Abstract
Prior studies have suggested that biomodels enhance patient education, preoperative planning and intra-operative stereotaxy; however, the usefulness of biomodels compared to regular imaging modalities such as X-ray, CT and MR has not been quantified. Our objective was to quantify the surgeon’s perceptions on the usefulness of biomodels compared to standard visualisation modalities for preoperative planning and intra-operative anatomical reference. Physical biomodels were manufactured for a series of 26 consecutive patients with complex spinal pathologies using a stereolithographic technique based on CT data. The biomodels were used preoperatively for surgical planning and customising implants, and intra-operatively for anatomical reference. Following surgery, a detailed biomodel utility survey was completed by the surgeons, and informal telephone interviews were conducted with patients. Using biomodels, 21 deformity and 5 tumour cases were performed. Surgeons stated that the anatomical details were better visible on the biomodel than on other imaging modalities in 65% of cases, and exclusively visible on the biomodel in 11% of cases. Preoperative use of the biomodel led to a different decision regarding the choice of osteosynthetic materials used in 52% of cases, and the implantation site of osteosynthetic material in 74% of cases. Surgeons reported that the use of biomodels reduced operating time by a mean of 8% in tumour patients and 22% in deformity procedures. This study supports biomodelling as a useful, and sometimes essential tool in the armamentarium of imaging techniques used for complex spinal surgery.
Keywords: Biomodelling, Complex spinal surgery, Rapid prototyping, Stereolithography, Spinal deformity, Spine surgery planning
Introduction
The process of stereolithographic biomodelling and its application to spinal surgery was first described by D’Urso et al. in 1999 [4]. Biomodelling accurately reproduces the morphology of a biologic structure from computerised tomography (CT) scans by using image processing software and a rapid prototyping apparatus to produce a physical copy in acrylate [1, 11].
Planar radiographic images used in planning spinal surgery can be difficult to interpret, especially in cases involving complex pathoanatomy. Three-dimensional CT imaging allows construction of a virtual anatomic overview enhancing the visualisation of spinal disease [2, 9, 13, 15]. However, the limitations of visualising three-dimensional (3D) models using two-dimensional prints—the method employed by most radiology departments making such images available for surgeons to use in practice—has limited its application [1]. Planning the placement of implants onto pathologic spinal structures is also very difficult using the standard 2D and 3D visualisation techniques available.
The preliminary investigation of spinal biomodelling in 1999 [4] based on five cases found that an accurate physical model of the spine is an effective tool to enhance patient education and consent, preoperative planning and intra-operative stereotaxy [7]. A study using biomodels in 45 complex cranio-maxillofacial surgeries found their use improved diagnosis and operative planning, facilitated informed consent and reduced operative time [5]. A subsequent study of six patients in 2001 [14] using biomodels concluded that they provided excellent understanding of the complex spinal pathology and assisted surgical planning and performance. However, the benefits of biomodels in complex spinal surgery have not been quantified to date. This study aims to quantify the surgeon’s perceptions on the usefulness of biomodels, compared to standard visualisation techniques for a series of complex spinal surgery patients.
Materials and methods
Biomodel manufacture and utilisation
The senior authors (GNA and RDL) employed 28 biomodels in a series of 26 patients with complex spinal disorders. Patients were selected for the technique if the pathoanatomy was not considered to be clearly displayed by standard imaging techniques. Helical CT scans of the spine were performed (GE Lightspeed Plus; General Electric Medical Systems, Milwaukee, WI, USA) to produce a series of axial images (16 bits, 512 × 512 pixels, 0.6–1 mm spacing). Scans were then transferred to an image processing system (Anatomics Biobuild; Anatomics Pty Ltd, Melbourne, Australia ), which produced a model suitable for manufacture by stereolithography (SLA250, 3D Systems, USA) [3]. Stereolithography involves a liquid-bed laser curing system, where a laser traces contours and polymerises a photosensitive liquid plastic monomer or resin [4, 10]. The accuracy of stereolithographic biomodels has been reported to be within 1 mm of the scanned anatomy [6]. Artificial struts are built into the model, if required, to position and hold separate structures in their anatomical positions. A typical model takes 18–30 h to build on the SLA apparatus, depending on geometry and volume. Cervical spine models or smaller spine segments can take between 12 and 16 h. The processing of CT data prior to building the model typically takes 1–2 h.
Following manufacture, the biomodels were used for:
Preoperative diagnosis and assessment of spinal pathology.
Patient and parent education about the nature of the deformity, possible surgical interventions and their risks prior to obtaining informed consent.
Preoperative surgical planning, to simulate surgery, practise implant placement and customise off-the-shelf implantable devices prior to the surgical procedure.
Intra-operative verification of bony anatomy, surgical navigation and instrumentation with visual and tactile feedback.
As a teaching aid in theatre, to explain the procedure to the entire surgical team.
Each biomodel was sterilised (first 12 by autoclave and subsequent 16 using Gamma radiation) prior to intra-operative use. Gamma radiation was used with the latter models to avoid thermal surface degradation due to autoclaving of the biomodel material.
Postoperative survey
In the early postoperative period following each operation, a detailed utility survey was completed by the senior surgeon (Appendix 1). The survey contained questions relating to the effect of the biomodel on: preoperative planning and implant selection, the surgical procedure and outcomes and the surgeon’s overall opinion on the usefulness and effectiveness of the biomodel. Informal telephone interviews with patients or consenting relatives were also conducted postoperatively to assess the effect of the biomodels in the consent process.
Results
Study cohort
Table 1 shows the cases selected for biomodelling between 1997 and 2005. All surgical procedures were performed by the senior authors (RDL and GNA). Both surgeons work exclusively in spinal orthopaedic surgery and predominantly with spinal deformity and have over 20 years of combined specialist experience. Spinal deformities or instability comprised 79% of the models (22 models in 21 patients), with the remaining 21% (6 models in 5 patients) comprising cervical tumours including osteoblastoma and chordoma.
Table 1.
Summary of the 28 consecutive complex cases selected for biomodelling
| ID | Model Date | Diagnosis | Sex | Age | Region surveyed |
|---|---|---|---|---|---|
| 1 | June-1997 | C2 osteoblastoma | M | 32 | Cervical |
| 2 | July-1997 | Fibromatosis C2 left longus colli involving C3 nerve root | F | 7 | Cervical |
| 3 | June-1998 | Condylicus Tertius, fracture dislocation of C1–2 | F | 8 | Cervical |
| 4 | August-1998 | Juvenile rheumatoid arthritis with atlanto-axial instability | F | 28 | Cervical |
| 5 | October-1998 | Osteoblastoma C5 | M | 20 | Cervical |
| 6 | November-1998 | Neurofibromatosis with kyphoscoliosis | F | 6 | Cervicothoracic |
| 7 | January-1999 | C2 chordoma | M | 50 | Cervical |
| 8 | March-1999 | Recurrent C2 chordoma post C1–3 posterior fusion | M | 50 | Cervical—second model |
| 9 | June-1999 | Congenital cervical spinal deformity with failure of segmentation of C1–3 | M | 6 | Cervical |
| 10 | January-2000 | Cervical hemivertebra with torticollis | M | 7 | Cervicothoracic |
| 11 | August-2000 | C2/3 Schwannoma | M | 35 | Cervical |
| 12 | September-2000 | Klippel-Feil / VACTERL | M | 10 | Cervical |
| 13 | September-2000 | Hemivertebra with thoracic scoliosis | M | 10 | Cervicothoracic—second model |
| 14 | July-2001 | C1/2 non-union of fusion for os odontoideum with brain stem compression and myelopathy | F | 24 | Cervical |
| 15 | October-2001 | Spina bifida meningomyelocoele with T9 kyphosis | F | 9 | Thoracolumbar to sacrum |
| 16 | March-2002 | Spina bifida meningomyelocoele with T7-L2 kyphosis | F | 8 | Thoracolumbar to Sacrum |
| 17 | October-2002 | Spondyloepiphyseal dysplasia congenita (SEDC) with cervical scoliosis | F | 4 | Cervical |
| 18 | May-2003 | Spina bifida meningomyelocoele with T10-L3 kyphosis | F | 10 | Thoracolumbar |
| 19 | September-2003 | Multiple congenital cervical spine abnormalities including C1–2 instability and lower cervical kyphosis | M | 2 | Cervical |
| 20 | February-2004 | SEDC with C1–2 instability with cord compression | M | 6 | Cervical |
| 21 | May-2004 | T10–11 hemivertebra with hyperkyphosis | M | 1 | Thoracic |
| 22 | May-2004 | Multiple congenital cervical and thoracic deformities including unsegmented bar T6–7 and Hemivertebra T1,4,7. | F | 5 | Cervicothoracic |
| 23 | May-2004 | Congenital kyphoscoliosis | F | 3 | Thoracolumbar |
| 24 | October-2004 | Facio-auriculovertebral syndrome. congenital cervical and thoracic kyphoscoliosis | F | 15 | CervicoThoracic |
| 25 | November-2004 | Congenital idiopathic juvenile thoracolumbar scoliosis | F | 7 | Thoracolumbar |
| 26 | November-2004 | Congenital kyphoscoliosis, VATER, T9–10 hemivertebra, large syrinx, fused ribs, tracheomalacia | F | 2 | Thoracolumbar |
| 27 | October-2005 | Congenital kyphoscoliosis | M | 13 | Thoracolumbar |
| 28 | October-2005 | Spina bifida meningomyelocoele with thoracic scoliosis | M | 9 | Cervicothoracic |
Two of the patients had a second biomodel made. The first had recurrence of a C2 chordoma, which necessitated a second model prior to a second surgical procedure. The second patient’s initial biomodel included the occiput and cervical spine only, so a second biomodel was ordered to allow visualisation of the patient’s extensive deformities, which included upper cervical instability, a C4–5 hemivertebra and cervico-thoracic scoliosis.
Tumour surgery
Five patients with tumours of the cervical spine were treated in the series using six biomodels resulting in six surgical procedures (Appendix 2). There were four males and one female with a mean age of 28.8 ± 16.2 (range 7–50). One patient had an early recurrence of tumour, so underwent revision surgery three months later with wide excision and reconstruction and has no evidence of recurrence 72 months following revision surgery. This group of patients has now been monitored postoperatively for a mean of 71.8 ± 4.1 months (range 65–75) and all patients are currently disease-free.
Deformity surgery
Thirteen cases of cervical and cervico-thoracic deformity and eight cases of thoracolumbar deformity were treated in the series (Appendix 3). These cases included 8 males and 13 females with a mean age of 8.7 ± 6.8 years (range 1–28). One patient with cervical hemivertebrae did not have a surgical procedure performed as a result of detailed preoperative examination of the biomodel, which showed the congenital deformity to be more benign than was indicated by other imaging modalities. In this case, the biomodel was also used to illustrate to the parents of this patient, aged seven, the reasons behind the decision not to operate. The deformity has since remained stable for the last 77 months. The spinal deformity group of patients has been monitored for a mean of 37.5 ± 24.8 (range 9–84) months.
Preoperative planning
A summary of other visual modalities used prior to requesting a biomodel during surgical planning revealed X-rays were ordered in 100% of cases, 2D CT scans in 67% of cases, 3D CT scans in 70% of cases and 2D MRI in 93% of cases. When comparing the anatomical details from the biomodel to that of other visualisation modalities, the information needed was less visible on the biomodel than on the images in 15.4% of cases (n = 4), there was no difference in 7.7% of cases (n = 2), the information needed was better visible on the biomodel than on the images in 65.4% of cases (n = 17) and the information needed was exclusively visible on the biomodel in 11.5% of cases (n = 3) (Figs. 1, 2, 3).
Fig. 1.
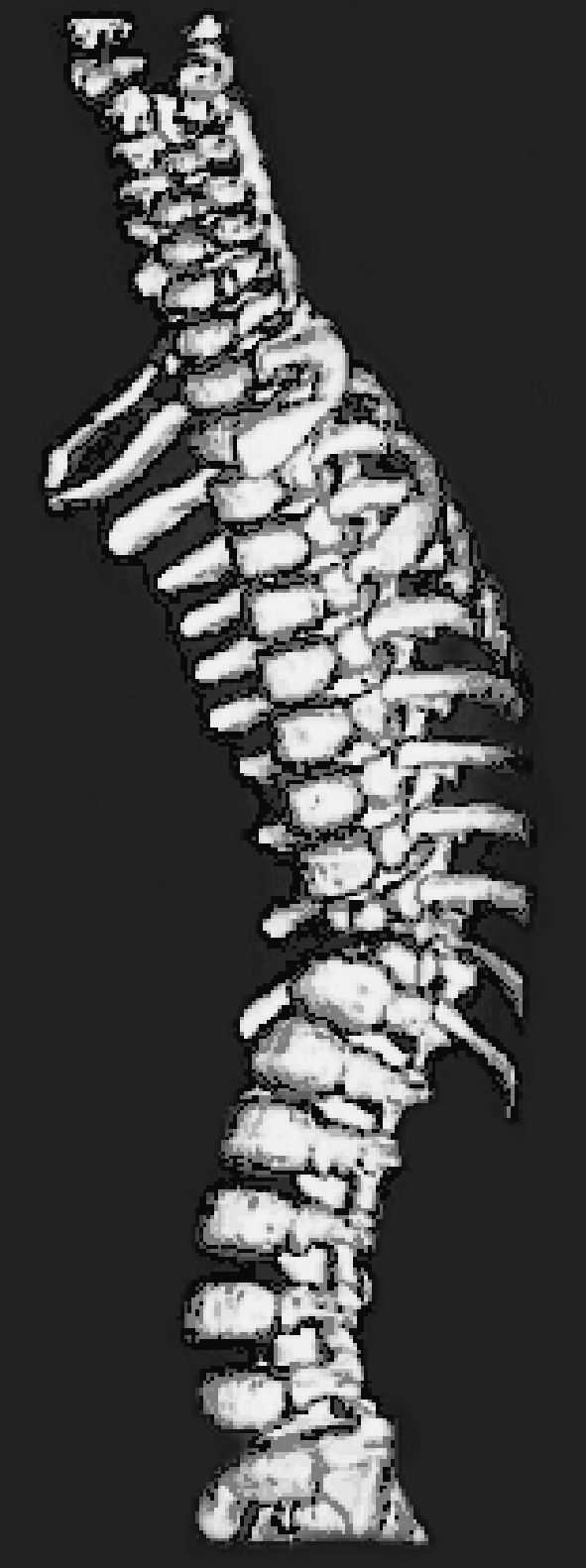
3D CT reconstruction of lower thoracic spine in a 22-month-old child (Case 21), who presented with severe thoracic kyphosis due to a suspected hemivertebra at T10/11
Fig. 2.
MRI sagittal slice of lower thoracic hemivertebra (Case 21) indicating that there may have been two hemivertebrae
Fig. 3.
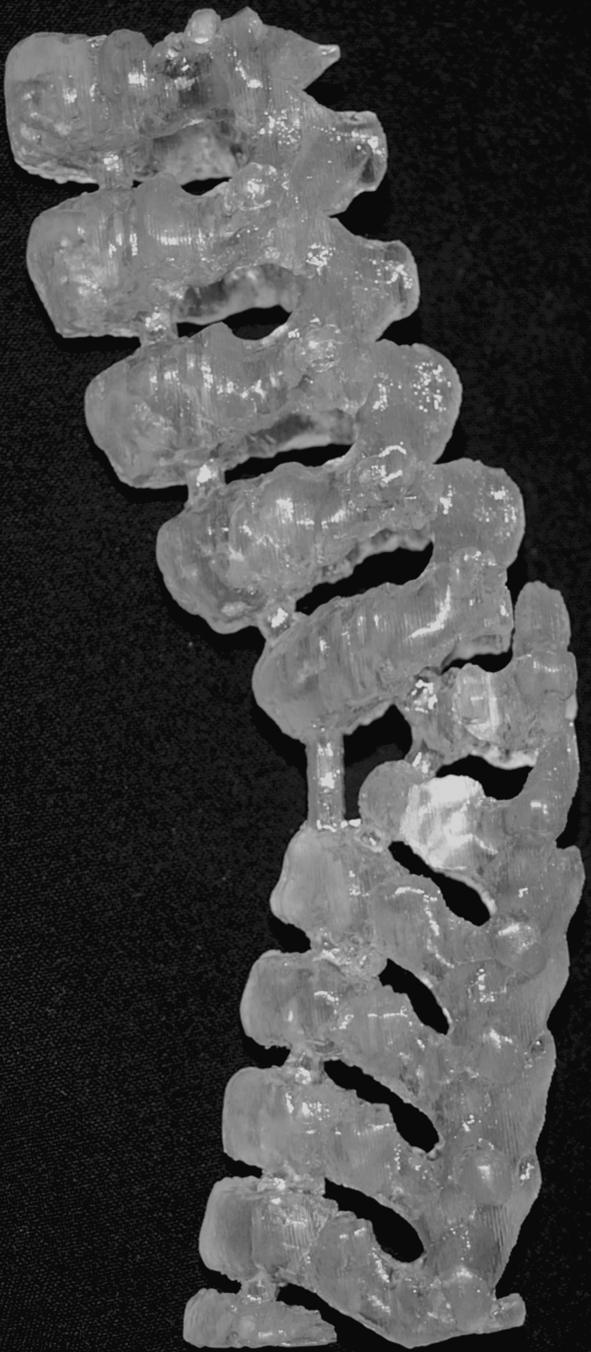
Lateral view of biomodel of lower thoracic hemivertebrae (Case 21) illustrating the two incomplete vertebral segments whose finer details were not clearly demonstrated with either the 3D CT or MRI examinations
In 22% of cases (n = 6), the detail revealed by the biomodel allowed multiple surgeries to be combined into one procedure with improved confidence. In 11% of cases (n = 3), the surgery was simulated preoperatively on the biomodel with reported benefits of a much better surgical outcome, having planned the precise positioning of the implant required (Fig. 4). The implant/instrumentation for the surgery was altered or custom made using the biomodel in three cases, which resulted in a perfect fit in two cases and a good fit with some minor bone remodelling in the third instance.
Fig. 4.
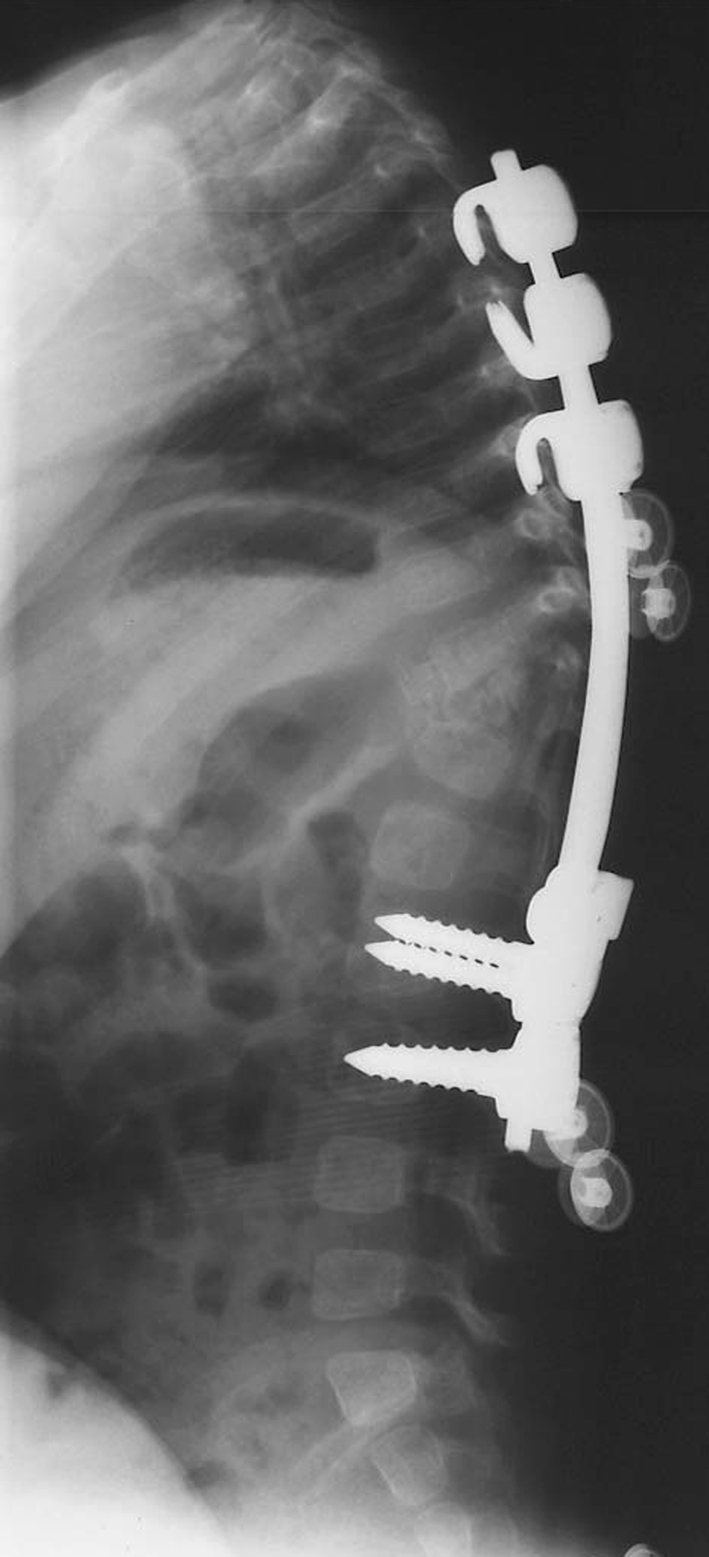
Postoperative sagittal X-ray after excision of both hemivertebrae (Case 21) and posterior instrumented fusion from T4-L3. During anterior surgery, the anatomical accuracy of the biomodel facilitated confident excision of both hemivertebrae, one of which was difficult to visualise within the intra-operative field. This allowed good surgical correction to be achieved
On comparing the biomodel to other visualisation modalities used for surgical planning, the biomodel was reported by the surgeons to be either ‘not as useful’ (39%), ‘the same’ (33%), or ‘more useful’ (20%) for diagnosis and detection of the problem, and was reported as ‘the same’ (15%), ‘more useful’ (37%), or ‘provided unique info’ (48%) for rectification of the problem. The surgeons reported that the biomodel was the most useful preoperative visualisation modality in the surgical planning process in 70% of cases (n = 19) and the second most useful modality in 19% (n = 5) of cases.
Of the 19 patients or relatives able to be contacted, all stated that the biomodels improved informed consent by improving anatomical understanding of the pathological condition, the planned procedure and the risks (Figs. 5, 6).
Fig. 5.
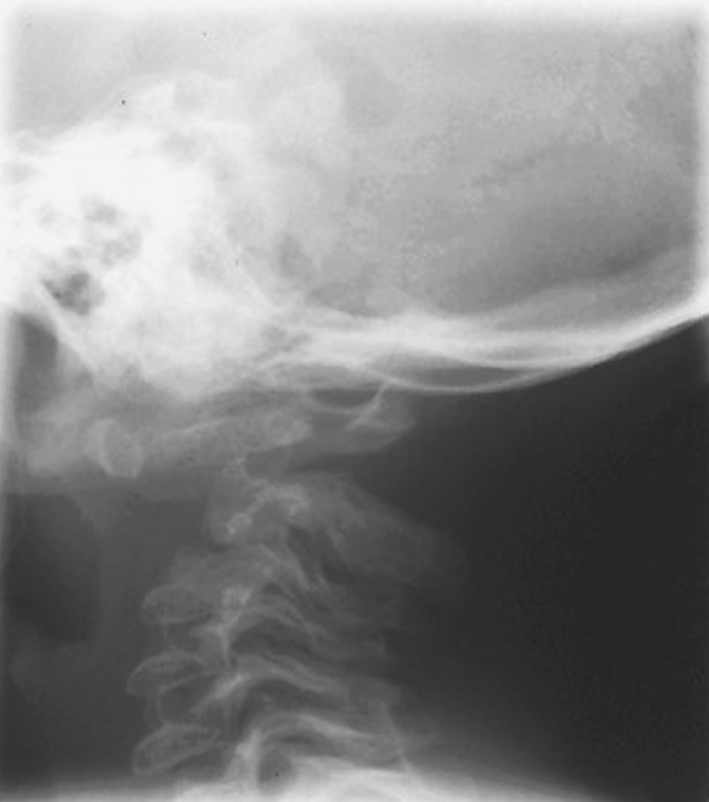
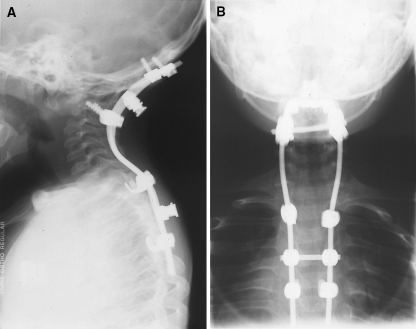
Sagittal X-ray of cervical spine of child (Case 20) with spondyloepiphyseal dysplasia congenita (SEDC) with atlanto-axial instablility and early neurological signs of spinal cord compression
Fig. 6.
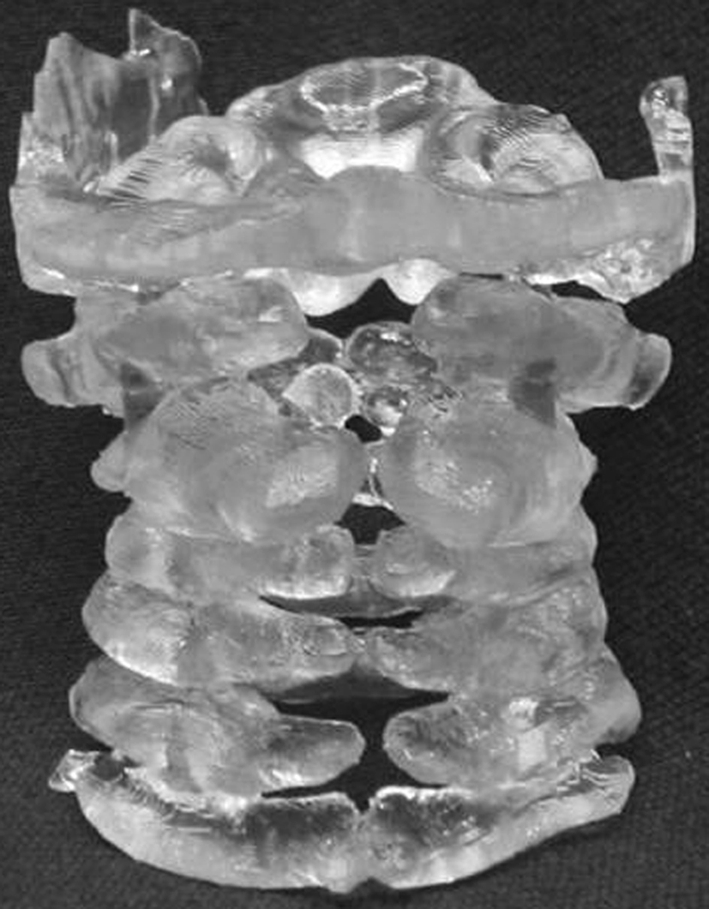
Posterior view of biomodel of cervical spine (SEDC in Case 20) illustrating the posterior bony deficits, which were not clearly demonstrated by other investigations. The biomodel allowed safer exposure of the spinal cord and facilitated the placement of posterior instrumentation to achieve fusion
Surgical procedure
The mean length of the surgical procedures for the tumour and deformity groups, respectively, were 610 ± 327 (range 240–960) min and 309 ± 220 (range 135–1,089) min. On the surgeon’s estimate, use of biomodels reduced operating time for the whole series by an average of 17% (63 min per case). For the tumour group, the estimated reduction in operating time was 8% (mean 46 ± 60 min per case, range 0–125), and 22% for the deformity group (mean 68 ± 40 min per case, range 30–180). Estimates of surgical time saved using the biomodels were made using historical reference data on surgical times for equivalent spinal deformity or tumour cases at the same centre. The reasons given in the survey for the reduction in surgery times were:
Detailed preoperative planning and improved confidence in the surgical intervention.
Better anatomical visualisation.
Easier and more accurate and efficient implant and screw positioning.
Easier and more accurate osteotomy and vertebrectomy.
Less frequent reference to other imaging resources.
Biomodel identifying a hidden hemivertebra prior to the surgery.
Reduced number of levels instrumented.
In 100% of cases, the surgeons reported that the accuracy of the model was either adequate (65.4%, n = 17) or more than needed (34.6%, n = 9) for the type of procedure. In 100% of cases, the surgeons reported that they would again order a biomodel should a similar patient present who required surgical correction.
Biomodel use facilitated better or much better intra-operative communication between the theatre staff and aided in trainee surgeon education in 89% of cases. Intra-operative anatomical details were found to be accurately represented by the biomodel and was reported as being almost the same in 58% of cases (n = 15), exactly the same in 39% of cases (n = 10) and somewhat different in the single remaining case.
The models were sterilised in all the cases and referred to extensively during the procedure (>10 times in 18 cases and 6–10 times in 8 cases). Intra-operatively, the surgeons reported the model as being the most useful visual modality in 89% of cases (n = 23) and in the top two most useful modalities in 96% of cases (n = 25).
When asked how much the use of a biomodel affected the choice of materials, instruments and devices used during surgery, the surgeons reported that the biomodel had no influence in 15% of cases (n = 4, due to the anatomy being adequately displayed by prior X-rays and scans), a slight influence in 8% of cases (n = 2), a moderate influence in 19% of cases (n = 5), a significant influence in 50% of cases (n = 13) and was extremely influential in 8% of cases (n = 2). The biomodel was reported as being extremely influential when use of the model resulted in custom or altered implants being arranged during preoperative planning (Figs. 7, 8).
Fig. 7.
Sagittal and posteroanterior X-ray views (Case 20) after decompression and posterior instrumented fusion from occiput-T5
Fig. 8.

Sagittal 3D CT reconstruction (Case 20) 6 months postsurgical instrumented fusion
Procedure outcomes
Regarding the effect of the biomodel on various surgical outcomes in comparison to other visualisation modalities, biomodel use changed the outcome of the procedure in 93% of cases (n = 25), with the biomodel improving surgical outcome (either ‘better’ or ‘much better’) in 78% of cases (n = 21) and the surgeons reporting that the desired surgical outcome was unachievable without the biomodel in 15% of cases (n = 4). The survey responses indicated that patient care was either ‘better’ or ‘much better’ in 96% of cases (n = 26) as a direct result of using a biomodel.
When asked to report their opinion on the effect the biomodel had on the cost of the procedure, the survey results indicated the cost was higher in 7.4% of cases (n = 2), the same in 33% of cases (n = 9), lower in 44% of cases (n = 12) and significantly lower in 15% of cases (n = 4). In the two cases where the biomodels increased the cost of the procedure, each patient had two biomodels made, and the reduction in surgery time was not enough to offset the cost of two biomodels. The Australian government funded Children’s Hospital paid for the biomodel in 58% of cases (n = 15), private health insurance in 27% of cases (n = 7) and the patient paid in 15% of cases (n = 4).
When asked to rank a series of eight attributes from most important (Score 1) to least important (Score 8) regarding the decision to use a biomodel, the surgeons ranked model accuracy as most important (Score 1) in 93% of cases (n = 25), while the ability to sterilise the model for intra-operative reference was listed in the top three most important attributes in 96% of cases (n = 26). The only other consistently nominated factor was rapid delivery time for the model, which was listed in the top three attributes in 89% of cases (n = 24).
Discussion
Biomodelling allows CT data to be displayed in accurate physical form. The data required is simple to acquire and can be transmitted to the manufacturer via the Internet or by postage of a computer disk. This enables physical biomodels to be used in countries where the manufacturing technique is not yet available. The biomodels were found to be highly accurate in relation to anatomy identified during the surgery and were especially helpful for cases with limited surgical access or with vital surrounding structures.
The utility survey responses in this study demonstrate that for complex spinal surgery cases, biomodels have advantages over the use of two-dimensional and three-dimensional imaging techniques both preoperatively and intra-operatively. The biomodel facilitates preoperative surgical planning and rehearsal by allowing simulated reconstruction and instrumentation. Of note, the surgeons reported that the desired surgical outcome was unachievable without the biomodel in 15% of cases. It is also possible to test custom made or off-the-shelf implants before surgery to ensure that they will be accurately implantable. During surgery, the anatomical reference provided by the biomodel allows safer dissection of tumours with a higher degree of surgical confidence during approach. These cases are technically challenging and the surgeon’s estimates of time saved (mean 46 min per case) provides substantial benefits to tumour patients.
Both the spinal deformity and tumour procedures have achieved satisfying results following biomodel-assisted surgery, with mean follow-up periods of around 3 years for the deformity group and 6 years for the tumour group. In one case, biomodelling of the pathoanatomy indicated that the deformity was unlikely to progress, and surgery was therefore not undertaken, with good clinical outcome.
The most challenging surgical group has been the subgroup of deformity patients with severe spinal dysraphism requiring long fusions. The surgical treatment of such patients often involves significant complication rates [8, 12]. One patient in this subgroup died one month postoperatively after a sudden unexplained increase in intracranial pressure on day 2 post surgery, resulting in a hypoxic brain incident (the spinal surgery itself was considered successful). One patient experienced some loss of correction following surgery. The remaining five patients achieved lasting correction of the deformity. Utility survey responses for this subgroup indicated that the biomodels facilitated planning of the kyphectomy and reduced operative time by a mean of 22% (58 min, range 30–120).
Biomodel cost is in the range of AUD $900–1,500 depending on the size and complexity of the model required. Surgeons report that for cases where a biomodel has been ordered, the total procedure cost is the same or lower than if a model were not ordered in 92% of cases. Reasons given for the offset of costs included reduction in surgery time due to; (1) more efficient operative technique (including cases where one surgical approach was required instead of two or a number of surgical interventions were combined into one procedure), (2) detailed preoperative planning and increased confidence with reduced reliance on other visualisation modalities, (3) fewer intra-operative complications, and (4) more accurate fixation requiring less extensive instrumentation. Due to the above justifications, the biomodel cost was billed directly to the patient in only four cases when the surgery was performed in a private hospital on uninsured patients. Otherwise, the biomodel costs were accepted by private health insurance companies or the government funded hospitals.
Survey results indicate that biomodels have also proved helpful as a communication tool, providing patients with a better appreciation of their condition and of the planned surgery and the subsequent risks involved. Further, biomodels facilitate communication within the surgical team both pre and intra-operatively. The main obstacle to more widespread biomodel use for educational purposes is the cost of production, but in the same way that demonstration spines with normal anatomy are used in outpatient clinics, it is possible to have an ‘exemplar’ biomodel of certain deformity conditions to explain the pathoanatomy to patients and students.
Conclusions
Our spinal unit has used biomodelling for tumour and deformity cases with the most challenging pathoanatomy and demonstrated it to be a useful tool in the armamentarium of imaging techniques used in complex spinal surgery.
A postoperative utility survey was completed by the surgeons, in which the biomodels were reported as the most useful visual modality in the preoperative planning process for 70% of cases and the most useful intra-operative visual modality in 89% of cases.
On comparing the anatomical details from the biomodel to that of other visualisation modalities, surgeons reported that the information needed was better visible on the biomodel than on other modalities in 65% of cases and was exclusively visible on the biomodel in 11% of cases.
Biomodels were found to be highly accurate in relation to anatomy identified during the surgery when compared with other visualisation modalities and resulted in improved communication with patients/parents and informed consent in 96% of cases.
Surgical times were reduced in 89% of cases by a mean of 63 min per case and therefore surgical costs were often subsequently reduced despite the additional cost of the biomodel.
In 100% of cases, the surgeons reported that the model had a positive effect on the outcome of the surgical procedure and that they would order a biomodel again should a similar case present.
Appendix 1: Biomodel utility survey
(Note: Possible responses for each question are given in parentheses)
Part A: Case information
Patient demographics, Surgeon, Surgery date, Hospital, Clinical problem, Type of procedure.
Part B: Preoperative planning
B1 Which visualisation modalities were used preoperatively for this case? (X-ray, 2DCT, 3DCT, 2DMRI, 3DMRI, other)
- B2 Was the biomodel used preoperatively for this patient? (Yes/No)
- B2.2 How did the information from the biomodel compare with that from other visualisation modalities?
- (Information needed was visible only on the images, not on the biomodel.)
- (Information needed was more visible on the images than on the biomodel.)
- (No difference.)
- (Information needed was better visible on the biomodel than on the images.)
- (Information needed was exclusively visible on the biomodel.)
- B3 Did the preoperative use of this biomodel lead to a different decision for:
- whether to operate or not (Yes/No)
- composition of the surgical team (Yes/No)
- skin incision (Yes/No)
- patient’s position on the operating table (Yes/No)
- choice of osteosynthetic material (Yes/No)
- choice of instrumentation/devices (Yes/No)
- implantation site of osteosynthetic material (Yes/No)
- sequence of surgery (Yes/No)
- other? please specify (Yes/No)
- B4 Was surgery simulated preoperatively on the biomodel? (Yes/No)
- B4.1 To what extent did the preoperative simulation affect the surgical outcome? (Much worse, Worse, Same, Better, Much better)
- B4.2 How was the outcome affected?
- B5 Was an off-the-shelf implantable device customised preoperatively using this biomodel? (Yes/No)
- B5.1 What type of implantable device was customised? How was the implantable device customised?
- B6 Was a custom implant made preoperatively using this biomodel? (Yes/No)
- B6.1 What type of implant was made? How well did the implant fit? (Poor, Fair, Good, Excellent, Perfect)
Part C: Surgical procedure
C1 How long did it take to perform this surgical procedure? (time spent performing the primary operation, min)
- C2 Was the biomodel used intra-operatively for this patient? (Yes/No)
- C2.1 How would you compare the use of this biomodel intra-operatively to other visualisation modalities with respect to (a) your diagnosis, (b) your surgical plan, (c) communication between members of the surgical team? (Much Worse, Worse, Same, Better, Much Better)
- C3 Was the biomodel sterilised? (Yes/No)
- C3.1 How was the biomodel sterilised? (Autoclave, Liquid Sterilised, ETO, Plasma, Gamma radiation, Other)
C4 To what extent were intra-operative findings accurately represented by the biomodel? (Totally different, Somewhat different, Similar, Almost the same, Exactly the same)
C5 How many times were the following modalities used intra-operatively? (a) Biomodel. (b) Other visualisation modalities. (0,1–2,3–5,6–10,10+)
- C6 Did the use of the biomodel have an effect on the time it took to perform the surgical procedure (compared to not using a biomodel)? (Yes/No)
- C6.1 What effect did the use of the biomodel have? (a) Estimated reduced time in min. How? (b) Estimated increased time in min. How? Was the increase justified? (Yes/No)
C7 How does the accuracy of the biomodel compare to that needed for this type of procedure? (Much less than needed, Less than needed, Adequate, More than needed, Much more than needed)
Part D: Outcomes
D1 In comparison to other visualisation modalities, how did the use of the biomodel affect (a) accuracy or quality of bone grafts, (b) accuracy or quality of osteotomy, (c) communication with colleagues, (d) communication with the patient, (e) degree of confidence during surgery? (Does not apply, Much less, Less, Same, More, Much more)
D2 In comparison to other visualisation modalities, how did the use of the biomodel change the outcome of this procedure? (Much worse, Worse, Same, Better, Much Better, Unachievable without the biomodel)
D3 How would you rate the usefulness of the biomodel? (Misleading, No real use, Useful, Very useful, Essential)
D4 What effect do you think the biomodel had on the total cost of this procedure? (Significantly higher cost, Higher cost, Same cost, Lower cost, Significantly lower cost) Why?
D5 To what extent do you think patient care was affected by the use of the biomodel? (Much worse, Worse, Same, Better, Much Better)
- D6 Did the use of the biomodel affect your estimate of the total number of surgical interventions that will be needed for this patient (compared to that made using other visualisation modalities)? (No/More/Fewer)
- D6.1 If more, more surgical interventions will be needed because (a) the biomodel indicated the problem was more complex than indicated by other visualisation modalities, (b) the biomodel indicated that the problem was more widespread than indicated by other visualisation modalities, (c) other.
- D6.2 If fewer, fewer surgical interventions will be needed because (a) the biomodel indicated the problem was less complex than indicated by other visualisation modalities, (b) the biomodel indicated that the problem was less widespread than indicated by other visualisation modalities, (c) the use of the biomodel allowed a number of interventions to be combined into one procedure, (d) other.
D7 What were the three most important outcomes arising from the use of the biomodel?
Part E: General
E1 What were your reasons for using a biomodel for this case? (a) Visualisation aid (to improve diagnosis, to improve surgical planning, to obtain informed patient consent). (b) Preoperative action (to simulate surgery preoperatively, to prepare an implant preoperatively, to prepare a template for resections). (c) For intra-operative reference. (d) Other.
E2 How much did the use of a biomodel for this patient affect the choice of materials, instruments or devices used or made available during surgery? (Not at all, Slightly, Moderately, Significantly, Extremely) How?
E3 Rate the most useful (1) to least useful (6) visualisation modalities used (a) preoperatively, (b) intra-operatively? (Frameless stereotaxy, X-ray, 2D CT, 3D CT, 2D MRI, 3D MRI, Biomodel)
E4 How did the biomodel compare to other visualisation modalities for the following? (a) Detection of the problem, (b) localisation of the problem, (c) diagnosis of the problem, (d) rectification of the problem. (Significantly inferior, Not as useful, Same, More useful, Provided unique info)
E5 Rank from most important (1) to least important (8) the importance of the following attributes in making your decision to use a biomodel for this patient. (Hardness, Density, Colour, Translucency, accuracy, Can sterilise, Price, Delivery time)
E6 Who will meet the cost of the biomodel? (Patient, Surgeon, Hospital, Insurance, Other)
E7 When treatment of this patient is completed, who will keep the biomodel? (Patient, Surgeon, Hospital, Other, Unsure)
E8 If another patient required similar surgery, would you use a biomodel again? (Yes/No) Why?
E9 In what ways could the biomodel be improved?
Appendix 2
Table 2
Table 2.
Tumour subgroup detailing the surgical procedure, follow-up, results and complications
| ID | Tumour | Surgical treatment | Follow-up | Result | Complication |
|---|---|---|---|---|---|
| 11 | Schwannoma C2/3 | Anterior resection and reconstruction, posterior fusion C2–5 | 65 months | No recurrence, solid fusion, excellent ranges of movement | Nil |
| 8 | Chordoma C2 × 2 | Anterior resection and reconstruction, posterior fusion occiput to C3 | 72 months | No evidence of recurrence following revision surgery | Recurrence—required the second biomodel with wide excision and reconstruction |
| 1 | Osteoblastoma C2 | Anterior resection and reconstruction, posterior fusion occiput to C3 | 75 months | No recurrence, solid fusion. | Some persistent numbness, right foot. |
| 5 | Osteoblastoma C5 | Anterior resection and reconstruction, posterior fusion C4–6 | 72 months | No recurrence | Nil |
| 2 | Fibromatosis C2 longus colli | Anterior resection and posterior fusion occiput to C3 | 72 months | No recurrence | Nil |
Appendix 3
Table 3
Table 3.
Spinal deformity subgroup detailing the surgical procedure, follow-up, results and complications
| ID | Cervical deformity/instability | Surgical treatment | Follow-up | Result | Complication |
|---|---|---|---|---|---|
| 3 | Condylicus tertius | Posterior occiput to C3 decompression and fusion | 84 months | Solid fusion, resolution of neurology | Nil |
| 17 | Spondyloepiphyseal dysplasia congenita (SEDC), cervical scoliosis | Posterior instrumented fusion occiput to C5 | 38 months | Solid fusion, good correction, pain resolved | Nil |
| 10 | Cervical hemivertebra with torticollis | No surgery | 77 months | Asymptomatic | Nil |
| 12 | Klippel-Feil/VACTERL | Anterior and posterior instrumented fusion occiput to T4, C4/5 vertebrectomy | 60 months | Solid fusion, good correction and spinal balance | Nil |
| 4 | Juvenile rheumatoid arthritis with atlanto-axial instability | Anterior and posterior decompression and instrumented fusion occiput to C5 | 80 months | Solid fusion, No progressive symptoms | Nil |
| 9 | Congenital cervical spinal abnormalities with failure of segmentation of C1–3 | Posterior fusion occiput to C2 | 60 months | Solid fusion, no adverse symptoms | Nil |
| 19 | Multiple congenital cervical spine abnormalities including lower cervical kyphosis | Posterior occiput to T4 decompression and fusion | 16 months | Solid fusion, no adverse symptoms | Nil |
| 20 | SEDC with cord compression | Decompression and instrumented fusion, posterior rod occiput to T5 | 12 months | Solid fusion, no adverse symptoms | Nil |
| 14 | C1/2 non-union of fusion for os odontoideum with brain stem compression | Anterior C1–3 and posterior occiput to C3 fusion and decompression | 57 months | Solid fusion, no progressive symptoms | Wound infection unilateral lingual numbness |
| ID | Cervicothoracic deformity | Surgical treatment | Follow up | Result | Complication |
|---|---|---|---|---|---|
| 6 | Neurofibromatosis with scoliosis | T3 vertebrectomy, decompression and instrumented fusion C2–T9 | 24 months | Solid fusion with satisfactory correction | Intra-operative cardiac arrest. Postop seizures from cerebral ischaemia |
| 28 | Spina bifida meningomyelocoele with thoracic scoliosis | Costoplasty T5–10 and insertion of growing rods | 18 months | Improved clinical appearance, no progression | Nil |
| 22 | Multiple congenital cervical and thoracic deformities, including unsegmented bar T6–7 and hemivertebra T1,4,7. | Thoracoplasty, hemiepihyseodesis, posterior instrumented fusion T1–6 | 23 months | Solid Fusion, clinically and radiologically stable | Nil |
| 24 | Facio-auriculovertebral syndrome, congenital cervical and thoracic kyphoscoliosis | Thoracoplasty, excision T2–6 transverse processes | 12 months | Solid fusion, good correction and cosmetic result | Nil |
| ID | Thoracolumbar deformity | Surgical treatment | Follow up | Result | Complication |
|---|---|---|---|---|---|
| 15 | Spina bifida meningomyelocoele with T9 kyphosis | Posterior kyphectomy at 3 levels and instrumented fusion T3 to pelvis | Died 1 month post surgery | Death | |
| 16 | Spina bifida meningomyelocoele with T7-L2 kyphosis | Posterior kyphectomy at 3 levels and instrumented fusion T3 to pelvis | 53 months | Loss of correction, but happy with sitting position | CSF leak with infection and wound sinus |
| 18 | Spina bifida menigomyelocoele with T10–L3 kyphosis | Posterior 2 level Kyphectomy and instrumented fusion T2 to pelvis | 36 months | Good sitting position, good correction and improved clinical appearance | Nil |
| 21 | Thoracic hemivertebra with hyperkyphosis | Anterior hemivertebrectomy and posterior instrumented fusion T4–L3 | 36 months | Solid fusion, good correction and clinical appearance | Nil |
| 23 | Congenital kyphoscoliosis | Posterior Instrumented fusion T3–L3 | 22 months | Solid fusion, good correction and cosmetic result | Nil |
| 25 | Congenital idiopathic juvenile thoracolumbar scoliosis | Anterior hemiepiphyseodesis, instrumented fusion T11–L2 | 9 months | Solid fusion, good correction and cosmetic result | Nil |
| 26 | VATER, hemivertebrae T9,T10 with kyphoscoliosis, fused ribs, tracheomalacia and syringomyelia | Anterior thoracotomy, T9 ,T10 vertebrectomy, posterior instrumented fusion T8–L1 | 16 months | Solid fusion, good cosmetic result, some Kyphosis progression. May require a long post fusion in future. | Nil. |
| 25 | Congenital kyphoscoliosis | Posterior osteotomy T11 and posterior instrumented fusion T8-L2 | 17 months | Solid fusion, good correction and cosmetic result | (R) Femoral nerve palsy postoperatively, resolved after 2 weeks |
Footnotes
Sources of support: No financial support was received for this study.
References
- 1.Barker TM, Earwaker WJS, Frost N, Wakeley G. Integration of 3-D medical imaging and rapid prototyping to create stereolithographic models. Aust Phys Eng Sci Med. 1993;16(2):79–85. [PubMed] [Google Scholar]
- 2.Bonnier L, Ayadi K, Vasdev A, Crouzet G, Raphael B. Three-dimensional reconstruction in routine computerized tomography of the skull and spine. J Neuroradiol. 1991;18(3):250–66. [PubMed] [Google Scholar]
- 3.D’Urso PS (1993) Stereolithographic modelling process [Australian Patent 684546; US Patent 5741215]
- 4.D’Urso PS, Askin GN, Earwaker WJS, Merry GS, Thompson RG, Barker TM, Effeney DJ. Spinal biomodeling. Spine. 1999;24(12):1247–1251. doi: 10.1097/00007632-199906150-00013. [DOI] [PubMed] [Google Scholar]
- 5.D’Urso PS, Barker TM, Earwaker WJS, Bruce LJ, Atkinson L, Lanigan MW, Arvier JF, Effeney DJ. Stereolithographic biomodeling in cranio-maxillofacial surgery: a prospective trial. J Craniomaxillofac Surg. 1999;27(1):30–37. doi: 10.1016/s1010-5182(99)80007-9. [DOI] [PubMed] [Google Scholar]
- 6.D’Urso PS, Hall BI, Atkinson RL, Weidmann MJ, Redmond MJ. Biomodel guided stereotaxy. Neurosurgery. 1999;44(5):1084–1094. doi: 10.1097/00006123-199905000-00080. [DOI] [PubMed] [Google Scholar]
- 7.D’Urso PS, Williamson OD, Thompson RG. Biomodeling as an aid to spinal instrumentation. Spine. 2005;30(24):2841–2845. doi: 10.1097/01.brs.0000190886.56895.3d. [DOI] [PubMed] [Google Scholar]
- 8.Furderer S, Hopf C, Schwarz M, Voth D. Orthopedic and neurosurgical treatment of severe Kyphosis in myelomeningocele. Neurosurg Rev. 1999;22(1):45–49. doi: 10.1007/s101430050008. [DOI] [PubMed] [Google Scholar]
- 9.Hadley MN, Sonntag VKH, Amos MR, Hodak JA, Lopez LJ. Three-dimensional computed tomography in the diagnosis of vertebral column pathological conditions. Neurosurgery. 1987;21(2):186–192. doi: 10.1097/00006123-198708000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Hull CW (1986) Apparatus for production of three-dimensional objects by stereolithography. US patent 4575330. 3D Systems, Valencia
- 11.Lohfeld S, Barron V, McHugh PE. Biomodels of bone: a review. Ann Biomed Eng. 2005;33(10):1295–1311. doi: 10.1007/s10439-005-5873-x. [DOI] [PubMed] [Google Scholar]
- 12.Niall DM, Dowling FE, Fogarty EE, Moore DP, Goldberg C. Kyphectomy in children with myelomeningocele: a long-term outcome study. J Pediatr Orthop. 2004;24(1):37–44. doi: 10.1097/00004694-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Starshak RJ, Crawford CR, Waisman RC, Sty JR. Three-dimensional CT of the pediatric spine. Appl Radiol. 1989;18:15–26. [Google Scholar]
- 14.Dijk M, Smit TH, Jiya TU, Wuisman PI. Polyurethane Real-size models used in planning complex spinal surgery. Spine. 2001;26(17):1920–1926. doi: 10.1097/00007632-200109010-00020. [DOI] [PubMed] [Google Scholar]
- 15.Virapongse C, Shapiro M, Gmitro A, Sarwar M. Three-dimensional computed tomographic reformation of the spine, skull and brain from axial images. Neurosurgery. 1986;18(1):53–58. doi: 10.1097/00006123-198601000-00009. [DOI] [PubMed] [Google Scholar]