Abstract
The rat L5/6 facet joint is multisegmentally innervated from the L1 to L6 dorsal root ganglia (DRG). Tumor necrosis factor (TNF) is a known mediator of inflammation. It has been reported that satellite cells are activated, produce TNF and surround DRG neurons innervating L5/6 facet joints after facet injury. In the current study, changes in TNF receptor (p55) expression in DRG neurons innervating the L5/6 facet joint following facet joint injury were investigated in rats using a retrograde neurotransport method followed by immunohistochemistry. Twenty rats were used for this study. Two crystals of Fluorogold (FG; neurotracer) were applied into the L5/6 facet joint. Seven days after surgery, the dorsal portion of the capsule was cut in the injured group (injured group n = 10). No injury was performed in the non-injured group (n = 10). Fourteen days after the first application of FG, bilateral DRGs from T13 to L6 levels were resected and sectioned. They were subsequently processed for p55 immunohistochemistry. The number of FG labeled neurons and number of FG labeled p55-immunoreactive (IR) neurons were counted. FG labeled DRG neurons innervating the L5/6 facet joint were distributed from ipsilateral L1 to L6 levels. Of FG labeled neurons, the ratio of DRG neurons immunoreactive for p55 in the injured group (50%) was significantly higher than that in the non-injured group (13%). The ratio of p55-IR neurons of FG labeled DRG neurons was significantly higher in total L1 and L2 DRGs than that in total L3, 4, 5 and 6 DRGs in the injured group (L1 and 2 DRG, 67%; L3, 4, 5 and 6 DRG, 37%, percentages of the total number of p55-IR neurons at L1 and L2 level or L3–6 level/the total number of FG-labeled neurons at L1 and L2 level or L3–6 level). These data suggest that up-regulation of p55 in DRG neurons may be involved in the sensory transmission from facet joint injury. Regulation of p55 in DRG neurons innervating the facet joint was different between upper DRG innervated via the paravertebral sympathetic trunks and lower DRG innervated via other direct routes.
Keywords: Sensory innervation, Lumbar facet joint, p55 tumor necrosis factor receptor, Dorsal root ganglion
Introduction
Many studies have reported the lumbar facet joints as a possible source of lower back pain in animals and humans [5, 10, 25]. Morphologically, the joint capsule is well innervated, receiving a nerve supply from the medial branches of the dorsal rami. The human L4/5 facet joint capsule is innervated by the medial branches of the dorsal rami from the L3 to L4 spinal nerves [2, 3]. The rat L5/6 facet joint is reported to be multisegmentally innervated by DRGs from L1 to L5 and nerve fibers from L1 to L2 DRGs pass through the paravertebral sympathetic trunks [11, 14, 15, 21, 22].
Proinflammatory cytokines such as tumor necrosis factor alpha (TNF) are well known mediators of the peripheral inflammatory response in animals and humans [1, 4, 9, 12]. In peripheral nerve injury, TNF expression is upregulated in endoneurial macrophages and Schwann cells in rats [23, 24]. There are two types of TNF receptors in humans and other vertebrates termed p55 and p75. The p55 receptor is responsible for mediating most of the cytodestructive actions of TNF, including apoptosis, cytokine production, pain transmission, and induction of a number of gene products [20]. The presence of TNF and p55 TNF receptor in satellite cells and neurons of the DRG in rats and mice following sciatic nerve injury has been reported [17, 18]. We have previously reported that glial fibrillary acidic protein immunoreactive satellite cells emerge and surround DRG neurons innervating the L5/6 facet joints after injury. These satellite cells are TNF immunoreactive [11]. However, TNF receptor expression in DRG neurons innervating the facet joints has not been investigated.
The aim of the current study is to examine changes in p55 TNF receptor expression in DRG neurons innervating the L5/6 facet joint before and after facet joint injury. The L1 and L2 DRG neurons innervating the L5/6 facet joint through sympathetic trunks and the L3, L4 and L5 DRG neurons innervating the L5/6 facet joint via other routes are discussed separately.
Materials and methods
Retrograde FG labeling
Twenty male Sprague-Dawley (SD) rats weighing 250–300 g were used. The protocols for animal procedures in these experiments followed the 1996 revision of the National Institutes of Health guidelines for the care and use of laboratory animals and received approval from the ethics committees of our institutions.
Rats were anesthetized with sodium pentobarbital (40 mg/kg, i.p.) and treated aseptically throughout the experiments. A midline dorsal longitudinal incision was made over the lumbar spine. The left L5/6 facet joint capsule was exposed. A 26-gauge needle whose tip was filled with two fluoro-gold crystals (FG; Fluorochrome, Denver, CO, USA) was advanced into the facet joint. The hole was immediately sealed with cyanoacrylate to prevent leakage of the FG. The fascia and skin were then closed.
Seven days after the application of FG, the rats were anesthetized with sodium pentobarbital (40 mg/kg, i.p.) again, and the dorsal portion of the capsule was cut for the injured group (injured group n = 10). Fourteen days after the first application of FG, all 20 rats were anesthetized with sodium pentobarbital (40 mg/kg, i.p.) again, and perfused transcardially with 500 ml of 4% paraformaldehyde in phosphate buffer (0.1 M, pH 7.4) (non-injured group, n = 10; injured group, n = 10). Bilateral DRGs from T13 to L6 levels were resected. The specimens were immersed in the same fixative solution overnight at 4°C. After storing in 0.01 M phosphate buffered saline (PBS) containing 20% sucrose for 20 h at 4°C, each DRG was sectioned at 10 μm thickness on a cryostat, and mounted on poly-l-lysine-coated slides.
Immunohistochemistry for p55 TNF receptor
The specimens were treated for 90 min in blocking solution, 0.01 M PBS containing 0.3% Triton X-100 and 3% skim milk, at room temperature. They were processed for p55 TNF receptor (p55) immunohistochemistry using a goat antibody to p55 receptor (1:1,000; R&D systems) for 20 h at 4°C, followed by incubation with biotinylated donkey anti-goat IgG (Vector Labs, Burlingame, CA, USA; 1:200 in blocking solution). Reactions were visualized with an Alexa 488 fluorescent antibody conjugate (1:400; Molecular Probes Inc., Eugene, OR, USA).
After each step, the sections were rinsed in 0.01 M PBS three times. The sections were observed with a fluorescence microscope. The number of FG labeled neurons, and that of FG labeled p55-immunoreactive (IR) neurons were counted. We counted the number of FG labeled neurons, and that of FG labeled p55-immunoreactive (IR) neurons in all serial sections and then averaged these numbers for each animal. Split neurons were counted as a single FG positive neuron. This procedure was performed in a blinded manner.
Statistical analysis
The data were compared using non-paired Student’s t test. A P value of less than 0.05 was considered statistically significant.
Results
FG labeled neurons innervating the L5/6 facet joints
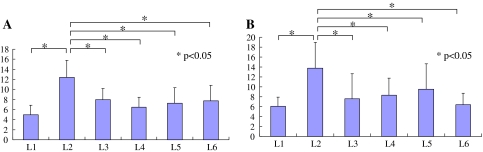
FG labeled DRG neurons innervating the L5/6 facet joint were distributed from ipsilateral L1 to L6 levels in non-injured and injured groups (Table 1; Fig. 1). No labeled neurons were observed in the bilateral T13 DRGs or in the contralateral DRGs from L1 to L6. Of the FG-labeled neurons, 63 and 62% were seen in the L3, L4 and L5 DRGs in the non-injured and injured group, respectively, while the remaining 37 and 38% were present in the L1 and L2. There was no significant difference in the number of FG-labeled neurons between non-injured and injured groups (P > 0.05). The numbers of FG labeled neurons in L2 DRGs were significantly higher than those of L1, L3, L4, L5, and L6 DRGs in both groups (P < 0.05) (Fig. 1).
Table 1.
The number of FG-labeled DRG neurons innervating L5/6 facet joints in each rat in non-injured and injured groups, the number of FG-labeled and p55-IR DRG neurons innervating L5/6 facet joints in each rat in non-injured and injured groups
| L1 | L2 | L3 | L4 | L5 | L6 | Total | |
|---|---|---|---|---|---|---|---|
| Number of FG labeled neurons innervating L5/6 facet joint | |||||||
| Non-injured group | |||||||
| Rat 1 | 4 | 8 | 4 | 4 | 5 | 7 | |
| 2 | 6 | 16 | 9 | 7 | 11 | 6 | |
| 3 | 4 | 9 | 8 | 5 | 12 | 7 | |
| 4 | 5 | 11 | 11 | 8 | 3 | 6 | |
| 5 | 7 | 12 | 12 | 9 | 9 | 5 | |
| 6 | 5 | 17 | 10 | 9 | 6 | 8 | |
| 7 | 7 | 14 | 7 | 4 | 5 | 9 | |
| 8 | 5 | 12 | 4 | 6 | 8 | 11 | |
| 9 | 4 | 13 | 5 | 6 | 6 | 8 | |
| 10 | 3 | 12 | 10 | 7 | 8 | 10 | |
| Total | 50 | 124 | 80 | 65 | 73 | 77 | 469 |
| Injured group | |||||||
| Rat 11 | 5 | 12 | 5 | 7 | 7 | 4 | |
| 12 | 7 | 21 | 6 | 9 | 8 | 5 | |
| 13 | 8 | 15 | 7 | 7 | 18 | 3 | |
| 14 | 9 | 16 | 9 | 5 | 5 | 7 | |
| 15 | 4 | 19 | 5 | 5 | 5 | 8 | |
| 16 | 5 | 9 | 21 | 4 | 4 | 8 | |
| 17 | 8 | 7 | 3 | 12 | 7 | 5 | |
| 18 | 6 | 8 | 5 | 15 | 12 | 6 | |
| 19 | 4 | 20 | 8 | 11 | 18 | 11 | |
| 20 | 5 | 11 | 7 | 8 | 11 | 7 | |
| Total | 61 | 138 | 76 | 83 | 95 | 64 | 517 |
| Number of FG labeled and p55-IR neurons innervating L5/6 facet joint | |||||||
| Non-injured group | |||||||
| Rat 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2 | 1 | 2 | 0 | 2 | 1 | 2 | |
| 3 | 0 | 2 | 1 | 1 | 2 | 1 | |
| 4 | 0 | 1 | 1 | 0 | 1 | 3 | |
| 5 | 0 | 2 | 2 | 2 | 1 | 0 | |
| 6 | 0 | 0 | 2 | 1 | 2 | 0 | |
| 7 | 1 | 1 | 1 | 1 | 0 | 0 | |
| 8 | 2 | 2 | 0 | 1 | 1 | 1 | |
| 9 | 0 | 2 | 0 | 1 | 2 | 1 | |
| 10 | 2 | 3 | 0 | 1 | 2 | 1 | |
| Total | 6 | 15 | 7 | 10 | 12 | 9 | 59 |
| Injured group | |||||||
| Rat 11 | 3 | 10 | 3 | 3 | 3 | 2 | |
| 12 | 5 | 12 | 3 | 3 | 3 | 2 | |
| 13 | 5 | 13 | 3 | 2 | 4 | 2 | |
| 14 | 7 | 10 | 2 | 4 | 2 | 3 | |
| 15 | 3 | 15 | 4 | 2 | 2 | 3 | |
| 16 | 3 | 5 | 3 | 1 | 3 | 2 | |
| 17 | 2 | 5 | 2 | 5 | 4 | 2 | |
| 18 | 5 | 6 | 3 | 5 | 4 | 3 | |
| 19 | 2 | 12 | 3 | 3 | 4 | 3 | |
| 20 | 3 | 8 | 3 | 3 | 4 | 2 | |
| Total | 38 | 96 | 29 | 31 | 33 | 24 | 251 |
Fig. 1.
Distribution of the average number of FG-labeled DRG neurons innervating L5/6 facet joints. These neurons were observed from L1 to L6 DRG. Error bars represent the SEM. The numbers of FG labeled neurons in L2 DRGs in both groups were significantly higher than those of L1, L3, L4, L5, and L6 DRGs in both groups (P < 0.05). a Non-injured group, b injured group
p55 TNF receptor expression in DRG neurons innervating the facet joint before and after facet capsule injury
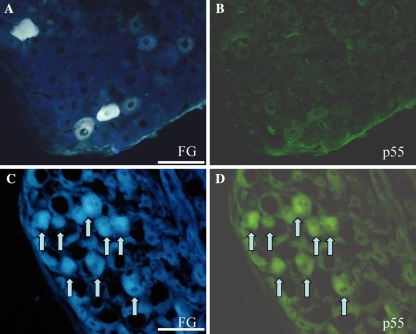
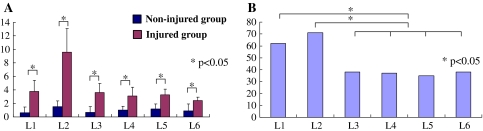
p55-IR cells were observed in both non-injured and injured groups. Immunoreactivity was observed only in neurons. p55-IR satellite cells were not seen in the current study. Of FG labeled neurons, the percentage of p55-IR neurons in the injured group was 50%. Of FG labeled neurons, the percentage of p55-IR neurons in the non-injured group was 13%. The percentage in the injured group was significantly higher than that in the non-injured group (Figs. 2, 3). The number of p55-IR neurons was significantly higher in L1 and 2 DRG than those in L3, 4, 5 and 6 DRG in the injured group (L1 and 2 DRG, 67%; L3, 4, 5 and 6 DRG, 37%: 67% is the percentage of the total number of p55-IR neurons in L1 and L2 level/the number of total FG-labeled neurons at L1 and L2 level. 37% is the percentage of the total number of p55-IR neurons from L3 to L6 level/the number of total FG-labeled neurons from L3 to L6 level) (Fig. 3).
Fig. 2.
Fluorescent photomicrographs show FG labeled DRG neurons, and p55-IR. a and c show the FG labeled neurons innervating the L5/6 facet joint. b, d Labeled cells in green show p55-IR neurons. a and b are the same section in the non-injured group. c, d The same section in the facet joint injury group. In the non-injured group, FG labeled neurons did not express p55 receptor. In the facet joint injury group, most FG labeled cells expressed the p55 receptor (arrows). Scale bar = 100 μm
Fig. 3.
a Distribution of the average number of FG-labeled and p55-IR cells at each level in both non-injured (blue) and facet joint injury (pink) groups. The number of FG-labeled and p55-IR cells in the facet joint injury group was significantly higher than that in the non-injured group (P < 0.05). b The ratios of FG-labeled and p55-IR cells at each level in the facet joint injury group. The ratio of FG-labeled and p55-IR cells in L1 and L2 DRG was significantly higher than those in L3, L4 and L5 DRG (P < 0.05)
Discussion
It has been reported that the rat L5/6 facet joint is innervated by DRGs from L1 to L6 levels via two pathways in rats. One is innervation from corresponding and adjacent segments and the other is from distant segments. In the latter type of innervation, sensory nerve fibers enter the paravertebral sympathetic trunks and reach L1 or L2 DRGs [11, 14, 15, 21, 22]. We did not examine this pathway in the current study; however, the results here do demonstrate that the rat L5/6 facet joint is innervated by ipsilateral DRGs from L1 to L6.
In the current study, a few p55 TNF receptor-expressing cells were observed in non-injured neurons; however, the number of p55 TNF immunoreactive FG labeled neurons innervating the facet joint was significantly increased after injury. p55 TNF receptor expression in DRG neurons innervating the facet joint was more frequently seen in upper DRGs rather than lower DRGs.
It has been reported that satellite cell activation in the DRG resulting from peripheral nerve injury produces hyperalgesia and allodynia in rats [7, 17, 26]. This activation is thought to be involved in the pathogenesis of neuropathic pain. TNF produced at nerve injury sites is axonally transported to DRG neurons where it correlates with the expression of p55 TNF receptor, which does not usually exist in the DRG neurons in rats. This may activate central cytokines in the pathogenesis of painful neuropathy in rats [18, 19]. This model does have precedent as sciatic nerve injury produces neuropathic pain, and TNF immunoreactive satellite cells were found to surround neurons expressing the TNF p55 receptors in mice [17]. It has been reported that in DRG neurons innervating the lumbar facet joints in rats, TNF-immunoreactive satellite cells activate and surround neurons innervating L5/6 facet joints after facet joint injury [11].
In the current study, there were two possible mechanisms of p55 TNF receptor upregulation in DRG neurons after facet joint injury. Facet joint capsule injury activates satellite cells in DRG, and the satellite cells produce TNF. Subsequently, satellite cells influence DRG neurons via the TNF and p55 TNF receptor pathway. The other is that TNF is up regulated by macrophages and other cells in the facet joint after injury. This TNF is then axonally transported to DRG neurons. Exposure of DRG neurons to TNF influences the production of p55 TNF receptors and through signal transduction pathways may increase signal intensity from the facet joint.
We have previously reported that activated satellite cells around DRG neurons innervating facet joints were co-labeled with TNF in rats. TNF expression in satellite cells around neurons innervating the facet joint was observed more frequently in upper DRGs compared to lower DRGs [11].
TNF induces substance P (SP) [6], and SP and calcitonin gene-related peptide (CGRP) are well known to be associated with inflammatory pain in animals and humans [8]. On the other hand, we have reported that DRG neurons innervating the rat facet joint are immunoreactive for SP and CGRP, and the ratios of the CGRP-IR L1 and L2 DRG neurons were significantly higher than L3, 4, and 5 DRGs in a model of facet joint inflammation [13, 16].
Suseki et al. [21] have reported that some neurons innervating the facet joint in rats were surrounded by CGRP-immunoreactive varicose fibers in paravertebral sympathetic trunks in rats. They find that upper DRG neurons innervating the facet joint via paravertebral sympathetic trunks were influenced by CGRP-immunoreactive nerve fibers in the trunks. We hypothesize that upper DRG neurons and lower DRG neurons innervating the facet joint may have several different sensory functions.
Facet joint pain and injury is recognized in the clinic. However, a limitation in this study may exist, since this facet capsule injury model does not absolutely correspond to human facet joint pain or injury. The present study suggests that TNF is potentially one of the molecules involved in the change in sensory transmission of facet joint capsule injury. These current findings contribute information that may help to elucidate the mechanism of change in sensory neurons innervating the facet joint after facet capsule injury.
Footnotes
Y. Sakuma and S. Ohtori contributed equally to this work.
References
- 1.Bartholdi D, Schwab ME. Expression of pro-inflammatory cytokine and chemokine mRNA upon experimental spinal cord injury in mouse: an in situ hybridization study. Eur J Neurosci. 1997;9:1422–1438. doi: 10.1111/j.1460-9568.1997.tb01497.x. [DOI] [PubMed] [Google Scholar]
- 2.Bogduk N, Long DM. The anatomy of the so-called “articular nerves” and their relationship to facet denervation in the treatment of low back pain. J Neurosurg. 1979;51:172–177. doi: 10.3171/jns.1979.51.2.0172. [DOI] [PubMed] [Google Scholar]
- 3.Bogduk N. The innervation of the lumbar spine. Spine. 1983;8:286–293. doi: 10.1097/00007632-198304000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Botchkina GI, Meistrell ME, Botchkina IL, Tracey KJ. Expression of TNF and TNF receptors (p55 and p75) in the rat brain after focal cerebral ischemia. Mol Med. 1997;3:765–781. [PMC free article] [PubMed] [Google Scholar]
- 5.Cavanaugh JM, el-Bohy A, Hardy WN, Getchell TV, Getchell ML, King AI. Sensory innervation of soft tissues of the lumbar spine in the rat. J Orthop Res. 1989;7:378–388. doi: 10.1002/jor.1100070310. [DOI] [PubMed] [Google Scholar]
- 6.Ding M, Hart RP, Jonakait GM. Tumor necrosis factor-alpha induces substance P in sympathetic ganglia through sequential induction of interleukin-1 and leukemia inhibitory factor. J Neurobiol. 1995;28:445–454. doi: 10.1002/neu.480280405. [DOI] [PubMed] [Google Scholar]
- 7.Fenzi F, Benedetti MD, Moretto G, Rizzuto N. Glial cell and macrophage reactions in rat spinal ganglion after peripheral nerve lesions: an immunocytochemical and morphometric study. Arch Ital Biol. 2001;139:357–365. [PubMed] [Google Scholar]
- 8.Hökfelt T. Neuropeptides in perspective: the last ten years. Neuron. 1991;7:867–879. doi: 10.1016/0896-6273(91)90333-U. [DOI] [PubMed] [Google Scholar]
- 9.Muñoz-Fernandez MA, Fresno M. The role of tumour necrosis factor, interleukin 6, interferon-gamma and inducible nitric oxide synthase in the development and pathology of the nervous system. Prog Neurobiol. 1998;56:307–340. doi: 10.1016/S0301-0082(98)00045-8. [DOI] [PubMed] [Google Scholar]
- 10.Mooney V, Robertson J. The facet syndrome. Clin Orthop Res. 1976;115:149–156. [PubMed] [Google Scholar]
- 11.Miyagi M, Ohtori S, Ishikawa T, Aoki Y, Ozawa T, Doya H, Saito T, Moriya H, Takahashi K. Up-regulation of TNF alpha in DRG satellite cells following lumbar facet joint injury in rats. Eur Spine J. 2006;15:953–958. doi: 10.1007/s00586-005-1031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myers RR, Wagner R, Sorkin LS (1999) Hyperalgesic actions of cytokines on peripheral nerves. In: Watkins LR, Maier SF (eds) Cytokines and Pain. Birkhauser, Boston, pp 133–157
- 13.Ohtori S, Takahashi K, Chiba T, Yamagata M, Sameda H, Moriya H. Substance P and calcitonin gene-related peptide immunoreactive sensory DRG neurons innervating the lumbar facet joints in rats. Auton Neurosci. 2000;86:13–17. doi: 10.1016/S1566-0702(00)00194-6. [DOI] [PubMed] [Google Scholar]
- 14.Ohtori S, Takahashi K, Chiba T, Yamagata M, Sameda H, Moriya H. Phenotypic inflammation switch in rats shown by calcitonin gene-related peptide immunoreactive dorsal root ganglion neurons innervating the lumbar facet joints. Spine. 2001;26:1009–1013. doi: 10.1097/00007632-200105010-00005. [DOI] [PubMed] [Google Scholar]
- 15.Ohtori S, Takahashi K, Moriya H. Inflammatory pain mediated by a phenotypic switch in brain-derived neurotrophic factor immunoreactive dorsal root ganglion neurons innervating the lumbar facet joints in rats. Neurosci Lett. 2002;323:129–132. doi: 10.1016/s0304-3940(02)00120-9. [DOI] [PubMed] [Google Scholar]
- 16.Ohtori S, Takahashi K, Moriya H. Calcitonin gene-related peptide immunoreactive DRG neurons innervating the cervical facet joints show phenotypic switch in cervical facet injury in rats. Eur Spine J. 2003;12:211–215. doi: 10.1007/s00586-003-0573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohtori S, Takahashi K, Moriya H, Myers RR. TNF-alpha and TNF-alpha receptor type 1 upregulation in glia and neurons after peripheral nerve injury: studies in murine DRG and spinal cord. Spine. 2004;29:1082–1088. doi: 10.1097/00007632-200405150-00006. [DOI] [PubMed] [Google Scholar]
- 18.Shubayev VI, Myers RR. Axonal transport of TNF-alpha in painful neuropathy: distribution of ligand tracer and TNF receptors. J Neuroimmunol. 2001;114:48–56. doi: 10.1016/S0165-5728(00)00453-7. [DOI] [PubMed] [Google Scholar]
- 19.Shubayev VI, Myers RR. Anterograde TNF alpha transport from rat dorsal root ganglion to spinal cord and injured sciatic nerve. Neurosci Lett. 2002;320:99–101. doi: 10.1016/S0304-3940(02)00010-1. [DOI] [PubMed] [Google Scholar]
- 20.Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 21.Suseki K, Takahashi Y, Takahashi K, Chiba T, Tanaka K, Moriya H. CGRP-immunoreactive nerve fibers projecting to lumbar facet joints through the paravertebral sympathetic trunk in rats. Neurosci Lett. 1996;221:41–44. doi: 10.1016/S0304-3940(96)13282-1. [DOI] [PubMed] [Google Scholar]
- 22.Suseki K, Takahashi Y, Takahashi K, Chiba T, Tanaka K, Morinaga T, Nakamura S, Moriya H. Innervation of the lumbar facet joints. Origin and functions. Spine. 1997;22:477–485. doi: 10.1097/00007632-199703010-00003. [DOI] [PubMed] [Google Scholar]
- 23.Wagner R, Myers RR. Schwann cells produce TNF-alpha: expression in injured and non-injured nerves. Neurosci. 1996;73:625–629. doi: 10.1016/0306-4522(96)00127-3. [DOI] [PubMed] [Google Scholar]
- 24.Wagner R, Myers RR. Endoneurial injection of TNF-alpha produces neuropathic pain behaviors. Neuroreport. 1996;7:2897–2901. doi: 10.1097/00001756-199611250-00018. [DOI] [PubMed] [Google Scholar]
- 25.Yamashita T, Cavanaugh JM, el-Bohy AA, Getchell TV, King AI. Mechanosensitive afferent units in the lumbar facet joint. J Bone Joint Surg Am. 1990;72:865–870. [PubMed] [Google Scholar]
- 26.Zhou XF, Deng YS, Chie E, Xue Q, Zhong JH, McLachlan EM, Rush RA, Xian CJ. Satellite-cell-derived nerve growth factor and neurotrophin-3 are involved in noradrenergic sprouting in the dorsal root ganglia following peripheral nerve injury in the rat. Eur J Neurosci. 1999;11:1711–1722. doi: 10.1046/j.1460-9568.1999.00589.x. [DOI] [PubMed] [Google Scholar]