Abstract
Circumferential cervical decompression and fusion (CCDF) is an important technique for treating patients with severe cervical myelopathy. While circumferential cervical decompression and fusion may provide improved spinal cord decompression and stability compared to unilateral techniques, it is commonly associated with increased morbidity and mortality. We performed a retrospective analysis of patients undergoing CCDF at the University of California, San Francisco (UCSF) between January 2003 and December 2004. We identified 53 patients and reviewed their medical records to determine the effectiveness of CCDF for improving myelopathy, pain, and neurological function. Degree of fusion, functional anatomic alignment, and stability were also assessed. Operative morbidity and mortality were measured. The most common causes of cervical myelopathy, instability, or deformity were degenerative disease (57%) and traumatic injury (34%). Approximately one-fifth of patients had a prior fusion performed elsewhere and presented with fusion failure or adjacent-level degeneration. Postoperatively, all patients had stable (22.6%) or improved (77.4%) Nurick grades. The average preoperative and postoperative Nurick grades were 2.1 ± 1.9 and 0.4 ± 0.9, respectively. Pain improved in 85% of patients. All patients had radiographic evidence of fusion at last follow-up. The most common complication was transient dysphagia. Our average clinical follow-up was 27.5 ± 9.5 months. We present an extensive series of patients and demonstrate that cervical myelopathy can successfully be treated with CCDF with minimal operative morbidity. CCDF may provide more extensive decompression of the spinal cord and may be more structurally stable. Concerns regarding operation-associated morbidity should not strongly influence whether CCDF is performed.
Keywords: Cervical, Circumferential, Fusion, Myelopathy
Introduction
Compression of the spinal cord results in sensory and motor dysfunction known as myelopathy [3]. Cervical myelopathy can be caused by degenerative disease such as spondylosis, disc herniation, and ossification of the posterior longitudinal ligament or ligamentum flavum. Other causes include trauma, primary or metastatic malignancies, and infection. Degenerative spine disease is the most common cause of cervical myelopathy and is increasingly prevalent given the increasing life expectancy.
Patients with cervical myelopathy can experience symptoms ranging from mild gait abnormalities and decreased arm dexterity to complete loss of sensory and motor function corresponding to the level of the disease. Bowel and bladder dysfunction can also be present. Concomitant impingement of the nerve roots can occur along with myelopathy, leading to a myeloradiculopathy. The natural history of cervical myelopathy can be variable, but many patients experience a gradual progression with neurologic deterioration [4]. Non-operative management and close observation is indicated in patients with mild and stable myelopathy, but patients with progressive or severe symptoms undergo surgical decompression with or without fusion [16]. When indicated, surgery should be performed within 6 months to 1 year of symptom onset for best results [4]. In patients undergoing extensive decompression for cervical myelopathy, fusion is sometimes indicated.
Circumferential cervical decompression and fusion (CCDF) is an important technique for treating cervical myelopathy, instability, or deformity. In this paper we describe our experience with circumferential cervical decompression and fusion for patients with cervical myelopathy, myeloradiculopathy, instability, or deformity. We determine if: (1) circumferential decompression and fusion are efficacious in treating myelopathy and cervical instability or deformity, and (2) whether CCDF can be performed with low operative morbidity and mortality.
Materials and methods
We performed a retrospective analysis of the 53 patients who underwent circumferential cervical fusion at the University of California, San Francisco (UCSF) between January 2003 and December 2004. All patients who underwent elective surgery were evaluated at the UCSF Spine Center. Their complete preoperative medical history was recorded, and a physical examination was performed. Imaging evaluation consisted of magnetic resonance imaging (MRI), plain radiographs, and computed tomography (CT) with bi-planar image reconstructions. Patients evaluated in the emergency department or transferred from a referring institution had the same imaging workup at the referring institution or at UCSF.
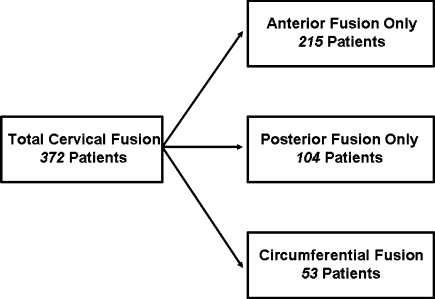
Of 372 patients undergoing cervical fusion, 53 patients underwent circumferential cervical fusion (Fig. 1). Patients were selected for circumferential cervical fusion if they met one of the following criteria: (1) anterior and posterior compression of the spinal cord with loss of lordosis, (2) anterior compression only with need for corpectomy of greater than two levels, or (3) anterior compression only with poor bone quality, preoperative instability, or deformity needing correction (including pseudoarthrosis from prior fusion). Monosegmental disease is generally not an indication for circumferential cervical fusion except in the setting of exceptionally poor quality of bone.
Fig. 1.
Schematic representation of the management of cervical stenosis at our institution by type of procedure performed. The majority of patients underwent anterior or posterior decompression and fusion, but 14.2% underwent circumferential fusion
Data was collected from the UCSF computerized clinical information system. Patient age, gender, etiology of presentation, preoperative pain, neurological function, and Nurick grade were collected by an independent clinical nurse coordinator and recorded [18]. Intra-operative data including levels fused, instrumentation used, and complications were also noted.
Outcome measures were evaluated by an independent nurse coordinator and included postoperative pain, neurological function, and Nurick grade. Pain was assessed by subjective patient self-assessments on a scale of increasing severity from 1 to 5, with 1 representing no pain, and 5 representing maximum pain. The severity of myelopathy was also assessed postoperatively using the Nurick grade [18]. Neurological motor function was assessed using the royal medical research council of Great Britain strength grading scale (Table 1). Evaluation for dysphagia or dysphonia was performed by reviewing the independent evaluation by speech and swallow pathologists, which was performed on every patient within 1 day of extubation. Patients with dysphagia or dysphonia underwent serial evaluations as inpatients and outpatients until their dysphonia or dysphagia was completely resolved. The presence or absence of dysphagia or dysphonia was considered transient if it resolved by 1 month after surgery and permanent if present 1 month after surgery. All patients wore rigid cervical collars for comfort, except for two patients who required halo external immobilization secondary to concern regarding the stability of fusion due to patient history and/or severe deformity. Halos were also used between surgeries when the circumferential cervical fusion was staged or if the patient was very unstable.
Table 1.
Royal medical research council of Great Britain strength grading scale
| Grade | Strength |
|---|---|
| 0 | No contraction |
| 1 | Flicker or trace of contraction |
| 2 | Active movement with gravity eliminated |
| 3 | Active movement against gravity |
| 4 | Active movement against resistance 4− slight resistance 4 moderate resistance 4+ strong resistance |
| 5 | Normal strength |
Radiographic evidence of hardware placement, vertebral column alignment, and fusion were evaluated by the surgeon along with review of neuroradiology reports transcribed by an independent radiologist in the medical record. Plain radiographs and CT scans were used in the evaluation of postoperative fusion. All patients were also specifically evaluated with long cassette scoliosis films to assess for global sagittal and coronal balance. Evidence of radiographic fusion included presence of bony extension or expansion into the space between the graft and the adjacent levels, and was considered excellent with evidence of fusion on CT scan. Two patients were deemed to have “satisfactory” fusion which involved having no evidence of bridging trabecular bone on CT scan; however, the patients did have evidence of lack of motion on flexion–extension radiographs and no evidence of lucency or hardware failure. Alignment was estimated based on radiographic findings and was considered excellent if the normal lordotic cervical curvature was restored or maintained and if no evidence of delayed kyphosis or scoliosis was seen. Alignment was considered satisfactory in one patient without return of normal lordosis but with radiographic improvement of neck alignment and no evidence of kyphosis or scoliosis. The success of hardware placement was evaluated and deemed excellent when there was no evidence of hardware subsidence, screw misplacement, or hardware failures. It was deemed satisfactory in one patient with breech of one pedicle screw, which was not clinically significant.
Results
Patient characteristics
Fifty-three patients (31 males, 22 females) underwent circumferential cervical fusion at UCSF between January 2003 and December 2004. During this period the spine surgery service at UCSF performed 215 anterior only and 104 posterior only cervical fusions (Fig. 1). While in an ideal setting, the patient population would be homogenous, the cause of cervical myelopathy or instability in our patient population ranged from infection in two patients (4%), trauma in 18 patients (34%), tumor in three patients (6%), and degenerative disease in 30 patients (57%). Twelve patients (23%) had undergone prior decompression and fusion at other institutions and presented with fusion failure or adjacent-level degenerative spondylosis. Nine patients had undergone an anterior or posterior cervical decompression without fusion and presented with recurrent symptoms (Table 2).
Table 2.
Patient characteristics and preoperative assessment
| Number of patients | 53 |
| Age (years) | |
| Mean | 51.9 ± 14.6 |
| Median | 51 |
| Range | 14–78 |
| Gender | |
| Male | 31 |
| Female | 22 |
| Preoperative presentation | |
| Infection | 2 (4%) |
| Trauma | 18 (34%) |
| Tumor | 3 (6%) |
| Degenerative | 30 (57%) |
| Prior cervical surgery | |
| Prior fusion | 12 (23%) |
| Prior decompression | 9 (17%) |
| Structural abnormality | |
| Instability | 19 (36%) |
| Deformity | 21 (40%) |
| Preoperative assessment | |
| Average pain score | 4.2 ± 1.1 |
| Average arm motor score | 4.1 ± 0.7/5 |
| Average leg motor score | 3.9 ± 1.1/5 |
| Average Nurick myelopathy grade | 2.1 ± 1.9 |
Circumferential cervical fusion
Most procedures were completed in one stage (89%), and all were performed with neuromonitoring using Cascade IONM (Cadwell laboratories, Kennewick, Washington, USA) [21]. Neuromonitoring was performed throughout the anterior and posterior components of the procedure with measured motor and sensory evoked potentials, and with the addition of EMG recordings when the procedure involved the C5 level or below. Of 53 patients, SEPs were lost in two patients (4%), a decrease in the amplitude of SEPs was recorded in five patients (9%), and one patient had a transient change recorded in MEPs. None of these changes manifested clinically. Anterior cervical fusions extended an average of 3.9 levels. Local bone was used for autograft tissue and packed into cages for most patients, unless there was underlying infection or malignancy. Iliac crest autograft material was used as a supplement in seven patients (13%) and bone morphogenic protein was used in four patients (13%) undergoing second cervical fusions (Table 3). A cage was used as an anterior structural construct in 39 patients (74%), a fibular allograft was used in 13 patients (25%), and in one patient no construct was used (Table 3). All fusion constructs were secured with dynamic plates; ABC® plates (Aesculap, Tuttlingen, Germany) were used in four patients (8%) and Atlantis® plates (Medtronic, Memphis, Tennessee, USA) were used in 49 patients (92%) (Table 3).
Table 3.
Operative technique
| Average levels fused | |
| Anterior | 3.9 |
| Posterior | 5.9 |
| Surgery | |
| 1-stage | 47 (89%) |
| 2-stage | 6 (11%) |
| 540 circumferential fusion | 3 (5.6%) |
| Revision fusion | 1 (1.9%) |
| Anterior construct | |
| Cage | 39 (74%) |
| Fibular allograft | 13 (25%) |
| Anterior plate | |
| ABC plate | 4 (8%) |
| Atlantis plate | 49 (92%) |
| Bone graft | |
| Bone morphogenic protein | 4 (8%) |
| Iliac-crest graft | 7 (13%) |
| Local-bone | 48 (91%) |
| Postoperative Halo | 2 |
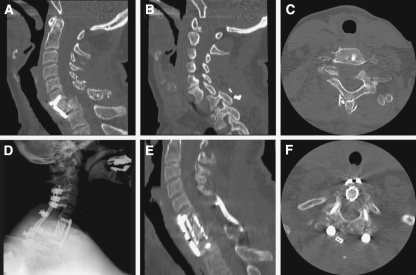
Posterior cervical fusions extended an average of 5.9 levels; lateral mass screws were used from C3 to C6 and pedicle screw fixation was used at C2 and from C7 to T4. The rods were secured with rod fixation. After instrumentation, the local bone was decorticated to promote bony fusion. Three patients underwent a 540° circumferential cervical fusion which consisted of a circumferential cervical decompression and fusion preceded by removal of posterior instrumentation. This procedure was performed when CCDF was indicated in a patient previously harboring posterior hardware with unfavorable alignment (Table 3). An example of a 540° circumferential fusion from our series is presented in Fig. 2.
Fig. 2.
A 47-year old female suffered a motor vehicle accident 1 year prior to presentation with C6–7 jumped facets and complete motor and sensory loss corresponding to the C7 level at that time. She underwent reduction and in situ fusion with anterior cervical discectomy and fusion and posterior intraspinous wiring at that time (a–c). She now presented with increasing neck pain and rapidly deteriorating triceps function. She underwent a 540° fusion with removal of posterior instrumentation, osteotomy of posterior fusion mass, complete facetectomy, and placement of lateral mass screws from C3 to C6 and pedicle screws from C7 to T3, followed by removal of anterior instrumentation, C7 complete corpectomy, reduction of deformity, anterior cage and plating from C6 to T1, followed by posterior compression and rod insertion (d–f)
Outcome
Mean clinical follow-up time was 27.5 ± 9.5 months, and mean radiographic follow-up time was 17.5 ± 11.5 months (Table 4). All patients were living at the end of the follow-up period, and had stable (23%) or improved (77%) neurological function. Although improvement is assessed as an estimated average comparison of patient preoperative and postoperative status, we nonetheless found that postoperative motor function improved in both the arms and legs to 4.6 ± 0.7 and 4.5 ± 0.8, respectively. Likewise, the average pain score improved from 4.2 ± 1.1 to 1.5 ± 0.9 (Tables 2, 4). The average preoperative and postoperative Nurick grades were 2.1 ± 1.9 and 0.4 ± 0.9, respectively (Tables 2, 4). Upon review of medical records, 96% had extensive evidence of fusion, as revealed by bony growth across fusion segments, while 4% had minimal fusion at the time of last follow-up. None of our patients required re-operation for non-fusion or pseudoarthrosis. Hardware placement was deemed excellent in nearly all (98%) patients at the time of last follow-up (Table 4).
Table 4.
Functional and fusion outcomes
| Functional outcomes | |
| Survival | 53 (100%) |
| Pain | |
| Average pain (scale from 1 to 5) | 1.5 ± 0.9 |
| Pain improved | 85% |
| Motor function | |
| Average arm functional score | 4.6 ± 0.7 |
| Average leg functional score | 4.5 ± 0.8 |
| Myelopathy | |
| Average postoperative Nurick Score | 0.4 ± 0.9 |
| Average Nurick score improvement | 1.7 |
| Myelopathy | |
| Improved | 41 (77.4%) |
| Stable | 12 (22.6%) |
| Radiographic outcomes | |
| Fusion | |
| Excellent | 51 (96.3%) |
| Satisfactory | 2 (3.8%) |
| Alignment | |
| Excellent | 52 (98.1%) |
| Satisfactory | 1 (1.9%) |
| Hardware | |
| Excellent | 52 (98.1%) |
| Satisfactory | 1 (1.9%) |
| Follow-up time | |
| Mean (months) | 17.5 ± 11.5 |
Morbidity and mortality
Postoperative complications included ten patients (19%) with transient dysphagia or dysphonia (Table 5). All patients experienced resolution of their dysphagia or dysphonia, but one patient required the placement of a percutaneous endoscopic gastric tube due to severe dysphagia and poor oral food intake—the tube had been removed at last follow-up. There were three patients (6%) with wound infections requiring wound revisions, one patient (2%) with a prominent spinous process below the segment of posterior decompression that required reduction, and one cachectic (2%) patient who had prominent hardware. One patient had respiratory depression requiring intubation due to over-sedation with self-administered opiates, and one patient developed adjacent spondylosis requiring extension of posterior fusion.
Table 5.
Morbidity
| Postoperative complications | |
| Transient dysphagia/dysphonia | 9 (19%) |
| Permanent dysphagia/dysphonia | 1 (1.9%) |
| Wound revision (infection) | 3 (6.0%) |
| Prominence spinous processes | 1 (1.9%) |
| Prominence of hardware | 1 (1.9%) |
Discussion
Indications and technique: circumferential cervical fusion
Choosing the right patient for a surgical procedure is as important as the expert performance of the operative technique itself. “Going after the pathology” dictates whether an anterior or posterior approach should be utilized. However, for patients in whom there is a loss of the normal lordotic curvature and cervical canal stenosis, posterior decompression alone can lead to progression of kyphosis and does not improve myelopathy [2, 19]. The need for extensive anterior or posterior decompression alone can lead to postoperative instability and progressive myelopathy, as well.
Several studies have shown that anterior cervical corpectomy or discectomy at more than one level is associated with decreased fusion rates and structural construct dislodgement [4, 27]. Presumably, this is because the number of surfaces requiring fusion increases with the number of levels of discectomy, thereby reducing the probability of adequate fusion [26, 29]. Circumferential cervical fusion has been shown to be beneficial in ameliorating the increased incidence of failure with anterior corpectomy and fusion involving more than two levels [13, 22, 28], and in decreasing anterior strut-graft dislodgement [14]. Therefore, anterior decompression of more than three levels should be accompanied by posterior stabilization [2, 22].
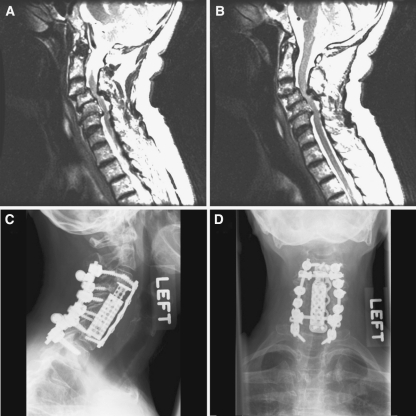
Posterior stabilization was generally done at the same levels as anterior fixation (e.g. a C3–C6 corpectomy was fused from C4–C7), however, if there is a long construct (e.g. from C4–C7) and if there is any evidence of degenerative change at the C7–T1 level, it is our preference to extend the fusion to cross the cervico-thoracic junction in order to prevent accelerated degeneration at the C7–T1 space [25]. Intraoperatively, if there is not adequate fixation at the T1 level, we will often extend to T2 for supplemental fixation with low threshold given that the T1–T2 levels are not generally mobile elements. We have not found any evidence of screw loosening or non-fusion at levels only posteriorly fused. Figure 3 presents a case of combined anterior and posterior pathology treated with a 2-level anterior corpectomy along with 5-level posterior stabilization, with instrumentation past the cervico-thoracic junction.
Fig. 3.
A 66-year old male presented with central cord syndrome with pain and numbness in both hands. An MRI revealed degenerative spine disease (a, b) with severe canal stenosis due to C3–4 anterolisthesis and C5–6 retrolisthesis. He underwent C4–6 corpectomy and placement of an anterior expandable cage with plating. Posteriorly, he underwent laminectomy followed by C3–C6 lateral mass screws and C7–T1 pedicle screws
Degenerative disease with instability adjacent to prior fusions has been an increasingly recognized phenomenon. This has been reported to occur in 3% of patients undergoing spinal fusion, with the adjacent disease most often occurring at C5–6 and C6–7 [14]. In our series we had three patients who required extension of a prior fusion due to adjacent-level disease. Two of these patients had previously been operated on elsewhere. The third received the first fusion at UCSF, extending posteriorly from C3 to C6; the new subluxation was at C6–7 and presented with neck pain within 3 months of the first operation. We have not seen any patients present with adjacent segment degeneration or junctional failure when fusion was extended past the cervico-thoracic junction.
The use of dynamic anterior plates has decreased the incidence of strut-graft dislodgement seen with static anterior plates and buttress plates without posterior fusion; therefore, we exclusively use dynamic plating.
We prefer to place lateral mass screws in the cervical spine from C2–C6 with bicortical purchase and pedicle screws in C7 and the upper thoracic spine. For C7 and upper-thoracic screws, a CT-guided StealthStation® (Medtronic, Memphis, Tennessee, USA) is sometimes used to confirm the trajectory of the pedicle screws, especially in patients who have already undergone prior cervical-spine operations.
We prefer to perform circumferential cervical fusion in one stage whenever possible. Nevertheless, in select patients with comorbidities that would put them at significant risk with increase in operative length, it is safer to stage the procedure. We do not routinely recommend the use of external halo stabilization after circumferential fusion unless there is significant concern regarding the stability of the fusion due to a history of previous non-fusion, severe deformity, or a fusion spanning several levels is performed where significant strain will be placed on the hardware. Epstein reported the use of halo stabilization in all patients, likely due to the use of posterior interlaminar wiring as opposed to screw and rod instrumentation [7]. Halo immobilization is uncomfortable for patients, has associated complications and morbidity, and, in our opinion, is unnecessary in most cases.
Fusion, alignment, and hardware placement
All patients underwent postoperative imaging to evaluate for bony fusion. Bony fusion was determined by bridging bone and lack of motion on flexion and extension. We obtained excellent fusion rates with no occurrences of pseudoarthrosis (Table 4). These fusion rates are the same or better than those achieved in published series of anterior-only or posterior-only fusions. Although comparisons between series is difficult, our experience demonstrates that good fusion rates can be obtained even when extensive bony decompressions are performed [1, 5, 8–12, 15, 17]. We used fibular allograft or cages for our anterior construct, and did not notice a difference in fusion rates, but found expandable cages easier to work with. Whenever possible, we used local autograft material to pack the allograft or cage. In seven of our patients, we used the iliac crest as a source of autograft material. Although this adds an incision and can increase morbidity, we feel that it significantly improves fusion rates and should be used when local bone cannot be used due to the presence of tumor or infection. With careful operative technique and respect for regional anatomy, we did not have any increased morbidity from iliac-crest autograft harvest [20]. In four patients with previous non-fusion, we also used bone morphogenic protein.
Improvement in myelopathy
Excellent outcomes can be obtained with circumferential cervical fusion with respect to functional and radiographic criteria without increased complication rates. No deaths occurred from the procedure. The mean postoperative Nurick grade was 0.4 ± 0.9 with a mean follow-up of 27.5 ± 9.5 months. This compares favorably to the only other large published series of circumferential cervical fusion in the literature, which studied 47 patients undergoing circumferential cervical fusion for an ossified posterior longitudinal ligament [7]. In that series, Epstein reported a Nurick grade of 0.4 after 2 years of follow-up, with an improvement of 2.8 points on the Nurick classification 1 year after surgery and 3.2 points 2 years after surgery [7]. Our patients had an improvement of 1.7 points on the Nurick classification and will likely continue to improve with further follow-up, as was seen by Epstein. All of our patients also had improvements in motor function and pain that are comparable to Epstein’s series [7]. In ongoing prospective analyses of patients at UCSF undergoing circumferential cervical fusion, we are instituting patient-based assessments including the SF-36 assessment scale.
Circumferential decompression may be associated with improved myelopathy [23]. In comparing patients who underwent anterior decompression alone with those undergoing circumferential decompression, we have anecdotally found that the improvement in myelopathy was higher in those undergoing circumferential decompression. As one might expect, those undergoing only anterior decompression had a less severe myelopathy preoperatively. Nevertheless we have been surprised by the amount of residual spinal cord impingement after extensive anterior-only decompression in many of our patients.
Morbidity and mortality
Despite the variety of indication, we had a low overall operative morbidity rate. Common morbidities associated with anterior cervical fusion are dysphagia and dysphonia, especially in the treatment of multi-level disease [4, 5, 6, 17, 24]. Dysphagia or dysphonia occurred in ten of our patients, but improved in all but one by the time of discharge; one patient required feeding-tube placement for a short time. Postoperative airway obstruction due to hematoma is also an important complication reported in the literature [4]. We had no patients with postoperative cervical hematomas; one possible reason is that we leave drains in the surgical bed postoperatively until the drain output is less than 100 cc over 24 h (usually by postoperative day 1 or 2). This is especially important if the patient has a transient coagulopathy from the operation. We had no vertebral-artery injuries in our series, with the major source of patient blood loss incurred from the muscle exposure of the posterior procedure and from physiological cancellous bone bleeding of the corpectomy in the anterior component of the procedure. Postoperative wound infections occurred in three patients (all posterior), which is comparable to infection rates reported in the literature [5, 15].
Although retrospective series tend to overestimate clinical improvement and underestimate morbidity associated with a surgical intervention, comparison of our study with other retrospective studies reveals that CCDF can be performed with good outcomes and minimal morbidity. For instance, the percentage of patients who had an improvement in myelopathy, as assessed by the Nurick scale, was comparable to other retrospective studies for anterior [1, 5, 17] and posterior [15, 30] decompression. Nevertheless, the rate of fusion, re-stenosis, subsidence, or hardware failure that we observed with circumferential fusion was less than that seen by other series evaluating anterior [1, 5, 17] or posterior [15] fusion only. These comparisons are limited because different methods were used to measure outcome variables in the different studies, yet our experience demonstrates that indeed CCDF provides extensive decompression with minimal morbidity. Dysphagia and dysphonia are well-characterized possibilities after anterior approaches to the cervical spine. The rates reported in the literature vary significantly [5, 17, 24]. This variation is likely due to the timing of dysphagia and dysphonia assessments, as well as operative techniques in attaining and maintaining exposure. The transient nature of dysphagia and dysphonia in one of five patients in our series, suggests this may result from retraction injury. Only one patient had persistent problems although this patient eventually improved as well. We prefer to bluntly dissect the neck with scissors and use bipolar cautery when necessary. Our soft tissue dissection is extended only far enough to visualize all levels being treated. When retracting we prefer to only expose two vertebral bodies at one time and move the self-retaining retractor appropriately when working at a different level.
Conclusions
Analysis of our results demonstrates excellent outcomes in patients undergoing circumferential cervical spinal fusion in terms of improving myelopathy and pain, and providing stable fusion with correct alignment. A circumferential approach, although more invasive, does not appear to contribute to significant morbidity and mortality in appropriately selected patients.
References
- 1.Baba H, Furusawa N, Imura S. Late radiographic findings after anterior cervical fusion for spondylotic myeloradiculopathy. Spine. 1993;18:2167–2173. doi: 10.1097/00007632-199311000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Deutsch H, Haid RW, Rodts GE. Postlaminectomy cervical deformity. Neurosurg Focus. 2003;15:E5. doi: 10.3171/foc.2003.15.3.5. [DOI] [PubMed] [Google Scholar]
- 3.Dvorak J, Sutter M, Herdmann J. Cervical myelopathy: clinical and neurophysiological evaluation. Eur Spine J. 2003;12(Suppl 2):S181–S187. doi: 10.1007/s00586-003-0631-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards CC, Riew KD, Anderson PA. Cervical myelopathy: current diagnostic and treatment strategies. Spine J. 2003;3:68–81. doi: 10.1016/S1529-9430(02)00566-1. [DOI] [PubMed] [Google Scholar]
- 5.Eleraky MA, Llanos C, Sonntag VK. Cervical corpectomy: report of 185 cases and review of the literature. J Neurosurg Spine. 1999;90:35–41. doi: 10.3171/spi.1999.90.1.0035. [DOI] [PubMed] [Google Scholar]
- 6.Epstein NE. Anterior cervical discectomy and fusion without plate instrumentation in 178 patients. J Spinal Disord. 2000;13:1–8. doi: 10.1097/00002517-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Epstein NE. Circumferential cervical surgery for ossification of the posterior longitudinal ligament: a multianalytic outcome study. Spine. 2004;29:1340–1345. doi: 10.1097/01.BRS.0000127195.35180.08. [DOI] [PubMed] [Google Scholar]
- 8.Freeman BJ, Licina P, Mehdian SH. Posterior lumbar interbody fusion combined with instrumented postero-lateral fusion: 5-year results in 60 patients. Eur Spine J. 2000;9:42–46. doi: 10.1007/s005860050007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gertzbein SD, Betz R, Clements D (1996) Semirigid instrumentation in the management of lumbar spinal conditions combined with circumferential fusion. A multicenter study. Spine 21:1918–1925, discussion 1925–1926 [DOI] [PubMed]
- 10.Gertzbein SD, Hollopeter MR, Hall S (1998) Pseudarthrosis of the lumbar spine. Outcome after circumferential fusion. Spine 23:2352–2356, discussion 2356–2357 [DOI] [PubMed]
- 11.Grob D, Scheier HJ, Dvorak J. Circumferential fusion of the lumbar and lumbosacral spine. Arch Orthop Trauma Surg. 1991a;111:20–25. doi: 10.1007/BF00390187. [DOI] [PubMed] [Google Scholar]
- 12.Grob D, Scheier HJ, Dvorak J. Circumferential fusion of the lumbar and lumbosacral spine: comparison of two techniques of anterior spinal fusion. Chir Organi Mov. 1991b;76:123–131. [PubMed] [Google Scholar]
- 13.Herman JM, Sonntag VK. Cervical corpectomy and plate fixation for postlaminectomy kyphosis. J Neurosurg. 1994;80:963–970. doi: 10.3171/jns.1994.80.6.0963. [DOI] [PubMed] [Google Scholar]
- 14.Hilibrand AS, Carlson GD, Palumbo MA. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999;81:519–528. doi: 10.2106/00004623-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Huang RC, Girardi FP, Poynton AR. Treatment of multilevel cervical spondylotic myeloradiculopathy with posterior decompression and fusion with lateral mass plate fixation and local bone graft. J Spinal Disord Tech. 2003;16:123–129. doi: 10.1097/00024720-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Kadanka Z, Bednarik J, Vohanka S (2000) Conservative treatment versus surgery in spondylotic cervical myelopathy: a prospective randomised study. Eur Spine J 9:538–544, discussion 545–546 [DOI] [PMC free article] [PubMed]
- 17.Mayr MT, Subach BR, Comey CH. Cervical spinal stenosis: outcome after anterior corpectomy, allograft reconstruction, and instrumentation. J Neurosurg Spine. 2002;96:10–16. doi: 10.3171/spi.2002.96.1.0010. [DOI] [PubMed] [Google Scholar]
- 18.Nurick S. The natural history and the results of surgical treatment of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:101–108. doi: 10.1093/brain/95.1.101. [DOI] [PubMed] [Google Scholar]
- 19.Schnee CL, Freese A, Weil RJ, Marcotte PJ. Analysis of harvest morbidity and radiographic outcome using autograft for anterior cervical fusion. Spine. 1997;22:2222–2227. doi: 10.1097/00007632-199710010-00005. [DOI] [PubMed] [Google Scholar]
- 20.Schultz KD, McLaughlin MR, Haid RW. Single-stage anterior–posterior decompression and stabilization for complex cervical spine disorders. J Neurosurg Spine. 2000;93:214–221. doi: 10.3171/spi.2000.93.2.0214. [DOI] [PubMed] [Google Scholar]
- 21.Sebastian C, Raya JP, Ortega M. Intraoperative control by somatosensory evoked potentials in the treatment of cervical myeloradiculopathy. Results in 210 cases. Eur Spine J. 1997;6:316–323. doi: 10.1007/BF01142677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh K, Vaccaro AR, Kim J (2003) Biomechanical comparison of cervical spine reconstructive techniques after a multilevel corpectomy of the cervical spine. Spine 28:2352–2358, discussion 2358 [DOI] [PubMed]
- 23.Slosar PJ, Reynolds JB, Schofferman J. Patient satisfaction after circumferential lumbar fusion. Spine. 2000;25:722–726. doi: 10.1097/00007632-200003150-00012. [DOI] [PubMed] [Google Scholar]
- 24.Smith-Hammond CA, New KC, Pietrobon R. Prospective analysis of incidence and risk factors of dysphagia in spine surgery patients: comparison of anterior cervical, posterior cervical, and lumbar procedures. Spine. 2004;29:1441–1446. doi: 10.1097/01.BRS.0000129100.59913.EA. [DOI] [PubMed] [Google Scholar]
- 25.Steinmetz MP, Miller J, Warbel A, Krishnaney AA, Bingaman W, Benzel EC. Regional instability following cervicothoracic junction surgery. J Neurosurg Spine. 2006;4:278–284. doi: 10.3171/spi.2006.4.4.278. [DOI] [PubMed] [Google Scholar]
- 26.Swank ML, Lowery GL, Bhat AL. Anterior cervical allograft arthrodesis and instrumentation: multilevel interbody grafting or strut graft reconstruction. Eur Spine J. 1997;6:138–143. doi: 10.1007/BF01358747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thongtrangan I, Balabhadra RS, Kim DH. Management of strut graft failure in anterior cervical spine surgery. Neurosurg Focus. 2003;15:E4. doi: 10.3171/foc.2003.15.3.4. [DOI] [PubMed] [Google Scholar]
- 28.Vaccaro AR, Falatyn SP, Scuderi GJ. Early failure of long segment anterior cervical plate fixation. J Spinal Disord. 1998;11:410–415. [PubMed] [Google Scholar]
- 29.Wang JC, McDonough PW, Endow KK. Increased fusion rates with cervical plating for two-level anterior cervical discectomy and fusion. Spine. 2000;25:41–45. doi: 10.1097/00007632-200001010-00009. [DOI] [PubMed] [Google Scholar]
- 30.Wiggins GS (2004) Posterior approach to cervical degenerative disease. In: HR W (ed) Youmans neurological surgery. Saunders, Philadelphia, pp 4409–4430