Abstract
The objective of this study was to describe clinical and radiological features of a series of patients presenting with Brown-Sequard syndrome after blunt spinal trauma and to determine whether a correlation exists between cervical plain films, CT, MRI and the clinical presentation and neurological outcome. A retrospective review was done of the medical records and analysis of clinical and radiological features of patients diagnosed of BSS after blunt cervical spine trauma and admitted to our hospital between 1995 and 2005. Ten patients were collected for study, three with upper- and seven with lower-cervical spine fracture. ASIA impairment scale and motor score were determined on admission and at last follow-up (6 months–9 years, mean 30 months). Patients with lower cervical spine fracture presented with laminar fracture ipsilateral to the side of cord injury in five out of six cases. T2-weighted hyperintensity was present in seven patients showing a close correlation with neurological deficit in terms of side and level but not with the severity of motor deficit. Patients with Brown-Sequard syndrome secondary to blunt cervical spine injury commonly presented T2-weighted hyperintensity in the clinically affected hemicord. A close correlation was observed between these signal changes in the MR studies and the neurologic level. Effacement of the anterior cervical subarachnoid space was present in all patients, standing as a highly sensitive but very nonspecific finding. In the present study, craniocaudal extent of T2-weighted hyperintensity of the cord failed to demonstrate a positive correlation with neurological impairment.
Keywords: Brown-Sequard, Cervical spine trauma, MRI, Prognosis, Spinal cord Injury
Introduction
The clinical presentation of Brown-Sequard syndrome (BSS) includes ipsilateral weakness with contralateral loss of pain and temperature sensation below the level of the lesion. It is assumed that BSS is caused by cord hemisection resulting in disruption of the descending lateral corticospinal tracts and the ascending lateral spinothalamic tracts which cross within one or two levels of the dorsal root entrance. BSS is most commonly caused by penetrating spinal trauma [21] whereas injuries caused by blunt trauma are much less common [20]. Patients with BSS may recover almost full motor function within 6 months of the injury and prognosis for recovery seems better for patients who have sustained a blunt rather than a penetrating trauma [24].
Although, recent investigations correlate clinical and radiological features in spinal cord injured patients [5, 9], little is known about CT and MR characteristics of patients with BSS after blunt spinal trauma.
The object of the present work is to describe the radiological features of a series of ten patients presenting with BSS after blunt spinal trauma and to determine whether a correlation exists between radiological characteristics and the clinical presentation and neurological outcome.
Materials and methods
We retrospectively reviewed data obtained from patients presenting with BSS secondary to blunt cervical spinal cord injury admitted to our hospital between 1995 and 2005. We selected 10 of the 12 patients in whom clinical findings were consistent with BSS on admission. Two patients were excluded from the study because radiological studies were not available. Clinical characteristics are summarized in Table 1. There were seven men and three women. Mean age was 37.6 years (range 21–52 years). Anteroposterior and lateral radiographs of the cervical spine were obtained in every patient on admission. High cervical fractures were classified as reviewed by Menezes et al. [18]. Lower cervical spine fractures were classified as described by Alen et al. [1]. Cervical CT scan was obtained in every patient presenting with a cervical spine fracture. Motor deficit was evaluated using the ASIA impairment classification and the motor score [7]. The cervical spine was also examined in every patient using a 1.5 Tesla MR imager. In the sagittal and axial plane, T1- and T2-weighted images were obtained. Additionally, gradient echo sequences were obtained in the axial plane in five patients. In one patient, T2-weighted images could not be retrieved for the study. The type of fracture in plain films and CT, and the areas of signal change in MR were compared with the clinical features on admission and at last follow-up. Nonparametric correlations (r Spearman) were calculated using SPSS 12.0 software with a level of significance set at a probability value of 0.05.
Table 1.
Clinical characteristics
| Case no. | Age | Sex | Mechanism of trauma | Level of fracture | Side affected | Motor levela | ASIA on admissiona | Motor score on admission | Treatment | Follow-up | ASIA at last follow-upa | Motor score at last Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 21 | M | Rugby | C2 | Left | C4 | C | 70 | External reduction + halo brace | 6 years | D | 90 |
| 2 | 37 | M | Knocked down by car | C0–C1 | Left | C4 | B | 50 | Philadelphia collar | 6 months | C | 60 |
| 3 | 28 | M | MVA | C4–C5 | Left | C5 | D | 85 | Corpectomy, graft and plate | 2 years | D | 95 |
| 4 | 52 | F | MVA | C5–C6 | Left | C5 | D | 82 | Corpectomy, graft and plate | 6 months | D | 95 |
| 5 | 44 | M | Assault | C4–C5 | Left | C4 | D | 90 | Philadelphia collar | 8 months | E | 100 |
| 6 | 40 | M | MVA | C5–C6 | Left | C6 | C | 76 | Corpectomy, graft and plate | 9 months | D | 90 |
| 7 | 35 | M | Diving | C5–C6 | Right | C6 | D | 85 | Corpectomy, graft and plate | 9 years | E | 100 |
| 8 | 46 | F | MVA | C6–C7 | Left | C5 | C | 65 | Discectomy, graft and plate | 4 years | C | 79 |
| 9 | 22 | F | MVA | C5–C6 | Right | C4 | B | 50 | Corpectomy, graft and plate | 1 year | C | 85 |
| 10 | 49 | M | Knocked down by car | C2 | Left | C4 | B | 50 | Philadelphia collar | 7 months | C | 75 |
Applicable to the side of motor deficit
M Male, F Female, MVA Motor vehicle accident
Results
Clinical characteristics are summarized in Table 1. Among the ten patients, five were involved in motor vehicle accidents, two pedestrians were knocked down by a car, one sustained spinal injury while playing rugby, one while diving, and one was victim of an assault. Three patients sustained trauma to the upper cervical spine (odontoid type III fracture, occipitoatlantal dislocation and miscellanous C2 fracture) (Fig. 1). In the seven patients with lower cervical spine injury, flexion and/or compression were the mechanisms of injury in six cases (Fig. 2).
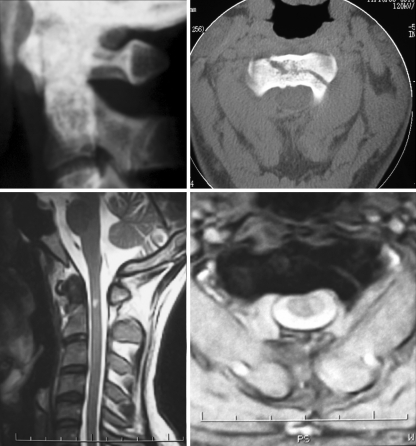
Fig. 1.
Images corresponding to case number 1. Upper left Plain lateral radiograph shows odontoid type III fracture. Upper right CT scan shows extension of fracture into the body of the axis. Lower left Sagittal T2-weighted MRI shows spinal cord hyperintensity at C2 level. Lower right Axial MRI depicts the extension of spinal cord hyperintensity limited to the left hemicord
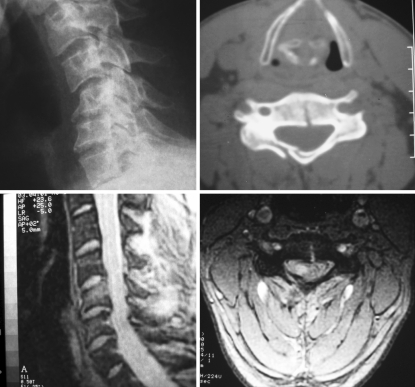
Fig. 2.
Images corresponding to case number 7. Upper left Plain lateral radiograph shows C5-C6 flexion-compression stage 3 fracture. Upper right CT scan shows fracture of the right lamina and through the body of C5. Lower left Sagittal MRI shows hyperintensity at the body of C5 and cord compression at C5-C6 without signs of hemorrhage. Lower right Axial T2-weighted MRI depicts the extension of spinal cord hyperintensity limited to the right hemicord
Three patients were classified as ASIA B, three as ASIA C and four as ASIA D category on admission. The most common motor level in the affected side at presentation was C4.
Every patient with lower cervical spine fracture underwent anterior decompression and fusion with no complications. Among patients with upper cervical spine injury, two were managed conservatively with Philadelphia collar and one underwent external reduction and immobilization with halo brace.
Clinical follow-up ranged from 6 months to 9 years (mean 30 months, median 9 months). At the last follow-up, every patient had improved significantly compared to the initial examination. Patients in ASIA grade D on admission recovered complete or almost complete motor function, while patients in ASIA grades B and C on admission recovered incompletely from initial motor deficit.
Clinical impairment on admission significantly correlated with ASIA and motor score at last follow-up (r > 0.8, P < 0.05).
Radiological findings are summarized in Table 2. Unilateral vertebral arch fracture ipsilateral to the side of the hemiparesis was identified in the cervical CT scan in five of the six patients with lower cervical spine fractures. Seven patients presented with spinal cord abnormalities consisting of an isointense signal on T1-weighted sequences and hyperintensity on T2-weighted sequences.
Table 2.
Radiological findings
| Case no. | Type of fracture | Laminar fracture on CT | Time to MRI | T2 cord hyperintensity | Center of MRI changes | Craniocaudal extent of edema (mm) | Axial extent of edema | Effacement of anterior subarachnoid space | Other |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Odontoid III | Absent | 5 days | Present | C2 | 7 | Left hemicord | Present | |
| 2 | Occipito-cervical Distraction | Absent | 2 days | Absent | C2 | – | – | Present | Epidural hematoma |
| 3 | Flexion- compression | Left C4 | 8 h | Present | C5 | 10 | Left hemicord | Present | |
| 4 | Vertical compression | Left C5 | 6 h | Not available | C6 | – | – | Present | Complete ligamental failure |
| 5 | Not applicable | Absent | 1 day | Absent | C4-C5 | – | – | Present | Cervical spondylosis |
| 6 | Flexion-compression | Absent | 3 h | Present | C4-C5 | 10 | Left hemicord | Present | Cervical spondylosis |
| 7 | Flexion compression | Right C5 | 6 days | Present | C5 | 20 | Right hemicord | Present | |
| 8 | Flexion-distraction | Left C7 | 7 days | Present | C6 | 12 | Left hemicord | Present | |
| 9 | Flexion-compression | Left C5 | 9 h | Present | C6 | 5 | Right hemicord | Present | |
| 10 | Miscellaneous C2 | Absent | 2 days | Present | C2 | 30 | Left hemicord | Present |
Based on the timing of MR acquisition, this abnormality cannot be related to a hemorrhagic lesion. Gradient echo sequences in five patients confirmed the absence of hemorrhage in every case. This hyperintensity on T2-weighted sequences is believed to be secondary to ischemia and/or edema. The anatomic level of this edema correlated with the neurologic level, although this correspondence was not absolutely precise. The craniocaudal extent of edema did not correlate with the degree of impairment on admission or at last follow-up (r < 0.15, P > 0.7). The axial extent of the edema was limited to the clinically affected hemicord in all cases. Of the three patients with no spinal cord signs of edema, one presented with an epidural hematoma at C2, one displayed signs of cervical spondylosis more marked in the side of hemiparesis, and one showed complete ligament failure and laminar fracture ipsilateral to the motor deficit: this was the case where T2-weighted sequences could not be retrieved for study. Finally, all patients displayed effacement of the anterior subarachnoid space in axial MR studies.
Discussion
Brown-Sequard described the classic syndrome of ipsilateral hemiplegia and loss of propioceptive sensation with contralateral loss of pain and temperature sensation following spinal cord hemisection [4]. This injury causes disruption of the descending lateral corticospinal tracts, the ascending dorsal columns (both of which decussate in the medulla), and the ascending lateral spinothalamic tracts, which cross within one or two levels of the dorsal root entrance. Common causes of BSS include penetrating trauma, syringomyelia, extramedullary spinal neoplasms and blunt trauma, including disc herniation [11, 14, 20, 21]. Other causes include spinal cord herniation, viral myelitis, or demyelinating disease [2, 5, 23, 25]. The most common cause is by far penetrating trauma, while BSS secondary to blunt injury is much less common [8, 20, 21].
Oller et al. described in 1991 a series of three cases of blunt cervical spine injury presenting with BSS [20]. The same year, Roth et al. reviewed the clinical features of 38 patients with traumatic cervical BSS: 22 injuries were caused by road traffic accidents, 8 by penetrating injuries, 5 by diving injuries and 3 by other causes [24]. We are unaware of further series focusing on BSS secondary to blunt cervical spine injury.
Patients with BSS generally have a good prognosis for neurological and functional improvement, and many recover almost full motor function within 6 months of the injury [16, 24]. Prognosis for recovery is usually better for patients who have sustained a blunt rather than a penetrating injury [20, 24]. The pathogenesis of cord injury in cases of blunt trauma may include vascular compromise, compression by bone, disc or epidural hematoma, or longitudinal stretching of the cord.
Although, plain cervical spine radiographs and CT scan play a major role in the initial management of spine-injured patients and are extremely useful for detecting bony abnormalities such as fracture or dislocation, MR images are superior to depict the extent of injury to the spinal cord, the surrounding soft tissues, the overall spinal alignment and the damage of the intervertebral discs.
MR is therefore the technique of choice to evaluate the nature of BSS [12, 19, 22]. Additionally, several prognostic studies have shown a correlation between the degree of traumatic spinal cord damage as depicted by MR and neurological clinical presentation and outcome [3, 5, 6, 9, 13, 17, 26–28]. Some of these studies have focused on particular clinical categories, such as central cord syndrome, or SCIWORA [5, 6, 13]. However, we have not found previous series addressing the clinical and radiological correlations in BSS patients after blunt cervical spine injury. Isolated case reports provide scarce information about this clinical entity [8, 15, 29].
Our findings enclose some interesting observations. First, MR images showed obliteration of the anterior cervical subarachnoid space at the level of the injury in all patients. This anterior effacement constitutes a highly sensitive radiological finding although very nonspecific at the same time. Second, flexion and/or compression were implicated in the mechanism of injury in six out of seven lower fractures. This mechanism of injury has been previously related to BSS by other studies [10, 15, 29]. However, the high number of vertebral arch fractures observed in our patients and the fact that these lesions are typically associated with hyperextension injuries [1] points to the fact that different lines of force follow one another at the moment of trauma. Third, we found a close correlation between the radiologically affected hemicord by edema and the motor deficit on admission, which confirms a precise damage underlying BSS, unlike centralcord syndrome [5]. In lower cervical spine fractures, this selective damage is frequently associated with the presence of ipsilateral laminar fracture, observed in five out of six patients. Nevertheless, we failed to demonstrate a positive correlation between the extent of spinal cord edema and the severity of neurological impairment either on admission or at last follow-up.
Our study also encloses several limitations. Most importantly, the low number of cases precludes us from taking definitive conclusions. However, many of the correlations observed are relatively new, appear clinically relevant and may deserve further research. Second, many of the clinical and radiological data (motor impairment, extent of signal changes in MR, type of fracture on plain radiographs) are subjective, and therefore they are a potential source of bias. Rating interobserver variability would enhance the quality of our methodology. Third, follow-up widely ranged from 6 months to 9 years. Since clinical improvement takes place mainly in the first 6 months after injury, it would be ideal to extend follow-up to at least 1 year.
Conclusions
Patients with BSS secondary to blunt cervical spine injury in our series commonly presented T2-weighted hyperintensity in the clinically affected hemicord. A close correlation was observed between MR signal changes and neurologic level. Patients with lower cervical spine fracture presented laminar fracture ipsilateral to the side of cord injury in five out of six cases. Effacement of the anterior cervical subarachnoid space was present in all patients, standing as a highly sensitive but very nonspecific finding. In the present study, craniocaudal extent of T2-weighted hyperintensity of the cord failed to demonstrate a positive correlation with neurological impairment. Further studies are necessary to confirm these preliminary results.
References
- 1.Allen BL, Jr, Ferguson RL, Lehmann TR, O’Brien RP. A mechanistic classification of closed, indirect fractures and dislocations of the lower cervical spine. Spine. 1982;7:1–27. doi: 10.1097/00007632-198200710-00001. [DOI] [PubMed] [Google Scholar]
- 2.Berlit P. Rare causes of the Brown-Sequard syndrome. NMR tomographic findings. Dtsch Med Wochenschr. 1988;113:1844–1846. doi: 10.1055/s-2008-1067899. [DOI] [PubMed] [Google Scholar]
- 3.Bondurant FJ, Cotler HB, Kulkarni MV, McArdle CB, Harris JH., Jr Acute spinal cord injury. A study using physical examination and magnetic resonance imaging. Spine. 1990;15:161–184. doi: 10.1097/00007632-199003000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Brown-Sequard CE (1973): De la trasmission des impressions sensitives par la moelle epiniere-CR Soc Biol 1849:1:192–194. In: Wilkins RH, Brody IA, (eds) English translation in neurological classics. Johnson Reprint Corporation, New York, pp 49–50
- 5.Collignon F, Martin D, Lenelle J, Stevenaert A. Acute traumatic central cord syndrome: magnetic resonance imaging and clinical observations. J Neurosurg. 2002;96(1 Suppl):29–33. doi: 10.3171/spi.2002.96.1.0029. [DOI] [PubMed] [Google Scholar]
- 6.Dare AO, Dias MS, Li V. Magnetic resonance imaging correlation in pediatric spinal cord injury without radiographic abnormality. J Neurosurg. 2002;97(1 suppl):33–39. doi: 10.3171/spi.2002.97.1.0033. [DOI] [PubMed] [Google Scholar]
- 7.DiTunno JF, Young W, Creasey G. International standards for neurological and functional classification of spinal cord injury: revised 1992. Paraplegia. 1994;32:70–80. doi: 10.1038/sc.1994.13. [DOI] [PubMed] [Google Scholar]
- 8.Edwards A, Andrews R. A case of Brown-Sequard syndrome with associated Horner’s syndrome after blunt injury to the cervical spine. Emerg Med J. 2001;18:512–513. doi: 10.1136/emj.18.6.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flanders AE, Spettell CM, Friedman DP, Marino RJ, Herbison GJ. The relationship between the functional abilities of patients with cervical spinal cord injury and the severity of damage revealed by MR imaging. Am J Neuroradiol. 1999;20:926–934. [PMC free article] [PubMed] [Google Scholar]
- 10.Fuentes JM, Bloncourt J, Vlahovitch B, Castan P. Tear drop fractures. Contribution to the study of the mechanism of osteo-discoligamentous lesions. Neurochirurgie. 1983;29:129–134. [PubMed] [Google Scholar]
- 11.Kobayashi N, Asamoto S, Doi H, Sugiyama H. Brown-Sequard syndrome produced by cervical disc herniation: report of two cases and review of the literature. Spine J. 2003;3:530–533. doi: 10.1016/S1529-9430(03)00078-0. [DOI] [PubMed] [Google Scholar]
- 12.Lee RR. MR imaging and cervical spine injury. Radiology. 1996;201:617–618. doi: 10.1148/radiology.201.3.8939205. [DOI] [PubMed] [Google Scholar]
- 13.Liao CC, Lui TN, Chen LR, Chuang CC, Huang YC. Spinal cord injury without radiological abnormality in preschool-aged children: correlation of magnetic resonance imaging findings with neurological outcomes. J Neurosurg. 2005;103(1 Suppl):17–23. doi: 10.3171/ped.2005.103.1.0017. [DOI] [PubMed] [Google Scholar]
- 14.Lim E, Wong YS, Lo YL, Lim SH. Traumatic atypical Brown-Sequard syndrome: case report and literature review. Clin Neurol Neurosurg. 2003;105:143–145. doi: 10.1016/S0303-8467(03)00009-X. [DOI] [PubMed] [Google Scholar]
- 15.Lipper MH, Goldstein JH, Do HM. Brown-Sequard syndrome of the cervical spinal cord after chiropractic manipulation. Am J Neuroradiol. 1998;19:1349–1352. [PMC free article] [PubMed] [Google Scholar]
- 16.Little JW, Halar E. Temporal course of motor recovery after Brown-Sequard spinal cord injuries. Paraplegia. 1985;23:39–46. doi: 10.1038/sc.1985.7. [DOI] [PubMed] [Google Scholar]
- 17.Marciello MA, Flanders AE, Herbison GJ, Schaefer DM, Friedman DP, Lane JI. Magnetic resonance imaging related to neurologic outcome in cervical spinal cord injury. Arch Phys Med Rehabil. 1993;74:940–946. [PubMed] [Google Scholar]
- 18.Menezes AH, Piper JG (1993) Anatomy and radiographic pathology of injury to the occipito-atlanto-axial complex. In: Rea GL, Miller CA (eds) Spinal Trauma: current evaluation and management. Neurosurgical topics. American Association of Neurological Surgeons, Illinois, pp 1–17
- 19.Mirvis SE, Geisler FH, Jelinek JJ, Joslyn JN, Gellad F. A cute cervical spine trauma: evaluation with 1.5-T MR imaging. Radiology. 1988;166:807–816. doi: 10.1148/radiology.166.3.3277249. [DOI] [PubMed] [Google Scholar]
- 20.Oller DW, Boone S. Blunt cervical spine Brown-Sequard injury. A report of three cases. Am Surg. 1991;57:361–365. [PubMed] [Google Scholar]
- 21.Peacock WJ, Shrosbree RD, Key AG. A review of 450 stab wounds of the spinal cord. S Afr Med J. 1977;51:961–964. [PubMed] [Google Scholar]
- 22.Richards PJ. Cervical spine clearance: a review. Injury. 2005;36:248–269. doi: 10.1016/j.injury.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 23.Rivas JJ, la Lama A, Gonza Lez P, Ramos A, Zurdo M, Alday R. Spontaneous spinal cord herniation. Neurocirugia. 2004;15:484–489. [PubMed] [Google Scholar]
- 24.Roth EJ, Park T, Pang T, Yarkony GM, Lee MY. Traumatic cervical Brown-Sequard and Brown-Sequard-plus syndromes: the spectrum of presentations and outcomes. Paraplegia. 1991;29:582–589. doi: 10.1038/sc.1991.86. [DOI] [PubMed] [Google Scholar]
- 25.Schott GD. MRI demonstration of an anterolateral plaque at C1: a note on some sensory changes including analgesia. J Neurol Neurosurg Psychiatr. 1993;56:827–829. doi: 10.1136/jnnp.56.7.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silberstein M, Hennessy O. Implications of focal spinal cord lesions following trauma: evaluation with magnetic resonance imaging. Paraplegia. 1993;31:160–167. doi: 10.1038/sc.1993.31. [DOI] [PubMed] [Google Scholar]
- 27.Silberstein M, Tress BM, Hennessy O. Prediction of neurologic outcome in acute spinal cord injury: the role of CT and MR. Am J Neuroradiol. 1992;13:1597–1608. [PMC free article] [PubMed] [Google Scholar]
- 28.Yamashita Y, Takahashi M, Matsuno Y, Kojima R, Sakamoto Y, Oguni T, Sakae T, Kim EE. Acute spinal cord injury: magnetic resonance imaging correlated with myelopathy. Br J Radiol. 1991;64:201–209. doi: 10.1259/0007-1285-64-759-201. [DOI] [PubMed] [Google Scholar]
- 29.Zorn PJ. Brown-Sequard syndrome following cervical spine compression fracture. Del Med J. 1991;63:549–553. [PubMed] [Google Scholar]