Abstract
A biomechanical study comparing simulated lytic vertebral metastases treated with laser-induced thermotherapy (LITT) and vertebroplasty versus vertebroplasty alone. To investigate the effect of tumor ablation using LITT prior to vertebroplasty on biomechanical stability and cement fill patterns in a standardized model of spinal metastatic disease. Vertebroplasty in the metastatic spine is aimed at reducing pain, but is associated with risk of cement extravasation in up to 10%. Six pairs of fresh-frozen cadaveric thoracolumbar spinal motion segments were tested in axial compression intact, with simulated metastases and following percutaneous vertebroplasty with or without LITT. Canal narrowing under load, pattern of cement fill, load to failure, and LITT temperature and pressure generation were collected. In all LITT specimens, cement filled the defect without extravasation. The canal extravasation rate was 33% in specimens treated without LITT. LITT and vertebroplasty yielded a trend toward improved posterior wall stability (P = 0.095) as compared to vertebroplasty alone. Moderate rises in temperature and minimal pressure generation was seen during LITT. In this model, elimination of tumor by LITT, facilitates cement fill, enhances biomechanical stability and reduces the risk of cement extravasation.
Keywords: Laser-induced thermotherapy, Vertebroplasty, Spinal metastases, Tumor ablation
Introduction
The spinal column is the most common site of skeletal metastases for tumors such as breast, kidney, lung and prostate and is a significant cause of morbidity due to pain, pathologic fracture, and neurologic compromise [8, 14, 35]. Osteolytic metastases structurally weaken affected vertebrae. Axial loading of metastatically involved vertebrae has been shown to cause internal pressurization, increased surface tensile hoop strains, and narrowing of the spinal canal due to bulging of the vertebral body [31]. Even at low loading rates, vertebrae with lytic metastases have an elevated risk of burst fracture through the mechanism of internal pressurization which in some cases can cause bone and tumor tissue to be retropulsed into the spinal canal [31]. Prophylactic intervention in the metastatic spine to prevent burst fracture is important as resulting neurologic compromise may be irreversible.
Prior to the occurrence of a pathologic fracture, pain is the most common symptom of metastatic disease and is potentially a result of mechanical instability created by the lytic destruction of spinal elements [5, 10]. Non-operative interventions used to treat spinal metastases are multimodal and include analgesia, corticosteroids, radiation therapy and chemotherapy [29]. These treatments provide pain relief, but do not deliver immediate improvement in biomechanical stability. Conventional surgical options include decompression and stabilization with instrumentation either from an anterior, posterior, or a combined approach. Surgery can provide significant improvement in biomechanical stability, decompression of neurologic elements, and pain relief after surgical healing has occurred [6, 13, 15]. However, these operations have significant morbidity related to the surgical approach, potential blood loss, and extensive dissection. More recently, minimally invasive surgical interventions have been utilized in treating vertebral metastases [18, 20, 30].
Percutaneous vertebroplasty (PVP) is a minimally invasive, radiologically guided therapeutic procedure that is performed to reduce pain and stabilize structurally weakened vertebrae through the injection of cement into the vertebral body. Complication rates in metastatic vertebroplasty occur at up to 10% [7, 9]. However, cement extravasation outside of the vertebral body has been shown to occur in up to 85.7% of patients with osteolytic metastases [21]. Fortunately, only a small percentage of leakages result in neurologic sequelae [9]. Other complications include embolic events due to marrow, fat, tumor, or cement. The high rate of cement extravasation may be attributed to the tumor tissue behaving as an incompressible space-occupying lesion [24]. The presence of the tumor tissue may lead to inadequate fill of the bony defect and suboptimal mechanical stabilization [24].
We hypothesize that ablation of the tumor, resulting in a volume reduction of this tissue, prior to PVP may improve both cement fill patterns and mechanical stabilization of the vertebra. Laser-induced thermotherapy (LITT) is a minimally invasive technique that provides predictable tumor tissue destruction [16, 22, 28]. The purpose of this study was to investigate the effect of tumor ablation using LITT prior to PVP on biomechanical stability and cement fill patterns of metastatically involved spinal motion segments.
Materials and methods
Six cadaveric thoracolumbar spines were harvested and radiographed to rule out pathological lesions or fractures (five males, one females, age 62–96 years, mean 80.5 ± 13.7 years). Specimens were frozen in an electronically monitored storage freezer at −20°C. Prior to testing, specimens were thawed at 3°C overnight and then placed into a saline bath at 37°C for 2 h prior to testing. Specimen temperature was monitored during testing using a 0.5 mm clear tipped Luxtron temperature probe (Luxtron Corporation, Santa Clara, CA, USA).
Two spinal motion segments were obtained from each spine (T12-L1-L2, L3-L4-L5) and were assumed to behave as paired samples for comparison during analysis. The posterior elements were removed from the superior and inferior vertebrae and anatomic measurements were taken on the mid vertebra of each segment. Motion segments from each pair were randomly assigned into one of two groups: LITT prior to PVP and PVP alone.
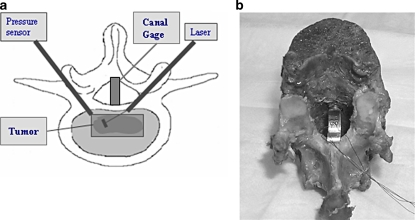
A canal displacement gauge (Fig. 1a, b) consisting of a thin curved brass beam instrumented with two uniaxial strain gages was placed in the spinal canal between the posterior arch and the midpoint of the posterior vertebral body wall of the central vertebrae of each spinal motion segment to measure canal narrowing under load (CN). The physical measurement of spinal canal narrowing under load (CN) is a non-destructive test of biomechanical stability of the posterior wall and represents the bulge of the posterior vertebral body wall into the canal when an axial load is applied to the vertebra [32]. Larger CN values represent greater instability of the posterior vertebral body wall and as such an increased potential for the initiation of burst fracture. The creation of an in vitro model of simulated lytic vertebral metastases was described by Whyne et al. and used to validate a three dimensional poroelastic finite element model of a metastatically involved spinal motion segment [32]. Results from this model based on quantifying radial vertebral bulge were shown to provide a clear threshold for burst fracture risk in a retrospective clinical study [25].
Fig. 1.
a Experimental set-up. An 800 μm laser fiber is inserted into one half of the tumor to deliver 1,750 J of energy (7 Watts, 250 s) at this location followed by an additional 1,750 Watts in the other half of the tumor. Pressures and temperatures were recorded during the ablation. A canal strain gage placed between the posterior arch and the midpoint of the posterior vertebral body wall of the central vertebrae of each spinal motion segment measures narrowing of the spinal canal under load. b A canal strain gage constructed with a thin curved brass beam instrumented with two uniaxial strain gages is shown placed within a vertebral specimen
To simulate the lytic lesion, a central core of cancellous bone was removed from the middle vertebral body’s lateral wall with a cylindrical boring trephine 16 mm in diameter, without breaking through the opposite lateral wall. The core was removed 3 mm anterior to the posterior vertebral body wall measured with a micrometer. In this study, the model previously developed by Whyne et al., was utilized to simulate a motion segment with a lytic lesion, near the posterior vertebral body wall without a fracture [32]. This can represent a clinical scenario where a patient has a painful lytic lesion in a vertebral body that has not fractured, but is at risk of potential burst fracture due to the proximity of the tumor to the posterior vertebral body wall. Placement of the tumor near the posterior vertebral body wall as shown by Tschirhart et al., using a finite element model, significantly increases the risk of pathologic burst fracture, independent from tumor volume [27].
Vertebral body dimensions including lateral vertebral body height, maximal width, and maximal depth of the vertebral body were used to calculate vertebral body volume. From the removed core of bone, enough central trabecular bone was removed to create a lytic tumor volume representing 12% of the vertebral body volume. Fresh soft tissue tumor, all metastatic uterine carcinoma, obtained from the pathologist, was used to fill the defect. Volume of tumor inserted was measured using a graduated vial and was equal to the 12% volume calculated for each vertebrae. The remaining end cap of bone was then replaced on the lateral wall and sealed into place with PMMA.
Two 11-gauge bone biopsy needles (Osteosite Bone Biopsy Needle, Cook, Canada) were inserted through a bipedicular approach into each vertebra with a simulated lesion. The positions of the biopsy needles were confirmed under fluoroscopy. A 600 μm core diameter pre-charred orb tipped surgical laser fiber with cladding (5501 ORB; Surgimedics, Houston, TX, USA) attached to a 810 nm diode laser (Diomed Ltd., Cambridge, UK) was inserted through the first cannula and introduced into the tumor tissue in those specimens assigned to the LITT prior to PVP group (Fig. 1a). To ablate the tumor tissue, 1,750 J of energy (7 Watts for 250 s) was administered to the distal half of the tumor. The laser fiber was then retracted into the proximal half of the tumor and an additional 1,750 J was administered over 250 s. This technique has been shown to volumetrically reduce tumors of this size by an average of 60%. A pressure transducer (CDX3; Cobe, Canada) was attached to the second cannula to measure the change in pressure during ablation and temperature was measured at the posterior vertebral body wall (0.5 mm clear tipped Luxtron probe).
Percutaneous vertebroplasty was performed on each motion segment using a transpedicular technique. Radio-opaque polymethylmethacrylate cement (Simplex P, Howmedica Osteonics, Mahwah, NJ, USA) was mixed using the recommended monomer-to-powder ratio (20 ml liquid monomer to 40 g powder) and 8 ml was injected into each specimen at a constant rate of 3 ml/min by an MTS 858 Bionix machine (MTS, Eden, MN, USA) using a fully automated procedure.
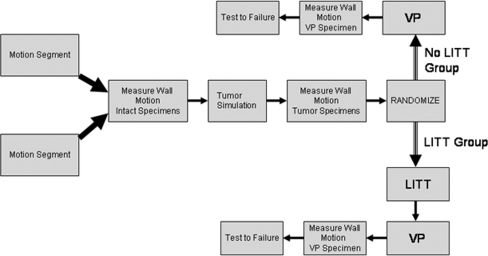
Specimens were tested under axial loading to 800 N at a rate of 1,600 N/s on a servohydraulic materials testing machine (MTS Bionix 858; Eden, MN, USA). Loading was performed at three points: intact, following tumor simulation, and following PVP (Fig. 2). CN was measured for each loading scenario. Finally, the post PVP specimens were tested to failure under axial compression. Following testing, vertebrae were axially sectioned to visualize the location of cement relative to the location of tumor and to assess for cement extravasation. To determine the difference in stabilization afforded by LITT prior to PVP in comparison to PVP alone, CN under load and load to failure were statistically analyzed using paired t-tests (SPSS, Version 10.0, Chicago, IL, USA).
Fig. 2.
Testing protocol flowchart: Two motion segments were excised from each spine for testing. Specimens were tested under axial loading intact, following creation of a simulated lesion and post-vertebroplasty after randomization to the LITT or non-LITT group
Results
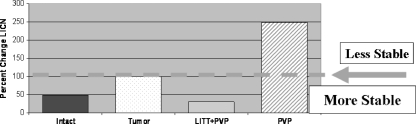
Vertebrae treated with LITT exhibited reduced posterior wall motion post-PVP; canal narrowing under load (%ΔCN) was 29.7 ± 27.1% as compared to the vertebrae with simulated metastases pre-treatment (Table 1). Specimens treated with PVP alone however, exhibited an increase in posterior wall motion, %ΔCN of 248.7 ± 253% as compared to pre-treatment values. A %CN value greater than 100% indicates a reduction in stability while a %ΔCN value less than 100% indicates a more stable posterior wall as compared to the simulated tumor scenario. (However, in comparing LITT + PVP to PVP alone yielded only a trend toward improved stability with LITT (P = 0.095) (Fig. 3).
Table 1.
The percent change in canal narrowing (CN), before and after treatment, is shown for metastatically involved vertebrae treated with PVP alone versus LITT and PVP
| Specimen no. | ΔCN post VP versus tumor vertebral level | Cement location | ||
|---|---|---|---|---|
| PVP alone | LITT + PVP | PVP alone | LITT + PVP | |
| 1 | 750% L1 | 18% L4 | L A V | L A P T |
| 2 | 79% L4 | 76% L1 | L A P | L A P T |
| 3 | 129% L4 | 6% L1 | L A | L A P T |
| 4 | 98% L1 | 6% L4 | L A P | L A P T |
| 5 | 173% L1 | 25% L4 | L A V | L A P T |
| 6 | 262% L4 | 47% L1 | L A | L A P T |
Cement location with respect to the lesion was documented as lateral (L), anterior (A), posterior (P), spinal canal (V), and within tumor (T) for both techniques. Note in specimens 2 and 4 that with PVP alone cement was documented posterior to the tumor. In these specimens lower values of %ΔCN were measured (<100%)
Fig. 3.
Percent change of load induced canal narrowing relative to the tumor model. Changes above the orange line indicate decreased stability of the posterior vertebral body wall with an increase in motion for a given axial load. Changes below the dashed line indicate increased stability and a reduction in posterior wall motion. Specimens treated with lasering prior to vertebroplasty exhibited a trend toward reduced posterior wall motion (P = 0.095) while specimens treated only with vertebroplasty had an increase in motion with a high standard deviation of 253%
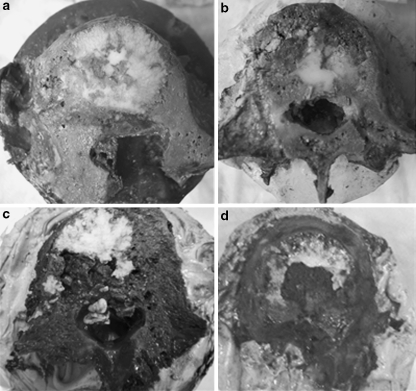
Cement was seen to permeate through the space previously occupied by the tumor in the specimens that received LITT treatment prior to PVP (Fig. 4a, b). In addition, cement was deposited near the posterior wall, yet, cement extravasation into the canal did not occur in any specimen treated with lasering prior to vertebroplasty. Cement was distributed lateral and anterior to the tumor in all specimens treated with PVP alone (Fig. 4c, d). Cement was present along the posterior vertebral body wall in two specimens and cement extravasation into the canal was noted in two cases (Table 1). The two specimens in the PVP alone group that had additional cement located posterior to the tumor exhibited reduced CN (79 and 98%) as compared to those specimens with no posterior cement presence (129–750%).
Fig. 4.
a, b Specimens treated with LITT prior to vertebroplasty. Cement has filled the area occupied by the tumor, along with areas anterior, lateral, and posterior to the tumor. No extravasation occurred. c, d Specimens not treated with LITT. Vertebroplasty has resulted in cement filling regions around the tumor, anteriorly, posteriorly and laterally. No filling of the defect occurred
LITT treatment caused a 12.3 ± 12.8°C rise in temperature along the posterior body wall, with an average maximum temperature of 35.3°C (range 23.0–59.7°C, standard deviation 13.1°C) and generated pressures of 1.6 ± 1.5 kPa of intravertebral pressure. No significant differences were observed when comparing load to failure for each group (PVP alone = 3,228 ± 1,168 N; LITT + PVP = 2,905 ± 927 N) (P = 0.540). All specimens failed at the middle column indicative of a burst fracture pattern.
Discussion
This study was designed to determine the influence of tumor ablation prior to PVP in the improvement of cement fill patterns and mechanical stabilization of the metastatic spine, prior to fracture, utilizing an in vitro model previously described and validated in the literature by Whyne et al. [32]. Clinically, this scenario can apply to patients with painful lytic lesions near the posterior vertebral body wall in unfractured vertebrae that can potentially benefit from vertebroplasty. However, injection of cement in this scenario can potentially lead to extravasation and its clinical sequelae such as neural compression or embolization. LITT was studied as a potential adjunct to vertebroplasty to help minimize this complication. Clinically, Halpin et al. and Masala et al. have already utilized radiofrequency ablation with vertebroplasty in patients to provide mechanical stabilization of the vertebrae with relief of pain [12, 19]. LITT is similar to radiofrequency ablation as it behaves as a heat source, but can provide more significant local tumor tissue ablation.
Using LITT consistent tumor tissue destruction was achieved within the vertebrae and a consistent reduction in canal narrowing (CN) under load was observed post-PVP. In the specimens treated with PVP alone however, 67% exhibited increased CN. Cement fill patterns following PVP were examined as a possible explanation for the differences in posterior wall stabilization. In all specimens treated with LITT, cement was distributed evenly throughout the vertebral body including the bony defect. However, in specimens treated with PVP alone, cement was not able to permeate through the lytic defect and was instead located anterior and lateral to the tumor in all cases. The specimens that exhibited reduced LICN in this group had additional cement located posterior to the tumor. The presence of a posterior rigid cement wall may reduce the posterior bulge of tumor under axial load while in contrast, the presence of rigid cement only anterior and lateral to the tumor may lead to an increase in the posterior bulge of the tumor, causing the posterior vertebral wall to be less stable than before PVP.
The findings from this biomechanical study concur with computational results from Tschirhart et al. who showed that cement distribution post-vertebroplasty is important in reducing the motion of the posterior vertebral body wall [27]. Using a parametric finite element model, the effect of vertebroplasty on vertebral bulge, a measure of posterior vertebral body wall motion as an indicator of burst fracture initiation, was assessed. In all cases, vertebroplasty reduced vertebral bulge, but the risk of the initiation of burst fracture was minimized with cement located posterior to the tumor, near the posterior vertebral body wall. Vertebral bulge in the model was found to decrease by up to 62% with 20% cement injection.
No difference was found for load to failure between the two groups; 2,905 N for the LITT specimens versus 3,227 N for PV alone (P = 0.61). Specimens in this study were comparable by spinal level, age, and bone mineral density for the two groups. Previous biomechanical studies in osteoporotic cadaveric specimens have shown that the strength of a vertebral body is not related to the volume of cement injected beyond 2cc [3]. Although this study examined cadaveric specimens with simulated lytic lesions, it is possible that vertebral body strength may be restored with a volume of cement smaller than the 8cc injected. The high volume of cement chosen in this study to permit filling of the lytic defect may explain the lack of difference in load to failure between the two tested groups. A smaller amount of cement may reduce cement extravasation in the PVP alone group. However the inability to fill the defect may affect the load to failure. Load to failure is a good assessment of the gross overall strength of the vertebral body; however, it does not provide information on the stability of select regions. CN specifically assesses the stability of the posterior vertebral body wall under an axial load which is potentially important in neurologic implications of burst fracture. In this study, CN did not correlate with load to failure. Specimens with a reduction in CN had an average load to failure of 3,207 N versus 3,019 N for specimens with an increase in CN (P = 0.76). In a vertebra without cement, this has been shown to be a good marker for the risk of the initiation of burst fracture [32]. However, following cement injection, this value only reflects the biomechanical stability of the posterior wall under an axial load and may not reflect load to failure.
Laser-induced thermotherapy may improve the safety of vertebroplasty for lytic metastases by reducing cement extravasation. The improvement in cement distribution by performing tumor ablation with LITT prior to VP results in the creation of a void allowing cement to fill the intended lytic defect rather than being displaced by the space occupying tumor tissue. Utilizing LITT prior to PVP, can result in a predictable fill of the structurally weakened region, while minimizing deposition of cement in regions not requiring structural augmentation. Prior cadaveric studies have demonstrated vertebrae with simulated metastases can generate higher intravertebral pressures during cement injection due to the relatively higher viscosity of tumor tissue as compared to the marrow, decreasing overall hydraulic permeability through the trabeculae [24]. This elevation in pressure may explain the higher complication rates seen clinically such as cement extravasation when vertebroplasty is used to stabilize lytic metastases. Ablation of this tumor tissue prior to vertebroplasty creates a void, leading to preferential filling of the defect, and decreasing the risk of cement extravasating out of the vertebral body. This creation of a void is similar in principle to the creation of an intravertebral cavity through the inflation of balloons during kyphoplasty for osteoporotic fractures [23]. Multiple clinical studies have shown the decreased rates of cement extravasation using kyphoplasty [17, 23] compared to vertebroplasty alone in compression fractures [1].
LITT is a treatment that has been used to treat metastases as destruction is reproducible and can be easily confined to the diseased area [16, 22, 28]. A potential concern with the use of LITT near the vertebral canal is the possibility of elevations in temperature. In this study, the temperature of the posterior wall during LITT rose by an average of 12.3°C during ablation. This temperature rise is comparable to the temperature rise found in cadaveric studies looking at heat generation in bone cement polymerization in vertebral bodies [26]. The peak absolute temperatures from LITT were similar or less than the reported values of 39°C to 110°C for polymerization temperatures at the bone–cement interface [2, 3, 4, 11, 26]. In vivo, such temperature rises along the posterior vertebral body wall will likely be reduced due to the circulatory effects of the blood and cerebrospinal fluid. This level of temperature rise has not been shown to cause any damage to the neurologic or vascular contents during vertebroplasty or more invasive spinal stabilization techniques that commonly utilize exothermically polymerizing bone cement.
In this work, the behavior of the upper (T12-L1-L2) and lower (L3-L4-L5) lumbar motion segments were assumed to yield similar behavior and were randomized into either LITT + PVP or PVP alone. Both treatment groups had an equal number of upper and lower lumbar specimens eliminating possible bias due to vertebrae from different levels. There was no statistical difference between groups in terms of bone mineral density, age of specimens, tumor size, and percentage of vertebral body volume occupied by tumor. There was no statistical relationship between these factors and load to failure. Because we utilized a repeated measures analysis, discrepancies due to anatomical differences in vertebral body size or shape should not affect our canal narrowing results. Each motion segment served as its own internal control for each step of data analysis for changes in posterior wall motion.
As vertebrae with metastatic defects were not available, the study was performed using simulated lytic lesions with fresh uterine metastatic tumor tissue. Though simulated lesions may not fully represent the behavior of naturally occurring metastases, this methodology previously described in the literature [32], allows consistent size and placement of the lytic lesions. Furthermore, parametric finite element analyses have shown that variations in biphasic material properties of lytic tumor specimens based on tumor type or cellularity do not have a large effect on outcomes related to the vertebral bone under load (strains and displacements) [33, 34]. This finding is explained by the much greater relative stiffness and permeability of the bone as compared to the whole range of properties found in metastatic tumor tissue. While in vitro cadaveric studies are important in understanding the mechanics of spinal metastases, subsequent testing is necessary with animal models and ultimately on human patients in clinical trials to assess the safety and efficacy of LITT.
Conclusion
The location of cement within the vertebral body following injection is an important factor in determining vertebral body wall motion. PVP is effective in decreasing CN for specimens with simulated lytic metastases if the tumor is surrounded posteriorly with cement. Utilizing this model, reducing tumor volume prior to cement injection creates a cavitary space which provides more reliable filling of the defect and CN reduction while eliminating the additional risk of cement extravasation into the spinal canal.
Acknowledgments
This work was supported by US Army Medical Research and Materiel Command Breast Cancer Research Award DAMD 17-00-1-0693. Simplex P Cement was provided by Stryker-Howmedica, Canada.
Contributor Information
Henry Ahn, Email: ahnh@smh.toronto.on.ca.
Cari Whyne, Phone: +1-416-480-5056, FAX: +1-416-480-5856, Email: cari.whyne@sunnybrook.ca.
References
- 1.Barr JD, Barr MS, Lemley TJ, McCann RM. Percutaneous vertebroplasty for pain relief and spinal stabilization. Spine. 2000;25:923–928. doi: 10.1097/00007632-200004150-00005. [DOI] [PubMed] [Google Scholar]
- 2.Belkoff SM, Malloy S. Temperature measurement during polymerization of polymethylmethacrylate cement used for vertebroplasty. Spine. 2003;28:1555–1559. doi: 10.1097/00007632-200307150-00015. [DOI] [PubMed] [Google Scholar]
- 3.Belkoff SM, Mathis JM, Jasper LE, Deramond H. The biomechanics of vertebroplasty. The effect of cement volume on mechanical behavior. Spine. 2001;26:1537–1541. doi: 10.1097/00007632-200107150-00007. [DOI] [PubMed] [Google Scholar]
- 4.Berman AT, Reid JS, Yanicko DR, Jr, Sih GC, Zimmerman MR. Thermally induced bone necrosis in rabbits. Relation to implant failure in humans. Clin Orthop Relat Res. 1984;186:284–292. [PubMed] [Google Scholar]
- 5.Boland PJ, Lane JM, Sundaresan N. Metastatic disease of the spine. Clin Orthop Relat Res. 1982;169:95–102. [PubMed] [Google Scholar]
- 6.Bridwell KH, Jenny AB, Saul T, Rich KM, Grubb RL. Posterior segmental spinal instrumentation (PSSI) with posterolateral decompression and debulking for metastatic thoracic and lumbar disease: limitations of the technique. Spine. 1998;13:13831394. doi: 10.1097/00007632-198812000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Chiras J, Depriester C, Weill A, Sola-Martinez MT, Deramond H. Percutaneous vertebral surgery. Technics and indications. J Neuroradiol. 1997;24:45–59. [PubMed] [Google Scholar]
- 8.Constans JP, Divitiis E, Donzelli R, Spaziante R, Meder JF, Haye C. Spinal metastases with neurologic manifestations: review of 600 cases. J Neurosurg. 1983;59:111–118. doi: 10.3171/jns.1983.59.1.0111. [DOI] [PubMed] [Google Scholar]
- 9.Cotten A, Dewatre F, Cortet B, Assaker R, Leblond D, Duquesnoy B, et al. Percutaneous vertebroplasty for osteolytic metastases and myeloma: effects of the percentage of lesion filling and the leakage of methyl methacrylate at clinical follow-up. Radiology. 1996;200:525–530. doi: 10.1148/radiology.200.2.8685351. [DOI] [PubMed] [Google Scholar]
- 10.DeWald RL, Bridwell KH, Prodromas C, Rodts MF. Reconstructive spinal surgery as palliation for metastatic malignancies of the spine. Spine. 1985;10:21–26. doi: 10.1097/00007632-198501000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Deramond H, Wright NT, Belkoff SM. Temperature elevation caused by bone cement polymerization during vertebroplasty. Bone. 1999;25(2 Suppl):17S–21S. doi: 10.1016/S8756-3282(99)00127-1. [DOI] [PubMed] [Google Scholar]
- 12.Halpin RJ, Bendok BR, Sato KT, Liu JC, Patel JD, Rosen ST. Combination treatment of vertebral metastases using image-guided percutaneous radiofrequency ablation and vertebroplasty: a case report. Surg Neurol. 2005;63:469–474. doi: 10.1016/j.surneu.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 13.Hammerberg KW. Surgical treatment of metastatic spine disease. Spine. 1991;17:11481153. doi: 10.1097/00007632-199210000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Harrington KD. Current concepts review: metastatic disease of the spine. J Bone Joint Surg Am. 1986;68:1110–1115. [PubMed] [Google Scholar]
- 15.Hatrick NC, Lucas JD, Timothy AR, Smith MA. The surgical treatment of metastatic disease of the spine. Radiother Oncol. 2000;56:335–339. doi: 10.1016/S0167-8140(00)00199-7. [DOI] [PubMed] [Google Scholar]
- 16.Izzo F. Other thermal ablation techniques: microwave and interstitial laser ablation of liver tumors. Ann Surg Oncol. 2003;10:491497. doi: 10.1245/ASO.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 17.Lieberman IH, Dudeney S, Reinhardt MK, Bell G. Initial outcome and efficacy of “kyphoplasty” in the treatment of painful osteoporotic vertebral compression fractures. Spine. 2001;26:1631–1638. doi: 10.1097/00007632-200107150-00026. [DOI] [PubMed] [Google Scholar]
- 18.Martin JB, Wetzel SG, Seium Y, Dietrich PY, Somon T, Gailloud P, et al. Percutaneous vertebroplasty in metastatic disease: transpedicular access and treatment of lysed pedicles—initial experience. Radiology. 2003;229:593–597. doi: 10.1148/radiol.2292020976. [DOI] [PubMed] [Google Scholar]
- 19.Masala S, Roselli M, Massari F, Fiori R, et al. Radiofrequency heat ablation and vertebroplasty in the treatment of neoplastic vertebral body fractures. Anticancer Res. 2004;24:3129–3133. [PubMed] [Google Scholar]
- 20.McGraw JK, Lippert JA, Minkus KD, Rami PM, Davis TM, Budzik RF. Prospective evaluation of pain relief in 100 patients undergoing percutaneous vertebroplasty: results and follow-up. J Vasc Interv Radiol. 2002;13:883–886. doi: 10.1016/S1051-0443(07)61770-9. [DOI] [PubMed] [Google Scholar]
- 21.Mousavi P, Roth S, Finkelstein J, Cheung G, Whyne C. Volumetric quantification of cement leakage following percutaneous vertebroplasty in metastatic and osteoporotic vertebrae. J Neurosurg Spine. 2003;99:56–59. doi: 10.3171/spi.2003.99.1.0056. [DOI] [PubMed] [Google Scholar]
- 22.Muralidharan V, Christophi C. Interstitial laser thermotherapy in the treatment of colorectal liver metastases. J Surg Oncol. 2001;76:73–81. doi: 10.1002/1096-9098(200101)76:1<73::AID-JSO1014>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Phillips FM, Ho E, Campbell-Hupp M, McNally T, Todd Wetzel F, Gupta P. Early radiographic and clinical results of balloon kyphoplasty for the treatment of osteoporotic vertebral compression fractures. Spine. 2003;28:2260–2265. doi: 10.1097/01.BRS.0000085092.84097.7B. [DOI] [PubMed] [Google Scholar]
- 24.Reidy D, Ahn H, Mousavi P, Finkelstein J, Whyne CM. A biomechanical analysis of intravertebral pressures during vertebroplasty of cadaveric spines with and without simulated metastases. Spine. 2003;28:1534–1539. doi: 10.1097/00007632-200307150-00011. [DOI] [PubMed] [Google Scholar]
- 25.Roth SE, Mousavi P, Finkelstein J, Chow E, Kreder H, Whyne CM. Metastatic burst fracture risk prediction using biomechanically based equations. Clin Orthop Relat Res. 2004;419:83–90. doi: 10.1097/00003086-200402000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Toksvig-Larsen S, Johnsson R, Stromqvist B. Heat generation and heat protection in methylmethacrylate cementation of vertebral bodies. A cadaver study evaluating different clinical possibilities of dural protection from heat during cement curing. Eur Spine J. 1995;4:15–17. doi: 10.1007/BF00298412. [DOI] [PubMed] [Google Scholar]
- 27.Tschirhart CE, Roth SE, Whyne CM. Biomechanical assessment of stability in the metastatic spine following percutaneous vertebroplasty: effects of cement distribution patterns and volume. J Biomech. 2005;38:1582–1590. doi: 10.1016/j.jbiomech.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 28.Vogl TJ, Muller PK, Mack MG, Straub R, Engelmann K, Neuhaus P. Liver metastases: interventional therapeutic techniques and results, state of the art. Eur Radiol. 1999;1999:675–684. doi: 10.1007/s003300050732. [DOI] [PubMed] [Google Scholar]
- 29.Wai EK, Finkelstein JA, Tangente RP, Holden L, Chow E, Ford M, et al. Quality of life in surgical treatment of metastatic spine disease. Spine. 2003;28:508–512. doi: 10.1097/00007632-200303010-00018. [DOI] [PubMed] [Google Scholar]
- 30.Weill A, Chiras J, Simon JM, Rose M, Sola-Martinez T, Enkaoua E. Spinal metastases: indications for and results of percutaneous injection of acrylic surgical cement. Radiology. 1996;199:241–247. doi: 10.1148/radiology.199.1.8633152. [DOI] [PubMed] [Google Scholar]
- 31.Whyne CM, Hu SS, Lotz JC. Biomechanically derived guideline equations for burst fracture risk prediction in the metastatically involved spine. J Spinal Disord Tech. 2003;16:180–185. doi: 10.1097/00024720-200304000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Whyne CM, Hu SS, Lotz JC. Burst fracture in the metastatically involved spine: development, validation, and parametric analysis of a three-dimensional poroelastic finite-element. Spine. 2003;28:652–660. doi: 10.1097/00007632-200304010-00007. [DOI] [PubMed] [Google Scholar]
- 33.Whyne CM, Hu SS, Workman KL, Lotz JC. Biphasic material properties of lytic bone metastases. Ann Biomed Eng. 2000;28:1154–1158. doi: 10.1114/1.1313773. [DOI] [PubMed] [Google Scholar]
- 34.Whyne CM, Hu SS, Lotz JC. Parametric finite element analysis of vertebral bodies affected by tumors. J Biomech. 2001;34:1317–1324. doi: 10.1016/S0021-9290(01)00086-0. [DOI] [PubMed] [Google Scholar]
- 35.Wong DA, Fornasier VL, McNab I. Spinal metastases: the obvious, the occult, and the imposters. Spine. 1990;15:1–3. doi: 10.1097/00007632-199001000-00001. [DOI] [PubMed] [Google Scholar]