Abstract
In an effort to augment the available grafting material as well as to increase spinal fusion rates, the utilization of a demineralized bone matrix (DBM) as a graft extender or replacement is common. There are several commercially available DBM substances available for use in spinal surgery, each with different amounts of DBM containing osteoinductive proteins. Each product may have different osteoinductivity potential due to different methods of preparation, storage, and donor specifications. The purpose of this study is to prospectively compare the osteoinductive potential of three different commercially available DBM substances in an athymic rodent spinal fusion model and to discuss the reasons of the variability in osteoinductivity. A posterolateral fusion was performed in 72 mature athymic nude female rats. Three groups of 18 rats were implanted with 1 of 3 DBMs (Osteofil, Grafton, and Dynagraft). A fourth group was implanted with rodent autogenous iliac crest bone graft. The rats were sacrificed at 2, 4, 6, and 8 weeks. A dose of 0.3 cm3 per side (0.6 cm3per animal) was used for each substance. Radiographs were taken at 2 weeks intervals until sacrifice. Fusion was determined by radiographs, manual palpation, and histological analysis. The Osteofil substance had the highest overall fusion rate (14/18), and the highest early 4 weeks fusion rate of (4/5). Grafton produced slightly lower fusion rates of (11/17) overall, and lower early 4 weeks fusion rate of (2/5). There was no statistically significant difference between the rate of fusion after implantation of Osteofil and Grafton. None of the sites implanted with Dynagraft fused at any time point (0/17), and there was a significantly lower fusion rate between the Dynagraft and the other two substances at the six-week-time point and for final fusion rate (P = 0.0001, Fischer’s exact test). None of the autogenous iliac crest animals fused at any time point. Non-decalcified histology confirmed the presence of a pseudarthrosis or the presence of a solid fusion, and the results were highly correlated with the manual testing. Although all products claim to have significant osteoinductive capabilities, this study demonstrates that there are significant differences between some of the tested products.
Keywords: Demineralized bone matrix, Spine fusion, Osteoinductivity
Introduction
Spinal surgery using autogenous bone graft is currently the standard method of fusion and is a common procedure for spinal pathology. Despite being the optimal graft solution, autogenous graft is associated with a certain rate of pseudarthrosis, and the potential complications and morbidity from the donor site harvesting [20, 27]. Because of these two major problems, bone graft extenders or replacements are commonly used to increase the biological potential for improvement in fusion success.
Demineralized bone matrix derived from human tissues has demonstrated the ability to aid in the stimulation of an osteoinductive response allowing for improved bone growth and fusion [3–5, 8, 16, 23]. In an effort to augment the available grafting material as well as increase fusion rates, the utilization of a demineralized bone matrix (DBM) as a graft extender or even as a graft substitute has become more common [4, 11, 12]. These materials contain the osteoinductive proteins derived from human bone and have the potential to aid in the formation of a spinal fusion.
There are several commercially available DBM substances available for use in spinal surgery, each with different amounts of DBM containing osteoinductive proteins. Additional variability exist in preparation of products from human donor bone, which may cause variation in their osteoinductivity.
The athymic rat spinal fusion model has been shown to be the ideal situation in which to test substances containing human DBMs as the rats cannot reject the human tissues and allow for each substance to be used in its direct, unaltered, “off the shelf” form. This permited a fair comparison of each material, which is used in the form available for use in human spine surgery in previous studies [6].
Several types of commercially available DBM products have been tested by using this animal model [9, 14] and a rabbit model [11, 12] revealing various fusion rates. The previous studies have concluded that significant differences may exist among commercially available DBMs in forming a spinal fusion. The different osteoinductive capability of these products has been explained as a consequence of the processing of the products and the carrier media used [9, 10, 14]. Lot variability due to the age, quality of bone and genetic background of the donor has also been claimed to be an important factor affecting the osteoconductivity but has not been studied extensively.
The purposes of this study were to compare the ability of three commercially available DBM products to fuse an athymic rat spine in a posterior-lateral intertransverse process model, and to discuss the possible reasons of variability of fusion rate in different commercially available DBM products.
Materials and methods
Overview
All protocols utilized for this experiment were approved by the animal research committee at our institution. Seventy-two mature (3–4-months old) athymic nude female rats were used in this study (175–240 g, Harlan Sprague Dawley, IN). A posterolateral fusion procedure was performed and each rat was implanted with one of three commercially available DBM products or autogenous bone graft taken from the iliac crest. Three different commercially available demineralized bone products were used: Dynagraft putty (GenSci Regeneration Sciences Inc., Irvine, CA), Grafton putty (Osteotech Inc., Eatontown, NJ), and Osteofil allograft bone paste (Medtronics Sofamor Danek, Memphis, TN). These demineralized bone matrices were obtained in the sterile, factory-sealed packaging for use in humans. Each was obtained directly from the operating room, unopened, and ready for implantation. All the products used from one company had the same lot number demonstrating that the material was prepared from the same cadaver bone. The autogenous bone graft was taken from iliac crest of the same animal. The following groups were tested: Group one had Osteofil paste (n = 18); Group two had Grafton putty (n = 17, one rat died from anesthesia complications in the post-operative period); Group three had Dynagraft putty (n = 17, one rat died from anesthesia complications in the post-operative period); Group four had autogenous iliac crest bone graft (n = 18). According to a pre set schedule, rats were sacrificed at 2, 4, 6, and 8 weeks after their posterolateral fusion and implantation procedure.
Surgical technique
The same surgeon performed all of the procedures. Athymic rats were anesthetized utilizing a combination of ketamine (0.054 mg/g) and xylazine (0.01 mg/g), and a posterior midline approach was used over the distal lumbar spine. Two separate fascial incisions were made 3 mm from the midline. A muscle-splitting approach was used lateral to the facet joints to expose the transverse processes of L4 and L5. A high-speed burr was used to decorticate only the transverse processes. The surgical site was then irrigated with sterile saline and an antibiotic solution.
Bone graft in the control group was harvested from the iliac crests. Through the fascial incisions noted above, the iliac crests were exposed. A ronguer was then used to harvest approximately 0.3 cm3 of corticocancellous bone from each iliac crest and the bone was later morcelized.
DBM graft materials were prepared and implanted between the transverse processes bilaterally in the paraspinal muscle bed in test animals while autograft was placed in the same manner in control animals. An aliquot equal to 0.3 cm3 was placed on each side of the prepared site for a total of 0.6 cm3 of graft material for each animal. The fascia and skin incisions were closed utilizing a 3–0 absorbable suture. The rodents were housed in separate cages, allowed to eat and drink ad libitum, while their health status was monitored on a daily basis.
Determination of fusion
Radiographs were taken at 2, 4, 6, and 8 weeks time. Characterization of the osseous union was determined by radiographic evidence of bridging, trabecular bone between the transverse processes.
Fusion was determined by manual palpation of the osseous mass between the transverse processes at the time of sacrifice. Explanted lumbar spines were manually tested for intersegmental motion by three independent observers blinded to the treatments. Any motion detected for either side between the facets or between the transverse processes of L4 and L5 by manual testing was considered a failure of fusion. The absence of motion (right and left) was considered successful fusion. These findings were correlated and confirmed by histological studies.
Histology
Non-decalcified longitudinal histological sections cut along the transverse processes of L4 and L5 and the intervening tissue were also taken of each specimen. Lumbar spine specimens were dissected down to individual fusion masses, and the entire lumbar spine was placed in 40% ethanol. They were sequentially dehydrated in ethanol solutions including 100% ethanol times three and then embedded in polymethylmethacrylate (PMMA).
Spines embedded in PMMA were sectioned sagitally using the Exakt saw with Precision Parallel 300 cP motorized specimen holder. Sections measuring approximately 120 μm were cut through the spinous processes. These were mounted to white acrylic slides using a thin layer of cyanoacrylate. The sections were thinned to approximately 50 μm using a Buehler grinding wheel and decreasing grades of silicon carbide grinding papers (240–600 grit), followed by a polishing pad and Gamma Micro polish® alumina 3B to achieve a highly polished appearance. The sections were etched using 10% Formic Acid for 1 min, rinsed in running water, stained with 1% aqueous Toluidine Blue stain for 3 min, and then rinsed under tap water until the water ran clear.
Following air drying, the sections were examined microscopically. A specimen was considered fused histologically if trabeculae were seen to span the region between the transverse processes. If a gap was noted, the specimen was deemed not fused. The histologists grading the specimens were blinded to treatment as well as the results of manual testing for fusion.
Statistical analysis
Fusion “rates” (proportion of rats with fusion) were compared using Fisher’s exact test since all such comparisons were between two independent groups of rats. No adjustments were made for multiple comparisons in this exploratory study. A P < 0.05 was considered significant for any one comparison.
Interobserver reliability was assessed by reporting both the observed agreement and by computing the kappa statistic. The kappa statistic corrects the observed agreement for possible chance agreement among observers.
Results
The results of the manual testing and non-decalcified histology fusion rates are presented in Table 1 and fusion rates as determined by radiographs are presented in Table 2. The animals implanted with Osteofil paste demonstrated slightly higher overall fusion rates when compared to the Grafton without statistical significance (P < 0.05), while no fusion was observed for rats implanted with Dynagraft putty at any time of sacrifce
Table 1.
Fusion rates as determined by manual testing and non-decalcified histology
| Sacrifice time (weeks) | Osteofil paste (n = 18) | Grafton putty (n = 17) | Dynagraft putty (n = 17) | Autograft (n = 18) |
|---|---|---|---|---|
| Fusions | Fusions | Fusions | Fusions | |
| 2 | 0/1 | 0/1 | 0/1 | 0/1 |
| 4 | 4/5 | 2/5 | 0/5 | 0/5 |
| 6 | 7/8 | 7/7 | 0/7 | 0/8 |
| 8 | 3/4 | 2/4 | 0/4 | 0/4 |
| Totals | 14/18 | 11/17 | 0/17 | 0/18 |
One each of the Grafton and Dynagraft rats died in the immediate post-operative period due to complications related to anesthesia
Table 2.
Fusion rates for the four groups as determined only by radiographs
| Sacrifice time (weeks) | Osteofil paste (n = 18) | Grafton putty (n = 17) | Dynagraft putty (n = 17) | Autograft (n = 18) |
|---|---|---|---|---|
| Fusions | Fusions | Fusions | Fusions | |
| 2 | 0/1 | 0/1 | 0/1 | 0/1 |
| 4 | 3/5 | 3/5 | 3/5 | 0/5 |
| 6 | 6/8 | 6/7 | 0/7 | 0/8 |
| 8 | 3/4 | 2/4 | 0/4 | 0/4 |
| Totals | 12/18 | 11/17 | 3/17 | 0/18 |
The results differ from those confirmed by histology primarily in the Dynagraft group at the four-week-time period where fusion appeared present on radiographs, but was not actually present
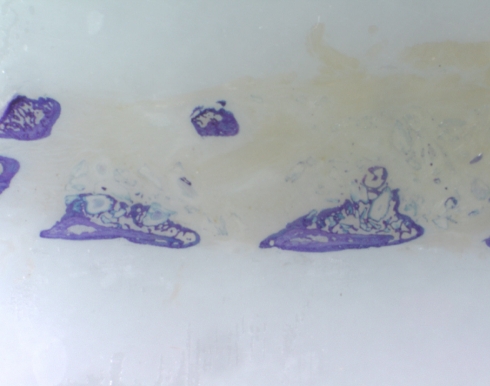
In the Osteofil group, no evidence of fusion was seen at the two-week-time point, but there were 4/5 sites fused by 4 weeks time. This fusion rate increased to 7/8 for rats sacrificed at the six-week-time point. A smaller group at 8 weeks time showed 3/4 fusion for a final fusion rate of 14/18 for all the animals receiving osteofil in this study. All fusions revealed by manual testing demonstrated radiographic healing which correlated with the histological findings. A radiograph of a successful fusion is shown in Fig. 1, and the histological section is shown in Fig. 2. Each rat with a pseudarthrosis appeared to have bone formation around each transverse process, but this bone failed to bridge the gap between L4 and L5 completely to restrict motion.
Fig. 1.

Anterior–posterior radiograph of a rat successfully fused with Osteofil at 6 weeks time. Note the large intertransverse osseous fusion at L4–L5
Fig. 2.
Sagittal histology section of the fusion mass connecting the transverse processes of L4–L5 after fusion with Osteofil. Note the new osseous bridging bone demonstrating a healed fusion
Grafton putty demonstrated similarly high fusion rates with a slightly lower but statistically insignificant (P < 0.05) final fusion rate compared to Osteofil paste. Again, there was no fusion at two-weeks-time. At the four-week-time point, the Grafton group had 2/5 fusions, which increased to 7/7 fusions at 6 weeks time. The smaller group at 8 weeks had a fusion rate of 2/4. Finally, the fusion rate for all the animals receiving Grafton in this study was 11/17. There was no statistically significant difference in the fusion rates between the animals receiving grafton and those receiving osteofil (P < 0.05) at any time point.
Again, the animals with a fusion had radiographs (Fig. 3) and histological studies (Fig. 4) demonstrating a solid osseous bridge between the transverse processes of L4 and L5. The animals with a pseudarthrosis typically had some new bone formation around each transverse process, but not a solid fusion.
Fig. 3.

Anterior-posterior radiograph of a rat successfully fused with Grafton at 6 weeks time. Note the large intertransverse osseous fusion at L4–L5
Fig. 4.
Sagittal histology section of the fusion mass connecting the transverse processes of L4–L5 after fusion with Grafton. Note the new osseous bridging bone demonstrating a healed fusion
Dynagraft putty did not demonstrate a solid fusion in any of the animals at any of the time points Each animal developed a pseudarthrosis, which had gross motion on manual palpation and a radiolucent line on the radiographs between the transverse processes of L4 and L5, an example of which is demonstrated in Fig. 5. The histological studies confirmed the presence of non-unions in all rats at all sacrifice times as shown in Fig. 6. There was a significant difference in the fusion rates between Dynagraft (fusion rate = 0) and the other two test substances at the six-week-time point and the final fusion rate (P < 0.001 for either comparison)
Fig. 5.

Anterior–posterior radiograph of a rat after attempted fusion with Dynagraft at 6 weeks time. Note the pseudoarthrosis and lack of bony bridging at L4–L5
Fig. 6.
Sagittal histology section of a pseudarthrosis between the transverse processes of L4–L5 after attempted fusion with Dynagraft. Note the paucity of bone formation and no bridging fusion at the L4–L5 level
The autogenous iliac crest bone grafted sites were not fused at any of the time points. The Osteofil and Grafton fusion rates were significantly higher than the autogenous graft group (P < 0.001 for either comparison) at the six-week-time point and the final fusion rate. The Dynagraft and autogenous fusion rates were both zero and therefore the same.
Fusion was judged by three observers by manual palpation, radiographs, and non-decalcified histology. There was a 93% agreement overall for the six- and -eight-week-time points of manual palpation versus histology (κ = 0.879), manual palpation versus radiographs (κ = 0.767), and radiographs versus histology (κ = 0.878).
On manual inspection, all spines that were manually fused contained a large fusion mass completely filling the intertransverse lateral “gutter” protruding well above the surface of the transverse processes.
Non-decalcified histology confirmed the presence of a pseudarthrosis or the presence of a solid fusion, and these results correlated with the manual testing.
Radiographic evaluation was less sensitive than histology in confirming the presence of a fusion by manual testing. Of interest, at the four-week-time point, over half of the Dynagraft animals were “overcalled” in that a fusion appeared present on radiographs while the spines were grossly loose on manual palpation. This “overcalling” a fusion mass did not occur at later time points. This discrepancy might be due to the radioopaque quality of the Dynagraft material itself, which may mimic induced bone formation early on. Kappa statistics correlated with this finding demonstrating the poor correlation of the radiographs versus the histology and manual palpation (κ = 0.4749). In addition, when the Dynagraft group was compared to the other groups in terms of agreement between the radiographs versus manual palpation and histology, the dynagraft group had significantly less correlation than the other two substances (P < 0.05).
Discussion
A direct comparison of “off the shelf” and unaltered form of three commonly used DBM products in the current study revealed differences in the osteoinductive potential. Among them, dynagraft putty has been tested for the first time in a spinal fusion animal model while the latter two have been tested several times. Dynagraft putty consistently failed to induce a sufficient osteoinductive response to bridge the transverse processes completely for fusion at any time. The Osteofil paste material appeared to induce a faster fusion at earlier time points, and a more reliable fusion at later time points. On the other hand, there was no significant difference between Osteofil and Grafton putty in fusion rates at any time points.
Controversy still exists concerning the influence of different variables in DBM osteoinductivity. Several factors are expected to influence the osteoinductive properties of a DBM product. Each product in the current study contained different amounts of DBM, and this amount did not correlate with the ability of each substance to consolidate a spinal fusion. The osteoinductive potential of each substance in the fusion area is most likely related to many factors such as differences in preprocess handling [2, 17, 26], varying demineralization times [7] final particle size [15, 18] terminal sterilization [19, 25] and the differences in the carrier [9, 14, 17, 21] all of which were the important variables in the products tested.
It is important to note that this model of spinal fusion is difficult to fuse and requires a material with significant osteoinductive ability to induce a solid arthrodesis. The results allow for a comparison of the three materials in their osteoinductive ability in a spinal fusion model, but do not necessarily demonstrate that the least effective material has no osteoinductive ability. We believe that interpretation of this data should note that the least effective DBM material was inferior to the other two substances in its osteoinductive potential, but was not worse than autogenous graft in the current study. Zero percent fusion rate in our study by using autograft was consistent with the same result of two previous studies [9, 24], while one study [6] reported 30% fusion rate evaluated by manual palpation. However, fusion rate was reported to be 10% in the same study according to the histological analysis. We believe that manual palpation only may not give the accurate fusion rate and should be consistent with the more objective results of histology as in the current and the two previous studies reporting 0% fusion rate [9, 24]. Therefore, we think that the fusion rate in the literature is pretty consistent as no or very little fusion with autogenous graft.
Two products (Osteofil and Grafton) have been tested in two previous studies using the same amount of DBM on the same animal model and the surgical technique [9, 14]. One other study using the same animal model but a different placement technique in a different anatomical place has also compared the osteoinductivity of Osteofil and Grafton products [21]. Peterson et al has tested three different kinds of DBM products in athymic rat spine fusion model [14]. The authors reported a 100% fusion rate by the use of grafton putty, which provided the highest fusion rate. The authors have used two different batches of grafton to prevent the lot variability. In another study from the same center, comparing eight different products using the athymic rat spine fusion model, Lee et al. reported a 60% fusion rate with osteofil and 80% fusion rate with grafton putty [9]. The authors have stated that there was a statistically significant difference in the rate of fusion among the groups (P = 0.04). The fusion rates for the two products in three studies including the current study, within exactly the same model, revealed 60–78% fusion rate for osteofil and 65–100% fusion rate for grafton putty. In a recent study comparing the osteoinductive properties of the two products in athymic rat model, the authors implanted both DBM products intramuscularly [21]. They reported a significantly higher osteoinductivity score for osteofil when compared to grafton (P < 0.01). These results in the same animal model are obviously conflicting and probably reflecting the variability in the efficacy of different batches of the same product considering that the preparation was unique for each batch of the same product. Concentration of osteoinductive proteins in the bone matrix of the individual donor, the intrinsic osteoinductive potential of the individual donor and genetical differences in expressing the bone morphogenetical proteins may play an important role for the osteoinductive properties of DBM products. Age of the donor is another controversial issue for the efficacy of DBM with conflicting reports in the literature [1, 13, 22]. Most commercial tissue banks do not generally evaluate the degree of bone forming potential of their DBM prior to distribution. There are several in vivo and in vitro ways of assesing the osteoinductive potentials of DBM, however there seems to be no reliable, easy to handle and rapid method to estimate accurately the bone forming potential of DBM. We believe that the lot differences may play an important role in the fusion rate of each DBM product of the same company and this should be more extensively studied in the future. Moreover the need for a specific, sensitive and reliable screening assay of the osteoinductive properties of DBM and objective information of each product’s osteoinductivity is obvious.
In conclusion, our study comparing three different products of DBM demonstrated different efficacies of osteoinductivity most likely due to the different processing procedures. We also observed that two products tested in this study performed differently when compared to the results of other studies in the pertinent literature testing the same product prepared with the same processing procedures. We hypothesize that the difference in efficacy of the same products in the same animal model may reflect the variable osteoinductivity potential of different donors despite the same processing procedures. This fact should further be analyzed in well-designed studies. The differences in osteoinductivity between the different products and inconsistencies of fusion rates between the different lots of the same products, question the reliable use of DBM in spinal surgery.
References
- 1.Becerra J, Andrades JA, Ertl DC, Sorgente N, Nimni ME. Demineralized bone matrix mediates differentiation of bone marrow stromal cells in vitro: effect of age of cell donor. J Bone Miner Res. 1996;11:1703–1714. doi: 10.1002/jbmr.5650111114. [DOI] [PubMed] [Google Scholar]
- 2.Boyce T, Edwards J, Scarborough N. Allograft bone. The influence of processing on safety and performance. Orthop Clin North Am. 1999;30:571–581. doi: 10.1016/S0030-5898(05)70110-3. [DOI] [PubMed] [Google Scholar]
- 3.Chesmel KD, Branger J, Wertheim H, Scarborough N. Healing response to various forms of human demineralized bone matrix in athymic rat cranial defects. J Oral Maxillofac Surg. 1998;56:857–863. doi: 10.1016/S0278-2391(98)90015-5. [DOI] [PubMed] [Google Scholar]
- 4.Cobos JA, Lindsey RW, Gugala Z. The cylindrical titanium mesh cage for treatment of a long bone segmental defect: description of a new technique and report of two cases. J Orthop Trauma. 2000;14:54–59. doi: 10.1097/00005131-200001000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Edwards JT, Diegmann MH, Scarborough NL. Osteoinduction of human demineralized bone: characterization in a rat model. Clin Orthop Relat Res. 1998;357:219–228. doi: 10.1097/00003086-199812000-00028. [DOI] [PubMed] [Google Scholar]
- 6.Grauer JN, Bomback DA, Lugo R, Troiano NW, Patel TC, Friedlander GE. Posterolateral lumbar fusions in athymic rats: characterization of a model. Spine. 2004;J4:281–286. doi: 10.1016/j.spinee.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Han B, Tang B, Nimni M. Quantitative and sensitive in vitro assay for osteoinductive activity of demineralized bone matrix. J Orthop Res. 2003;21:648–654. doi: 10.1016/S0736-0266(03)00005-6. [DOI] [PubMed] [Google Scholar]
- 8.Kado KE, Gambetta LA, Perlman MD. Uses of grafton for reconstructive foot and ankle surgery. J Foot Ankle Surg. 1996;5:59–66. doi: 10.1016/S1067-2516(96)80014-7. [DOI] [PubMed] [Google Scholar]
- 9.Lee YP, Jo M, Luna M, Chien B, Lieberman JR, Wang JC. The efficacy of different commercially available demineralized bone matrix substances in an athymic rat model. J Spinal Disord Tech Oct. 2005;18(5):439–444. doi: 10.1097/01.bsd.0000175696.66049.f7. [DOI] [PubMed] [Google Scholar]
- 10.Lomas RJ, Gillan HL, Matthews JB, Ingham E, Kearney JN. An evaluation of the capacity of differently prepared demineralised bone matrices (DBM) and toxic residuals of ethylene oxide (EtOx) to provoke an inflammatory response in vitro. Biomaterials. 2001;22:913–921. doi: 10.1016/S0142-9612(00)00255-6. [DOI] [PubMed] [Google Scholar]
- 11.Martin GJ, Jr, Boden SD, Titus L, Scarborough NL. New formulations of demineralized bone matrix as a more effective graft alternative in experimental posterolateral lumbar spine arthrodesis. Spine. 1999;24:637–645. doi: 10.1097/00007632-199904010-00005. [DOI] [PubMed] [Google Scholar]
- 12.Morone MA, Boden SD. Experimental posterolateral lumbar spine fusion with a demineralized bone matrix gel. Spine. 1998;23:159–167. doi: 10.1097/00007632-199801150-00003. [DOI] [PubMed] [Google Scholar]
- 13.Nishimoto SK, Chang CK, Gendler E, Stryker WF, Nimni ME. The effect of aging on bone formation in rats: biochemical and histological evidence for decreased bone formation capacity. Calcif Tissue Int. 1996;37:617–624. doi: 10.1007/BF02554919. [DOI] [PubMed] [Google Scholar]
- 14.Peterson B, Whang PG, Iglesias R, Wang JC, Lieberman RJ. Osteoinductivity of commercially available demineralized bone matrix. Preparations in a spine fusion model. J Bone J Surg (2004) 2004;86:2243–2250. doi: 10.2106/00004623-200410000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Reddi AH, Huggins CB. Influence of geometry of transplanted tooth and bone on transformation of fibroblasts. Proc Soc Exp Biol Med. 1973;143:634–647. doi: 10.3181/00379727-143-37381. [DOI] [PubMed] [Google Scholar]
- 16.Russell J, Scarborough N, Chesmel K. Re: ability of commercial demineralized freeze-dried bone allograft to induce new bone formation (1996; 67:918–26) [letter; comment] J Periodontol. 1997;68:804–806. [PubMed] [Google Scholar]
- 17.Russell JL, Block JE. Clinical utility of demineralized bone matrix for osseous defects, arthrodesis, and reconstruction: impact of processing techniques and study methodology. Orthopedics. 1999;22:524–531. [PubMed] [Google Scholar]
- 18.Sampath TK, Reddi AH. Importance of geometry of the extracellular matrix in endochondral bone differentiation. J Cell Biol. 1984;98:2192–2197. doi: 10.1083/jcb.98.6.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scarborough NL, White EM, Hughes JV, Manrique AJ, Poser JW. Allograft safety: viral inactivation with bone demineralization. Contemp Orthop. 1995;31:257–261. [PubMed] [Google Scholar]
- 20.Silber JS, Anderson DG, Daffner SD, Brislin BT, Leland JM, Hilibrand AS, Vaccaro AR, Albert TJ. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine. 2003;28:134–139. doi: 10.1097/00007632-200301150-00008. [DOI] [PubMed] [Google Scholar]
- 21.Takikawa S, Bauer TW, Kambic H, Togawa D. Comparative evaluation of the osteoinductivity of two formulations of human demineralized bone matrix. J Bone Miner Res. 2003;65(1):37–42. doi: 10.1002/jbm.a.10345. [DOI] [PubMed] [Google Scholar]
- 22.Traianedes K, Russell JL, Edwards JT, Stubbs HA, Shanahan IR, Knaack D. Donor age and gender effects on osteoinductivity of demineralized bone matrix. J Biomed Mater Res B Appl Biomater. 2004;70:21–29. doi: 10.1002/jbm.b.30015. [DOI] [PubMed] [Google Scholar]
- 23.Urist MR, Dowell TA. Inductive substratum for osteogenesis in pellets of particulate bone matrix. Clin Orthop. 1968;61:61–78. [PubMed] [Google Scholar]
- 24.Wang JC, Yoo S, Kanim EAL, Campbell PA, Berk A, Lieberman JA. Effect of regional gene therapy with bone morphogenetic protein -2-producing bone marrow cells on spinal fusion in rats. J Bone Joint Surg. 2003;85A:905–911. doi: 10.2106/00004623-200305000-00020. [DOI] [PubMed] [Google Scholar]
- 25.Wientroub S, Reddi AH. Influence of irradiation on the osteoinductive potential of demineralized bone matrix. Calcif Tissue Int. 1988;42:255–260. doi: 10.1007/BF02553752. [DOI] [PubMed] [Google Scholar]
- 26.Yazdi M, Bernick S, Paule WJ, Nimni ME. Postmortem degradation of demineralized bone matrix osteoinductive potential. Effect of time and storage temperature. Clin Orthop. 1991;262:281–285. [PubMed] [Google Scholar]
- 27.Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192–195. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]