Abstract
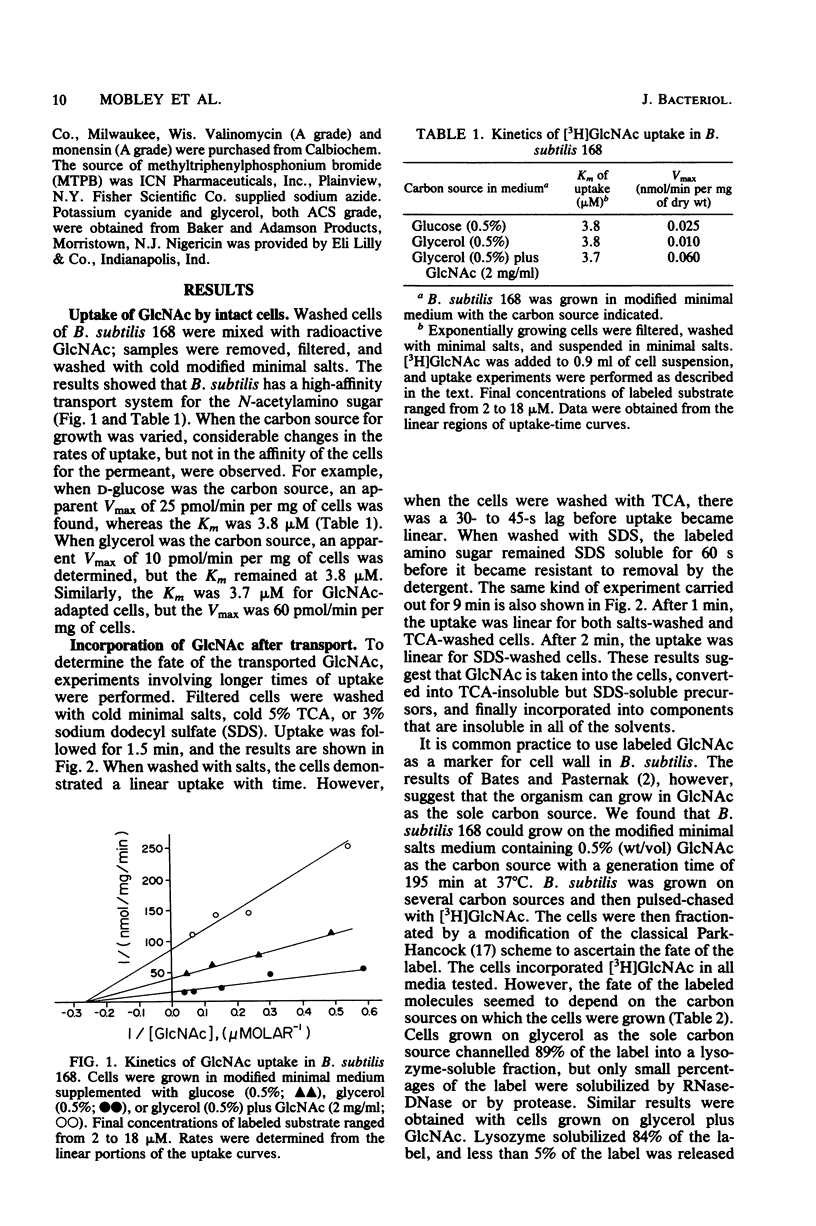
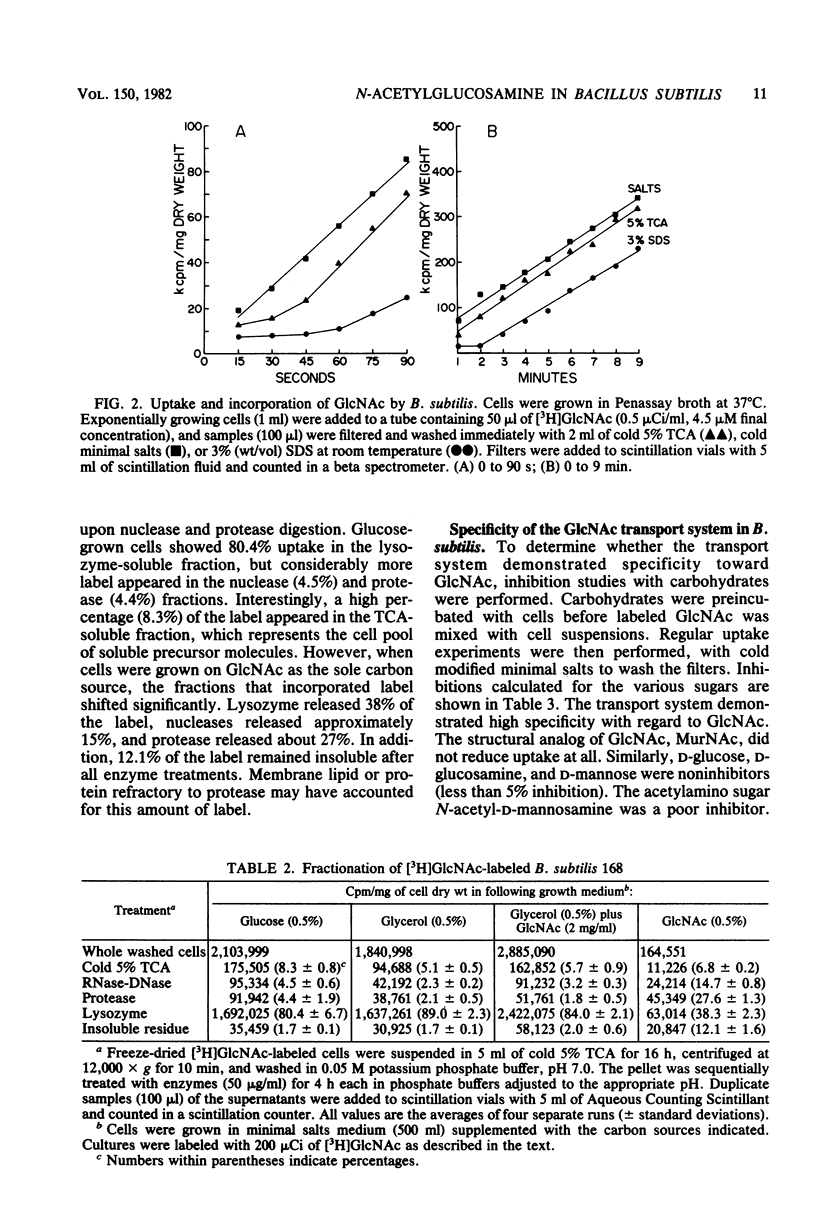
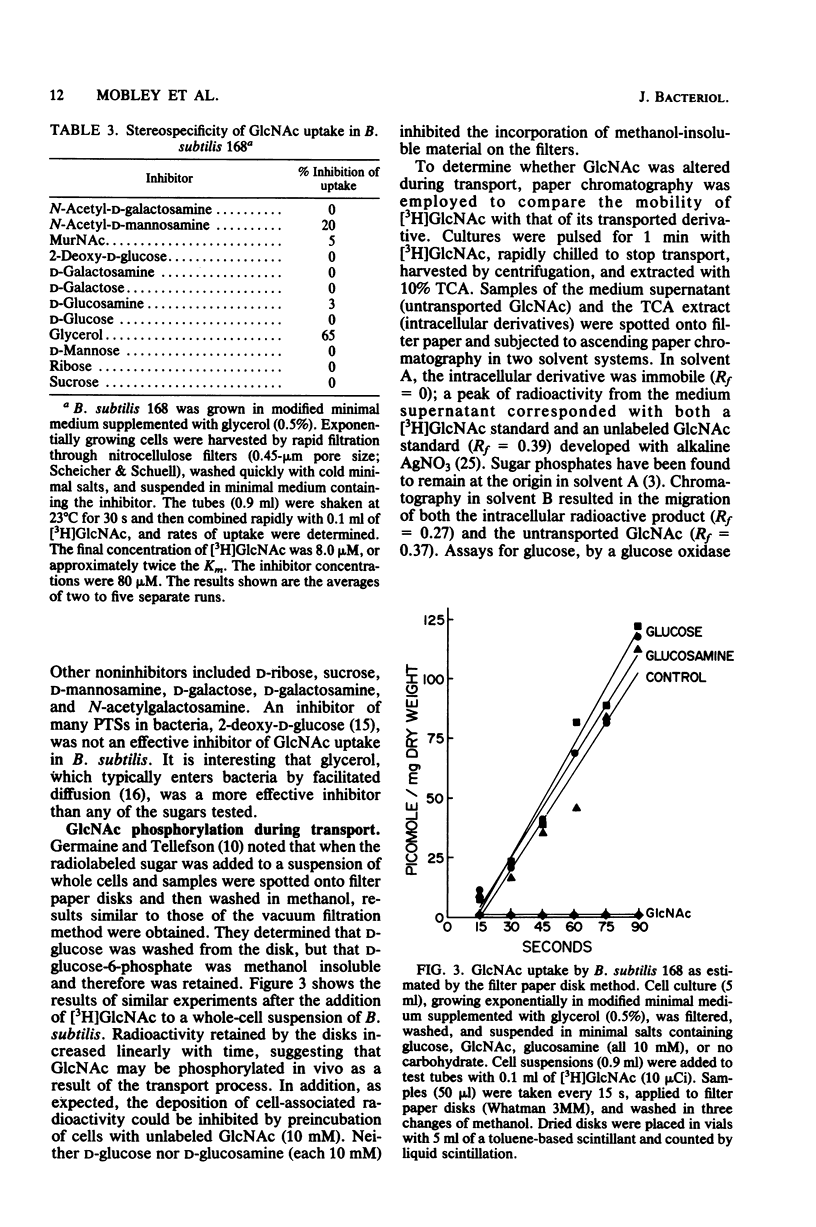
Bacillus subtilis 168 has been found to possess a high-affinity transport system for N-acetyl-D-glucosamine (GlcNAC). The Km for uptake was approximately 3.7 microM GlcNAc, regardless of the nutritional background of the cells. Apparent increases in Vmax were noted when the bacteria were grown in the presence of GlcNAc. The uptake of GlcNAc by B. subtilis was highly stereoselective; D-glucose, D-glucosamine, N-acetyl-D-galactosamine, D-galactose, D-mannose, and N-acetylmuramic acid did not inhibit GlcNAc uptake. In contrast, glycerol was an effective inhibitor of [3H]GlcNAc transport and incorporation. Partial inhibition of GlcNAc uptake was observed with azide, fluoride, and cyanide anions, carbonyl cyanide-m-chlorophenyl hydrazone, methyltriphenylphosphonium bromide, N,N'-dicyclohexylcarbodiimide, gramicidin, valinomycin, monensin, and nigericin. Two anions, arsenite and iodoacetate, were potent inhibitors of the uptake of GlcNAc in B. subtilis. Results from paper chromatography showed that there was no intracellular pool of free GlcNAc and that the acetylamino sugar was probably phosphorylated during transport. A modification of the Park-Hancock cell fractionation scheme indicated that cells grown on glycerol or D-glucose incorporated [3H]GlcNAc primarily into the cell wall fraction. When GlcNAc was used as the sole carbon source, label could be demonstrated in fractions susceptible to protease and nuclease, as well as lysozyme, showing that the N-acetylamino sugar was utilized in macromolecular synthesis and energy metabolism.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BATES C. J., PASTERNAK C. A. FURTHER STUDIES ON THE REGULATION OF AMINO SUGAR METABOLISM IN BACILLUS SUBTILIS. Biochem J. 1965 Jul;96:147–154. doi: 10.1042/bj0960147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BATES C. J., PASTERNAK C. A. THE INCORPORATION OF LABELLED AMINO SUGARS BY BACILLUS SUBTILIS. Biochem J. 1965 Jul;96:155–158. doi: 10.1042/bj0960155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhumiratana A., Anderson R. L., Costilow R. N. Trehalose metabolism by Bacillus popilliae. J Bacteriol. 1974 Aug;119(2):484–493. doi: 10.1128/jb.119.2.484-493.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKE J. S., PASTERNAK C. A. The regulation of amino sugar metabolism in Bacillus subtilis. Biochem J. 1962 Jul;84:185–191. doi: 10.1042/bj0840185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calmes R. Involvement of phosphoenolpyruvate in the catabolism of caries-conducive disaccharides by Streptococcus mutans: lactose transport. Infect Immun. 1978 Mar;19(3):934–942. doi: 10.1128/iai.19.3.934-942.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle R. J., Streips U. N., Imada S., Fan V. S., Brown W. C. Genetic transformation with cell wall-associated deoxyribonucleic acid in Bacillus subtilis. J Bacteriol. 1980 Dec;144(3):957–966. doi: 10.1128/jb.144.3.957-966.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P., Beckman B. E., Gardner-Eckstrom H. L. Mode of cell wall synthesis in gram-positive bacilli. J Bacteriol. 1975 Sep;123(3):1157–1162. doi: 10.1128/jb.123.3.1157-1162.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachelin G. A new assay of the phosphotransferase system in Escherichia coli. Biochem Biophys Res Commun. 1969 Feb 21;34(4):382–387. doi: 10.1016/0006-291x(69)90392-1. [DOI] [PubMed] [Google Scholar]
- Germaine G. R., Tellefson L. M. Simple filter paper procedure for estimation of glucose uptake via group translocation by whole-cell suspensions of bacteria. Appl Environ Microbiol. 1981 Mar;41(3):837–839. doi: 10.1128/aem.41.3.837-839.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFEE P., ENGLESBERG E. Effect of metabolic activity on the glucose permease of bacterial cells. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1759–1765. doi: 10.1073/pnas.48.10.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe L. K., Doyle R. J., Streips U. N. The energized membrane and cellular autolysis in Bacillus subtilis. Cell. 1981 Sep;25(3):753–763. doi: 10.1016/0092-8674(81)90183-5. [DOI] [PubMed] [Google Scholar]
- Jones-Mortimer M. C., Kornberg H. L. Amino-sugar transport systems of Escherichia coli K12. J Gen Microbiol. 1980 Apr;117(2):369–376. doi: 10.1099/00221287-117-2-369. [DOI] [PubMed] [Google Scholar]
- Kornberg H. L., Miller E. K. Role of phosphoenolpyruvate-phosphotransferase in glucose utilization by bacilli. Proc R Soc Lond B Biol Sci. 1972 Sep 19;182(1067):171–181. doi: 10.1098/rspb.1972.0073. [DOI] [PubMed] [Google Scholar]
- Lin E. C. Glycerol dissimilation and its regulation in bacteria. Annu Rev Microbiol. 1976;30:535–578. doi: 10.1146/annurev.mi.30.100176.002535. [DOI] [PubMed] [Google Scholar]
- PARK J. T., HANCOCK R. A fractionation procedure for studies of the synthesis of cell-wall mucopeptide and of other polymers in cells of Staphylococcus aureus. J Gen Microbiol. 1960 Feb;22:249–258. doi: 10.1099/00221287-22-1-249. [DOI] [PubMed] [Google Scholar]
- Romano A. H., Eberhard S. J., Dingle S. L., McDowell T. D. Distribution of the phosphoenolpyruvate: glucose phosphotransferase system in bacteria. J Bacteriol. 1970 Nov;104(2):808–813. doi: 10.1128/jb.104.2.808-813.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U. Coordinate regulation of adenylate cyclase and carbohydrate permeases by the phosphoenolpyruvate:sugar phosphotransferase system in Salmonella typhimurium. J Biol Chem. 1975 Sep 10;250(17):7078–7080. [PubMed] [Google Scholar]
- Schachtele C. F. Glucose transport in Streptococcus mutans: preparation of cytoplasmic membranes and characteristics of phosphotransferase activity. J Dent Res. 1975 Mar-Apr;54(2):330–338. [PubMed] [Google Scholar]
- Schäfer A., Schrecker O., Hengstenberg W. The staphylococcal phosphoenolpyruvate-dependent phosphotransferase system. Purification and characterisation of the galactoside-specific membrane-component enzyme II. Eur J Biochem. 1981 Jan;113(2):289–294. doi: 10.1111/j.1432-1033.1981.tb05065.x. [DOI] [PubMed] [Google Scholar]
- Simoni R. D., Nakazawa T., Hays J. B., Roseman S. Sugar transport. IV. Isolation and characterization of the lactose phosphotransferase system in Staphylococcus aureus. J Biol Chem. 1973 Feb 10;248(3):932–940. [PubMed] [Google Scholar]
- Slee A. M., Tanzer J. M. Phosphoenolpyruvate-dependent sucrose phosphotransferase activity in five serotypes of Streptococcus mutans. Infect Immun. 1979 Nov;26(2):783–786. doi: 10.1128/iai.26.2.783-786.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- White R. J. The role of the phosphoenolpyruvate phosphotransferase system in the transport of N-acetyl-D-glucosamine by Escherichia coli. Biochem J. 1970 Jun;118(1):89–92. doi: 10.1042/bj1180089. [DOI] [PMC free article] [PubMed] [Google Scholar]



