spinal cord ischemia by use of the evoked spinal cord potentials during aortic aneurysm surgery. J Vasc Surg. 1994;20:826–833. doi: 10.1016/s0741-5214(94)70171-7. [DOI] [PubMed] [Google Scholar]
- 27.Zivin JA, Waud DR. Quantal bioassay and stroke. Stroke. 1992;23:767–773. doi: 10.1161/01.str.23.5.767. [DOI] [PubMed] [Google Scholar]