Abstract
This systematic review updates the understanding of the evidence base for balloon kyphoplasty (BKP) in the management of vertebral compression fractures. Detailed searches of a number of electronic databases were performed from March to April 2006. Citation searches of included studies were undertaken and no language restrictions were applied. All controlled and uncontrolled studies were included with the exception of case reports. Prognostic factors responsible for pain relief and cement leakage were examined using meta-regression. Combined with previous evidence, a total of eight comparative studies (three against conventional medical therapy and five against vertebroplasty) and 35 case series were identified. The majority of studies were undertaken in older women with osteoporotic vertebral compression fractures with long-term pain that was refractory to medical treatment. In direct comparison to conventional medical management, patients undergoing BKP experienced superior improvements in pain, functionality, vertebral height and kyphotic angle at least up to 3-years postprocedure. Reductions in pain with BKP appeared to be greatest in patients with newer fractures. Uncontrolled studies suggest gains in health-related quality of life at 6 and 12-months following BKP. Although associated with a finite level of cement leakage, serious adverse events appear to be rare. Osteoporotic vertebral compression fractures appear to be associated with a higher level of cement leakage following BKP than non-osteoporotic vertebral compression fractures. In conclusion, there are now prospective studies of low bias, with follow-up of 12 months or more, which demonstrate balloon kyphoplasty to be more effective than medical management of osteoporotic vertebral compression fractures and as least as effective as vertebroplasty. Results from ongoing RCTs will provide further information in the near future.
Keywords: Kyphoplasty, Vertebral compression fractures, Osteoporosis, Systematic review, Meta-analysis
Introduction
An estimated 1,700,000 vertebral compression fractures (VCFs) occur every year in the US and in Europe, a figure that is likely to rise over the coming decades [5, 14, 15]. The burden of VCFs can be substantial, in particular chronic pain, a marked reduction in health-related quality of life and high healthcare costs [1, 5]. The development of minimally invasive surgical techniques such as balloon kyphoplasty (BKP) has been favoured by a large number of VCF patients remaining refractory to conventional treatments, including drugs, surgical braces and rehabilitation.
BKP was first performed in the 1998 and involves fracture reduction using inflation bone tamps (balloon) to restore vertebral height. The two bone tamps used bilaterally create a void in the vertebral body that can be filled under fine manual control and low pressure with high viscosity bone cement. Unlike vertebroplasty, BKP aims to not only secure fracture fixation and stabilization but also to correct and prevent the spinal deformity, thereby reducing the negative burden of VCFs [7].
We, and others, have undertaken systematic reviews of the efficacy and safety of BKP [2, 9, 17]. These reviews have shown that, following BKP, patients experience significant relief of short-term pain and improvement in function. In many patients, BKP appears to have the ability to partly restore vertebral height and thereby reducing kyphotic deformity. BKP is a comparably safe procedure and, compared to simple vertebroplasty, reported to cause less cement extravasation [6–8]. BKP seems to be an emerging medical technology, but the conclusions of previous reviews are limited by the lack of direct comparative evidence, comparing BKP to best medical care, or vertebroplasty.
This paper describes an update of a previous systematic review and meta-analysis of the efficacy and safety of BKP. In addition, factors related to patients and procedures, that are associated with the outcome of BKP, are explored.
Materials and methods
Studies were identified from previously published systematic reviews and meta-analyses [6–8]. This list of studies was updated by searching a number of databases, including MEDLINE (Ovid), MEDLINE (R) In-process citations, EMBASE (Ovid); Cochrane Library; and registers of ongoing research (Meta Register of Controlled Trials and ClinicalTrials.gov) up to April 2006. Search terms were selected in order to maximise both the search sensitivity and specificity. Index and text words representing the device/procedure were combined with terms for vertebral compression fractures. Hand searching of the reference lists of included studies was undertaken. The device manufacturer (Kyphon Inc.) was contacted to identify any studies that may have been missed that were ongoing or were unpublished. There were no language restrictions and foreign language papers were translated.
Selection of studies
Two reviewers independently scanned all the titles and abstracts and identified potentially relevant articles to be retrieved. Where there was uncertainty, full-text copies of papers were obtained. Studies were considered eligible for inclusion if they met the following criteria:
Study design: Experimental studies (i.e. randomised and non-randomised trials), observational studies (i.e. cohort studies, case control studies or cross sectional studies), and uncontrolled studies (i.e. case series).
Population: Patients with VCFs of osteoporotic or neoplastic (i.e. myeloma, metastasis or osteolysis) aetiology.
Intervention: BKP.
Comparator: Any invasive, semi-invasive or medical therapy.
Outcomes: Reported at least one of the following; efficacy, pain relief, functional capacity and health-related quality of life, deformity correction (height restoration, kyphotic angle correction), safety, cement leakage, incident (adjacent and non-adjacent) fractures, complications.
We excluded studies reporting on burst fractures and fractures due to trauma, including BKP combined with other invasive or semi-invasive intervention therapies, including patients undergoing repeat interventions, case reports, and studies published only in abstract form.
Quality assessment
As there is not an accepted instrument or standard approach to the assessment of the quality of case series or non-randomised comparative studies, quality was assessed quantitatively according to the four principal categories of study bias [10]:
Selection bias: i.e. Bias associated with the way the intervention or control groups were assembled.
Assessment bias: i.e. Bias as the result of assessment of the outcome.
Performance bias: i.e. Bias as the result of care provided to the participants in the intervention or control groups other than the interventions under investigation.
Attrition bias: i.e. Bias associated with withdrawal/loss to follow up from intervention or control groups.
On the basis of all the quality criteria, studies were judged to of ‘high’ (i.e. large number of individual biases present), medium (i.e. some biases present) or low (i.e. little or no bias present) risk of overall bias.
Data analysis
The principal characteristics of included studies were summarised in tabular form. In order to obtain a summary estimate of the efficacy and safety of BKP, the results of individual studies were combined, where possible [3]. Separate meta-analyses were undertaken for comparative and non-comparative studies and for each outcome. Dichotomous and continuous outcomes were summarised as proportions, rates or rate ratios (relative risks) and mean differences or standardised mean differences, respectively. Data were pooled as using a fixed-effects model, except where statistical heterogeneity existed (P < 0.100) according to the χ2-statistic, and a random-effects model was instead used [3]. Imputation methods were used to estimate outcome variances where not reported.
Meta-regression was used to examine the reasons for heterogeneity. This ‘subgroup analysis’ allows exploration of the influence of a variety of potential prognostic factors that might be associated with the efficacy or safety of BKP [16]. Meta-regression was performed on the most commonly reported efficacy and safety outcomes, i.e. the level of pain relief and level of cement leaks, respectively. The subgroups were defined a priori: type of study (comparative or non comparative); average duration of fracture or pain; sample size; study quality (low bias or not); study publication date; indication (i.e. osteoporotic or neoplastic VCFs); continent of study (i.e. USA or not); and duration of follow up (in months). For pain relief, an additional subgroup was added (average level of pain pre and post BKP).
Publication bias was assessed using funnel plots and the Egger test for those sufficiently reported outcomes (i.e. ≥10 studies) [14].
Data are expressed as means and 95% confidence intervals or medians and ranges. All analyses were performed using Stata Software (Stata 8, StataCorp LP, TX, USA).
Results
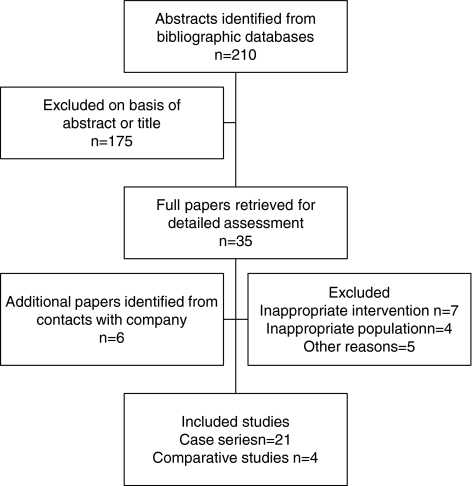
A total of 210 citations were obtained from updated searches of the various electronic bibliographies (March 2004–April 2006). A further six papers were identified through contact with the device manufacturer. Most abstracts and titles or full papers were excluded on the basis of an inappropriate intervention (e.g. vertebroplasty) or they were case reports. A total of four new comparative studies and 21 case series were judged to meet the inclusion criteria (see Fig. 1). Combining those studies in our original review (15 case series and five comparative studies) [17] and this update (21 case series and four comparative studies, a total of eight comparative studies (i–viii) and 35 case series were identified (ix–xliii, see Appendix). The publications of Grafe et al. (iii) and Ledlie and Renfro (xxvi) report additional follow ups on patient series included in our previous review. All included studies have been published with the exception of a large US multicentre registry, Kyphon Inc., making available their full report to the FDA. A version of the report is currently in press [8].
Fig. 1.
Summary of study selection and exclusion process (March 2004–April 2006)
Study characteristics and quality
Five studies directly compared BKP to vertebroplasty and three to conventional medical care across 481 fractures in 313 patients. Nussbaum et al. (vi), a comparison of BKP and vertebroplasty, reported adverse events listed in the FDA (Maude) database; no overall patient number was, therefore, available. The 35 case series included total of 2,047 patients undergoing BKP on 3,301 vertebral levels (Tables 1, 2).
Table 1.
Summary of characteristics and quality of BKP comparative studies
| Weisskopf et al. (2003) (viii) | Kasperk et al. (2004, 2005) (iii) | Komp et al. (2004) (iv) | Fourney et al. (2003) (i) | Grohs et al. (2005) (ii) | Masala et al. (2005) (v) | Pflugmacher et al. (2005) (vii) | Nussbaum et al. (2005) (vi) | |
|---|---|---|---|---|---|---|---|---|
| BK and control | BKP 22 (37) | BKP 40 (73) | BKP 19 (NR) | BKP 15 (32) | BKP 28 (35) | BKP NR (7) | BKP 22 (35) | BKP NR |
| Patient (fracture) | CMM 20 (35) | CMM 20 (33) | CMM 17 (NR) | VB 34 (65) | VB 23 (29) | VB NR (33) | VB 20 (32) | VB NR |
| Indications | Os, Ol | Os | Os | M, Mt, Ol | Os | Hg, M, Mt | Os | NR |
| Follow up++ | 3 months | 12 months | 6 months | 4.5 months | 24 months | 6 months | 12 months | NR |
| Design | Retrospective | Prospective | Prospective | Retrospective | Prospective | Retrospective | Prospective | Retrospective |
| Selection bias | ||||||||
| Inclusion/exclusion criteria specified | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Groups similar at baseline | No | Yes | Yes | No | Yes | NR | Yes | NR |
| Performance bias | ||||||||
| Concomitant therapies specified | No | Yes | No | No | No | No | No | No |
| Assessment bias | ||||||||
| Independent assessment of results | NR | Yes | NR | Yes | Yes | NR | NR | No |
| Attrition bias | ||||||||
| Intention to treat analysis* | NR | Yes | Yes | Yes | Yes | NR | Yes | Yes |
| Losses to follow up % | NR | 0% | 7.5% | 90% | 0% | NR | 0% | NR |
| Overall risk of bias | High | Low | Moderate | High | Low | High | Moderate | High |
* Patients allocated to groups to which they were assigned
NR Not reported, Hg hemangioma, M myeloma, Mt metastatic lesions, Ol osteolytic, Os osteoporosis, CMM conventional medical care, VB vertebroplasty
++Longest follow up point reported
Table 2.
Summary of characteristics of included BKP case series
| Author (year) country | N Patients (fracture) | Indication | Fracture age | Sex (% male) age-mean (SD or range) | Design (retrospective/prospective) | Follow up++ | Overall risk of bias+ |
|---|---|---|---|---|---|---|---|
| Atalay (2005) Turkey (ix) | 57 (77) | Os, M, Hg, Mm, T | <3 months | 33% 67.5 (48–92) |
NR | 6.5 months | High |
| Buisson et al. (2005) France (x) | 6 (12) | Os, M | NR | 83% 61.8 (37–82) |
Prospective | 6 months | High |
| Coumans et al. (2003) USA (xi) | 78 (188) | Os, Mm | NR | 42% 71 (44–89) |
Prospective | 18 months | Moderate |
| Crandall et al. (2004) USA (xii) | 47 (86) | Os | Acute ≤10 weeks Chronic ≥4 months |
26% 74 (47–91) |
Prospective | 24 months | Moderate |
| Darius et al. (2003) Belgium (xiii) | 7 (8) | Os | NR | 57% 64.7 (37–90) |
NR | 6 months | High |
| Dudeney et al. (2002) USA (xiv) | 18 (55) | Mm | NR | NR 63.5 (48–75) |
Prospective | 7.4 months | Moderate |
| Feltes et al. (2005) USA (xv) | 13 (20) | Os | NR | 46% 71.5 (±11) |
Retrospective | 1 month | High |
| Fribourg et al. (2004) USA (xvi) | 38 (47) | Os | NR | 26% 80.6 |
Retrospective | 31 months | Moderate |
| Gaitanis et al. (2005) Greece (xvii) | 32 (61) | Os, Mm, Mt, Hg | NR | 19% 68.2 (45–91) |
Prospective | 24 months | Moderate |
| Garfin et al. (2003, 2006) USA (xviii) | 155 (214) | Os, Mm | 4.6 months | 19% 77 (45–99) |
Prospective | 24 months | Moderate |
| Grohs et al. (2004) Germany (xix) | NR (101) | Os, Mt | NR | NR 70 |
Prospective | 12 months | High |
| Harrop et al. (2004) USA (xx) | 115 (225) | Os | NR | 34% 74 (45–89) |
Retrospective | 33 months | Low |
| Hillmeier et al. (2003) Germany (xxi) | 95 (165) | Os | NR | 20% 69 |
Retrospective | ≤12 months | Moderate |
| Hillmeier et al. (2004) Germany (xxii) | 102 (192) | Os | 172 VCFs were ‘old’; 20 VCFs ≤4 weeks | 25% 71 (56–88) |
Retrospective | 12 months | Moderate |
| Kasperk et al. (2003) Germany (xxiii) | 89 (NR) | Os | NR | NR NR |
Prospective | 6 months | Moderate |
| Khanna et al. (2006) USA (xxiv) | 211 (NR) | Os, M | NR | 37% 39.4 (35–89) |
Prospective | 10.8 months | High |
| Lane et al. (2004) USA (xxv) | 19 (46) | Mm | ≥3 months | 63% 60.4 (45–74) |
Prospective | 3 months | High |
| Ledlie and Renfro (2003, 2005, 2006) USA (xxvi) | 117 (151) | Os, Mm | 2.7 months | 28% 77 (51–93) |
Retrospective | 43.1 months | High |
| Libicher et al. (2005) Germany (xxvii) | 12 (23) | Os | NR | 25% 61 (9) |
Prospective | 12 months | Moderate |
| Lieberman et al. (2001) USA (xxviii) | 30 (70) | Os, Mm | NR | NR 68.6 |
Prospective | 24 months | Moderate |
| Lieberman et al. (2003) USA (xxix) | 63 (264) | Mm, Mt | NR | MR 58 |
Prospective | 4 months | High |
| Majd et al. (2005) USA (xxx) | 222 (360) | Os | 5.7 months | 28% 76 (28–98) |
Retrospective | 36 months | Moderate |
| Masala et al. (2004) Italy (xxxi) | 16 (16) | Os, Mt, Mm | NR | 44% 72.3 (63–82) |
NR | NR | High |
| Masala et al. (2005) Italy (xxxii) | 11 (11) | Os, M | 3 months | 45% 68.9 (63–78) |
Prospective | 3 months | High |
| Phillips et al. (2002) USA (xxxiii) | 20 (20) | Os | 4.5 months | NR 73.5 (51–90) |
Prospective | Postoperative | Low |
| Phillips et al. (2003) USA (xxxiv) | 29 (61) | Os | 3.8 months | NR 70 |
Prospective | 12 months | Moderate |
| Pradman et al. (2006) USA (xxxv) | 65 (85) | Os | NR | 35% 87 (45–84) |
Retrospective | 0 month | Moderate |
| Rhyne et al. (2004) USA (xxxvi) | 52 (82) | Os | 31.3 weeks | 21% 74 (49–89) |
Retrospective | 25 months | Moderate |
| Tang et al. (2005) (xxxvii) | 13 (37) | Os | NR | 0% 72.3 (NR) |
NR | 13.7 months | High |
| Theodorou et al. (2002) USA (xxxiiii) | 15 (24) | Os | 3.5 months | 27% 75 (41–86) |
NR | 8 months | Moderate |
| Villavicencio et al. (2005) USA (xxxxix) | 20 (24) | Os, Ol | NR | 45% 75.2–78.1 (39–99) |
Prospective | NR | High |
| Voggenreiter et al. (2004) Germany (xl) | 57 (87) | Os | NR | NR NR |
Prospective | Postoperative | Moderate |
| Voggenreiter et al. (2004) Germany (xli) | 30 (39) | Os | NR | 23% 70.1 (45–84) |
Prospective | Postoperative | High |
| Willhelm et al. (2003) Germany (xlii) | 34 (56) | Os | NR | 26% 75 (50–86) |
Prospective | 12 months | Moderate |
| Yang et al. (2005) China (xliii) | 58 (90) | Hg, M | 0.5–5 months | 35% 69.3 (32–98) |
Prospective | 48 months | High |
Based on evaluation of selection bias, assessment bias, performance bias and attrition bias
* Case series includes two cement types calcium phosphate [N = 6 (14)] and PMMA [N = 6 (9)]
NR not reported
Hg hemangioma, M myeloma, Mt metastatic lesions, Mm multiple myeloma, Ol osteolytic, Os osteoporosis, Traumatic; ++longest follow up point reported
The large majority of studies were undertaken in single centre setting either in the US or mainland Europe. Included BKP patients were predominantly older women (median 33% male and 70.1 years) who had experienced a symptomatic osteoporotic VCF (84%), the remainder having neoplastic lesions (10% multiple myeloma, 5% metastatic lesions and 1% haemangiomas). Six BKP studies were conducted exclusively in neoplastic patients (i, v, xiv, xxv, xxviiii and xliii). Although not always stated, the reported duration of pain (median 2.5 months) or fracture age (median 4.6 months) indicated that patients were generally refractory to medical treatment. In the study of Kasperk and colleagues, patients had experienced pain for at least 12-months. In contrast, the study reported by Yang et al. (xliii) included patients with fractures no older than 2 weeks. The duration of follow up varied considerably, ranging from immediately postprocedure to 3-years. Over 60% of studies reported on pain relief (73%), number of cement leaks (66%) and the change in vertebral height (61%). Some 18% of studies reported the impact of BKP on health-related quality of life and 36% of the studies reported mobility or functionality. The median duration of follow up ranged from hours postprocedure, up to 37 months after the procedure.
The limited level of reporting of methods hampered assessment of methodological quality (Table 2). However, where details were available, the quality of both comparative studies and case series was found to vary considerably. As studies provide no details on co-interventions (e.g. analgesic therapy; physiotherapy) and infrequently reported if blinding or independent outcome assessment was undertaken, the levels of performance and detection bias were potentially high. However, most studies used validated outcome measures and the majority of case series were prospective and consecutive so the levels of assessment and selection bias were relatively low. Across all quality dimensions, four studies (two comparative studies (ii and iii) and two case series (xx and xxxiii) were judged to have a low threat of bias, while 15 were assessed to have a high threat of bias.
Efficacy
Comparative studies
The results of three studies directly comparing the addition of BKP to comparative medical care (iii, iv and viii) are summarised in Table 3. VAS pain was significantly reduced with BKP at 3, 6, 12 and 36 months follow up (P < 0.001). These reductions in pain were greater (P < 0.0001) than those observed at the same point in time with medical care treatment alone. This gain in pain relief was associated with a reduction in pain related physician office visits (P < 0.05) , with BKP compared to control. Two studies assessed functional capacity using the EVOS (iii) and Oswestry disability index (iv). Functional capacity improved at 6 and 12 months following BKP (P < 0.0001). This improvement exceeded that of standard medical care at 6 months in both studies (P < 0.001), but did not reach statistical significance at 12 months (P = 0.574). The study by Kasperk and colleagues (iii) reported an increase in the vertebral height of patients treated with BKP that was maintained at 6 and 12 months (P < 0.0001), while the vertebral height of comparison patients was further reduced. At 6 months’ follow up, the mean kyphotic angle with BKP (mean 10.4, SD 7.4°) was lower (P < 0.0001) than controls (mean 20.4, SD 13.4°). Five studies compared BKP to vertebroplasty (i, ii, v, vi and vii). The report of Nussbaum et al. (vi) did not report efficacy outcomes, while Masala et al. (v) failed to report outcome results separately for BKP and vertebroplasty groups (Table 4). Both BKP and vertebroplasty reduced VAS pain and improved Oswestry disability index score up to 24 months postprocedure with no significant differences between procedures. Grohs and Pflugmacher et al. (ii, vii) reported improvement in vertebral height and kyphotic angle with BKP at follow up. These improvements exceeded that of vertebroplasty treated patients (P < 0.05).
Table 3.
BKP versus conventional medical care: efficacy results
| Outcome follow up (studies) | Studies with data | Balloon kyphoplasty N mean (SD) or n/N (%) |
Conventional medical care N mean (SD) or n/N (%) |
Effect size* (95% CI) P value |
|---|---|---|---|---|
| Prepost pain (VAS mm) | ||||
| 3 months (viii) | 1 | 22 –6.7 (2.7) | 20 –2.2 (2.5) | MD −4.5 (−6.1 to −2.9) <0.0001 |
| 6 months (iii and iv) | 2 | 59 –3.3 (1.6) | 37 0.6 (0.86) | MD −1.6 (−2.0 to −1.2) <0.0001 |
| 12 months(iv) | 1 | 40 –1.8 (0.37) | 20 –0.07 (0.88) | MD −1.7 (−2.1 to −0.3) <0.0001 |
| 36 months (iii) | 1 | 40 –2.0 (NR) | 20 –0.43 (NR) | MD −1.6 <0.0001 |
| Opiate medication at follow up | ||||
| 6 months (iv) | 1 | 22/40 (55%) | 13/20 (65%) | RR 0.88 (0.57 to 1.37) 0.596 |
| Pre-post functional capacity | ||||
| 6 months (iii and iv) | 2 | ** | ** | SMD −1.2 (−1.7 to −0.8) <0.0001 |
| 12 months (iv) | 1 | 40 –10.7 (34)+ | 20 –4.5 (43)+ | MD −6.2 (−27.8 to 15.4) <0.574 |
| Health-related quality of life | 0 | Not reported | ||
| Prepost vertebral height (% original height) | ||||
| 6 months (iii) | 1 | 73^ 7.6 (22.4) | 41^ –2.7 (32.4) | MD 10.3 (2.3 to 18.3) 0.012 |
| 12 months (iii) | 1 | 73^ 7.5 (20.5) | 41^ –5.1 (32.4) | MD 12.6 (4.8 to 20.4) 0.002 |
| Pre-post kyphotic angle (°) | ||||
| 6-months (iii) | 1 | 73^ −0.4 (10.2) | 41^ 4.9 (10.4) | MD −5.3 (−9.3 to −1.3) 0.009 |
| Satisfaction at follow up | ||||
| 6 months (iii) | 1 | 13/19 (68%) | 2/17 (12%) | RR 5.8 (1.5 to 22.1) 0.01 |
| Days in hospital (viii) | 1 | 22 10.4 (7.4) | 20 20.4 (13.4) | MD −10 (−16.7 to −3.3) 0.003 |
| Physician pain-related office visits | ||||
| 6 months (iii) | 1 | 40 3.3 (9.5) | 20 8.6 (7.6) | MD −5.3 (−9.7 to – 0.18) 0.019 |
| 12 months (iii) | 1 | 40 5.3 (5.1) | 20 11.6 (12) | MD −6.3 (−11.8 to –0.8) 0.025 |
* Random effect model, test for heterogeneity P ≤ 0.05
MD mean difference, SMD standardised mean difference, RR relative risk, NR not reported
** Different scales, +negative score indicates improvement in functional capacity, ^N of VCF’s
Table 4.
BKP versus vertebroplasty: efficacy results
| Outcome follow up (studies) | Studies with data | Balloon kyphoplasty N mean (SD) |
Vertebroplasty N mean (SD) |
Effect size (95% CI) P value |
|---|---|---|---|---|
| Prepre pain (VAS mm) | ||||
| 6 months (vii) | 1 | 22 –6.3 (3.71) | 20 –6.2 (3.98) | MD 0.1 (−2.4 to 2.2) 0.933 |
| 12 months (ii and vi) | 2 | 50 –5.3 (3.9) | 49 3.9 (4.4) | MD −1.4 (−3.0 to 0.22) 0.091 |
| 24 months (ii) | 1 | 28 –5.4 (3.9) | 29 –3.2 (6.5) | MD −2.2 (−5.2 to 0.8) 0.154 |
| Opiate medication at follow up | 0 | Not reported | ||
| Prepost functional capacity | ||||
| 6 months (vi) | 1 | 22 –51 (38.4)+ | 20–42.2 (35.4)+ | MD −8.8 (−31.1 to 12.5) 0.440 |
| 12 months (ii and vi) | 2 | 40 –33 (35)+ | 43–19 (25)+ | MD −6.7 (−19.3 to 5.8) 0.862 |
| 24 months (ii) | 1 | 28 –6 (32.1)+ | 23–9 (32.7)+ | MD 3.0.0 (−14.9 to 20.9) 0.742 |
| Health-related quality of life | 0 | Not reported | ||
| Prepost vertebral height (% original height or mm) | ||||
| Pos-operative (ii) | 1 | 28^ 5.8 (7.8) | 23^ 0 (0.1) | MD 5.8 (2.9 to 8.70) <0.0001 |
| 6 months (vii) | 1 | 35^ 3 (11.8) | 32^ 1 (11.3) | MD 2.0 (−3.5 to 7.5) 0.479 |
| 12 months (vii) | 1 | 35^ 3 (11.8) | 32^ 1 (11.3) | MD 2.0 (−3.5 to 7.5) 0.479 |
| Pre-post kyphotic angle (°) | ||||
| Postoperative (ii) | 1 | 28^ –6 (7) | 23^ 0 (0.3) | MD −6 (−9 to −3) <0.0001 |
| 6 months (ii and vii) | 1 | 35^ –7 (9.9) | 32^ –1 (10.5) | MD −6 (−11 to −1) 0.016 |
| 12 months (vii) | 1 | 35^ –6 (9.9) | 32^ –1 (10.7) | MD −5 (−8 to −1) 0.048 |
| Satisfaction at follow up | 0 | Not reported | ||
| Days in hospital | 0 | Not reported | ||
| Physician pain-related office visits | 0 | Not reported | ||
* Random effect model, test for heterogeneity P ≤ 0.05
MD mean difference, SMD standardised mean difference, RR relative risk
** Different scales, therefore not poolable, +negative score indicates improvement in functional capacity, ^ N° of VCF’s
Case series
All case series consistently reported a reduction in pain after BKP. In 14 studies reporting on VAS pain before and after BKP, a mean reduction of 5.4 mm (95% CI −6.3 to −4.4 mm, P < 0.0001, random effects) was observed (Table 5). Four studies reported their functional capacity findings to allow pooling. As these studies used a variety of outcome measures; Oswestry disability index (xi, xxiv and xxv), Roland Morris (xxxvi) questionnaire and the Index of back Function (xviii), they were pooled using standardised mean differences. Functional capacity was seen to improve by an average of 1.1 standard deviation units (95% CI 0.6–1.5, P < 0.0001, random effects) following BKP. Five studies assessed health related quality of life using the Short-Form 36 (SF-36) (xiv, xi, xviii, xxiv and xxviii) with significant improvements being seen in 6 of the 8 SF-36 domains. The studies that reported vertebral height (ix, x, xii, xiii, xvii, xxxiv, xxxvi, xxxix, xxxvii and xliii) and kyphotic angle correction (xxvi, xviii, xxxix, xliii and xli) consistently reported an effect significantly in favour of BKP. Across studies, there was an average improvement in vertebral height of 21% and a reduction of 6.3° in kyphotic angle.
Table 5.
Summary of BKP case series: efficacy results
| N | Mean (95% CI)* P value | Heterogeneity P value | |
|---|---|---|---|
| Change in pain (VAS 0–10 mm) | 14 | MD −5.4 mm (95% CI −6.3 to −4.4)* P < 0.0001 | <0.0001 |
| Change in functional capacity (different scales) | 5 | SMD 1.0 (0.6 to 1.5)* <0.0001 | <0.0001 |
| Change in quality of life (SF-36 0–100) | |||
| Physical functioning | 5 | MD −21 (−28 to −14)* <0.0001 | 0.036 |
| Role physical | 4 | MD −31 (−42 to −20)* <0.0001 | 0.009 |
| Bodily pain | 5 | MD −29 (−38 to −19)* <0.0001 | <0.0001 |
| General health | 5 | MD +4 (−2 to 10)* 0.266 | 0.013 |
| Vitality | 5 | MD −11 (−14 to −8) <0.0001 | 0.146 |
| Social functioning | 5 | MD −29 (−39 to −18)* <0.0001 | 0.001 |
| Role emotional | 4 | MD −18 (−39 to 4)* 0.109 | <0.0001 |
| Mental health | 5 | MD −11 (−17 to −5)* <0.0001 | 0.008 |
| Satisfaction at follow up (%) | 2 | MD 98 (90 to 100) <0.001 | 0.973 |
| Change in vertebral height (% original height) | 9 | MD 21 (15 to 26)* <0.0001 | <0.0001 |
| Change in kyphotic angle (°) | 12 | MD −6.3 (−5.8 to −6.7) <0.0001 | 0.826 |
* Random effects model
MD mean difference, SMD standardised mean difference
Further pooling of outcomes could not be performed, as many studies reported their results narratively or failed to report measures of variance such as standard deviations, intra-quartile ranges or maximum and minimum values.
Safety
Safety (or adverse) outcomes of BKP were combined for comparative studies and case series. Given the differences in follow up durations, results are expressed as both proportions and rates (see Table 6).
Table 6.
Summary of BKP comparative and case series: safety results
| N | Number of events probability (95% CI)** | Rate events per 1,000 patient or fracture years) | |
|---|---|---|---|
| Cement leakages* | |||
| Overall | 31 | 193/2,239 9.0% (7.4 to 11.0%)** |
81 |
| Symptomatic | 7 | 1/678 0.2% (0 to 0.3%)** |
0.9 |
| New vertebral fractures+ | |||
| Overall | 16 | 172/1,151 13.6% (9.0 to 20.7%)** |
111 |
| Adjacent | 10 | 110/871 13.8% (11.0 to 17.4%)** |
94 |
| Adverse events+ | |||
| Pulmonary embolism | 7 | 1/377 0.10% (0 to 0.17%) |
1.7 |
| Spinal cord compression | 8 | 1/431 0.2% (0 to 0.8%) |
1.6 |
| Nerve root pain/radiculopathy | 9 | 2/173 0.40% (0 to 1.2%)* |
1.7 |
| Mortality | |||
| Overall | 14 | 35/552 3.2% (0.7% to 5.6%)** |
44 |
| Peri-operative^ | 11 | 1/406 0.01% (0% to 0.64%) |
1.3 |
NA not applicable
* No. of events per vertebrae, +no. of events per patient, ++number of events/fracture/year or number of events/patient/year, ** random effects meta-analysis
A total of 28 studies provided details on the number of cement leakages and eight of these reported whether these leaks were symptomatic or not. A total of 189 (9.0%) cement leakages were reported in 2,239 vertebrae that underwent BKP. This corresponds to 81 cement leaks per 1,000 fractures undergoing BKP per year. One leak (0.001%) was reported to be symptomatic. In the case series of Majd et al. (2005), cement leakage resulted in a L1 radiculopathy, the patient recovered following nerve block and rehabilitation (xxx). A total of 171 new or incident fractures were reported in 1,151 patients across 16 studies, 110 (64%) of which occurred in the vertebrae adjacent to the procedure. This corresponds to 111 new fractures per year, per 1,000 individuals undergoing BKP.
Both Kasperk (iii) and Komp (iv) document the number of new vertebral fractures after BKP, compared to conventional medical care. These studies indicate that the level of new fractures with BKP to be significantly lower than those experienced by patients in the control group (relative risk 0.35, 95% CI 0.16–0.78, P = 0.01) at 1-year follow up. The study of Grohs et al. (ii) directly compared the incident fracture levels in BKP and vertebroplasty. At 3–4 months follow up, new fractures were seen in nine out of 58 (15.5%) patients receiving BKP, compared with one out of 40 (2.5%) patients receiving vertebroplasty. This difference was not statistically significant (P = 0.081).
The rates of serious adverse events reported with BKP are low. The overall rate of mortality of 3.2% reflects both the age of patients undergoing BKP, as well as the inclusion of patients with cancer. The peri-operative mortality rate was 0.01%. Based on the results of this review, for every 1,000 patients treated with BKP each year, 1.7 patients could experience a pulmonary embolism, 1.6 patients would experience a spinal cord compression, 1.7 patients could experience a radiculopathy, and 1.3 could die within the peri-operative period (30 days).
Heterogeneity/subgroup analysis
A substantial level of statistical heterogeneity was observed in both the level of pain relief (χ2 1195.56, df 17, P < 0.0001) and cement leaks (Q = 171.0, df 27, P < 0.0001) across studies. This statistical heterogeneity may reflect the variation in patient populations, differing periods of follow up, and methodological quality of studies. The results of the exploration of this heterogeneity are shown in Table 7.
Table 7.
Exploration of heterogeneity (subgroup analysis): change in VAS pain and cement leakage
| Pain relief | Univariate P value | Multivariate P value | Cement leakage | Univariate P value | Multivariate P value |
|---|---|---|---|---|---|
| Average pre/post mean VAS (n = 18) | 0.059 | 0.104 | |||
| Patient indication (osteoporosis vs. neoplastic) (n = 18) | 0.915 | 0.723 | Patient indication (osteoporosis vs. neoplastic) (n = 28) | <0.0001 | 0.013 |
| Average duration of pain or fracture duration (months) (n = 8) | 0.047 | Insufficient data | Average duration of pain or fracture duration (months) (n = 12) | 0.420 | Insufficient data |
| Continent of data collection (USA vs. non USA) (n = 18) | 0.735 | 0.719 | Continent of data collection (USA vs. non USA) (n = 28) | 0.553 | 0.744 |
| Study setting (single vs. multicentre) (n = 18) | 0.610 | 0.699 | Study setting (single vs. multicentre) (n = 28) | 0.641 | 0.544 |
| Publication year (n = 18) | 0.186 | 0.149 | Publication year (n = 28) | 0.409 | 0.731 |
| Average duration of follow up (months) (n = 18) | 0.889 | 0.655 | Average duration of follow up (months) (n = 28) | 0.148 | 0.325 |
| Study sample size (n = 16) | 0.760 | 0.996 | Study sample size (n = 31) | 0.118 | 0.442 |
| Study design (low bias vs. not) (n = 18) | 0.433 | 0.840 | Study quality (low bias vs. not) (n = 28) | 0.671 | 0.874 |
| Study design (case series vs. comparative) (n = 18) | 0.592 | 0.848 | Study design (case series vs. comparative) (n = 28) | 0.294 | 0.732 |
The only factor to show a significant association with the magnitude of BKP pain relief was the combined variable summarising the duration of pain or fracture age (P = 0.047). The longer the duration of pain/older the fracture, the smaller the magnitude of pain relief following BKP (correlation coefficient, r = −0.49). No factors were significant in multivariate analysis. Osteoporotic VCFs appeared to be associated with a higher rate of cement leakage with BKP compared to neoplastic VCFs (13.6 vs. 6.6%) both in univariate (P < 0.0001) and multivariate analysis (P = 0.013).
A small number of studies were identified that had undertaken a within study subgroup analysis (Table 8). There was little consistent evidence of an association between patient characteristics and BKP outcome.
Table 8.
Within study subgroup analyses
| Subgroup | Outcome measure | Analysis method | Conclusion | |
|---|---|---|---|---|
| Crandall (2004) | ‘Acute’ (<10 weeks old) versus chronic’ fractures (> 4 months old). | VAS pain post BKP ODI post BKP Kyphotic angle post BKP Vertebral height change |
t test | = = = Acute > chronic |
| Garfin (2003/6) | Non recent (>60 days) versus recent (<60 days) fractures | Change in pain Change in Index of back function Change in SF-36 Change in satisfaction |
ANOVA | = |
| Lane (2004) | Indication (osteoporosis vs. multiple myeloma) | Change in ODI Change in vertebral height (absolute) Change in vertebral height (%) Cement leakage |
t test | = = Osteoporosis > multiple myeloma = |
| Majd (2005) | Age of fracture | Change in VAS pain Change in vertebral height |
ANOVA | = = |
ODI Oswestry disability index, ANOVA analysis of variance, =no statistically significant difference between subgroups, >subgroup A has statistically significant superior outcome than subgroup B
Publication bias
There was evidence of significant funnel plot asymmetry for the each of the outcomes with a sufficient number of studies, i.e. VAS relief (P = 0.001), cement leakage (P = 0.004), and incident vertebral fractures (P = 0.005). Asymmetry can indicate publication bias (i.e. the omission of studies that are more negative in their conclusions). However, a number of other factors can cause asymmetry including the poor methodological quality of smaller studies, true heterogeneity; size of effect differs according to study size (for example, due to differences in the intensity of interventions, differences in underlying risk between studies of different sizes) or chance [4].
Discussion
Findings
This update review provides important new findings. First, a number of comparative studies of BKP have recently been published. As commented in a recent editorial, the availability of high quality direct (‘head-to-head’) comparative evidence is central in confirming BKP’s efficacy as seen in case studies [11]. There are now prospective studies of low bias, with follow up of 12 months or more, each of which have demonstrated BKP to be more effective than medical management of osteoporotic VCFs and that BKP is as least as effective as vertebroplasty (ii, iii).
Second, it has been suggested that a major adverse outcome of BKP could be an increase in the rate of incident fractures, particularly in those vertebrae adjacent to the treated fractures [6]. However, this observation is based on indirect comparison of the findings of BKP case series with natural history cohorts, where the case mix of the populations may be quite different. Using prospective direct comparative evidence, we, on the contrary, found a reduction (relative risk 0.35, 95% CI 0.16–0.78) in incident fractures in the 12 months following BKP compared to conventionally treated patients.
Third, an increased body of evidence provides the opportunity to comment more definitely on population factors that might be associated with the level of benefit or harm of BKP. It appears that the magnitude of pain relief following BKP is higher in studies recruiting either individuals with young fractures, and shorter periods of fracture-related pain compared to studies with older fractures or long durations of pain.
Finally, the low risk of complications identified in published studies in this review is in contrast to the retrospective analysis of the FDA safety database by Nussbaum et al. [13] that reported 21 serious adverse events associated with balloon kyphoplasty during the period 1999 to June 2003. This later analysis has received considerable subsequent criticism on its poor methodology, particularly the non-mandatory basis of reporting required by the FDA safety database and the inability to determine the true denominator (events or patients) to which the number of events can apply [12].
The precise mechanism by which BKP facilitates pain relief and improves the functionality of patients remains to be elucidated. It is often argued that BKP might be superior to other inventions, including vertebroplasty and non-operative care, as it works through the recovery of vertebral body height, which, in turn, improves vertebral alignment and, therefore, whole body function [7]. However, the evidence for the association between morphological changes and patient outcomes is limited. Kasperk et al. (iii), found no significant relationship between the change in vertebral height and the change in VAS pain. Similarly, although Crandall and Garfin both found recent fractures were more likely to lead to a gain in vertebral height than older fractures, there was no difference in pain relief between the two groups (xii and xviii). However, these studies were likely to be underpowered to detect such differences. Given the fact that our review was not a mechanistic one, we did have the opportunity to examine the association between the average morphological changes and average change in pain relief. We found some evidence of moderate correlations between the change in VAS pain with the change in vertebral height (r = 0.62, P = 0.184) and change in kyphotic angle (r = −0.68, P = 0.09). Given, that our analysis is at a study level, we acknowledge it is likely to have low power and also liable to confounding. Therefore this explains why a definite association between vertebral height and pain relief cannot be established from this analysis.
Strengths and limitations
The principal strength of this review is its comprehensiveness. We undertook exhaustive searches of the literature and sought all published and unpublished evidence. Inevitably, any review can be subject to publication bias, i.e. studies with ‘positive’ results are more likely to be reported and published, while side effects and adverse events are more likely to be underreported.
We recognise there are limitations to this study, both in its methods and also the nature of the evidence identified. Given the high level of statistical heterogeneity in both pain relief and cement leakage following BKP, we sought to explore this based on study level data (e.g. the mean age of patients, the proportion of males, the average duration of fracture pain). However, in view of the relatively limited number of included studies with numerical data, we recognise that it has limited power risk to identifying subgroup relationships. Nevertheless, the finding of few, if any, significant subgroups from our between-group analysis, was consistent within the identified study analyses.
The principal limitation in the interpretation of the findings of this review was the absence of randomised controlled trial (or ‘level I’) evidence. However, it is important to point out that although case series studies are relatively low in the hierarchy of evidence, well-conducted and adequately reported studies can provide useful data on ‘real world’ effectiveness and the safety of the procedure. Furthermore, as discussed above, this review has identified a growing number of direct, albeit non-randomised, comparative studies. It is recognised that because of non-random allocation of patients to intervention and control, studies are prone to substantial selection bias and confounding. Nevertheless, we sought to identify those comparative studies, where the risk of bias might be low, i.e. prospective studies that sought consecutive patients with evidence of similarity in baseline characteristics between comparisons groups, independent outcome assessment and low losses to follow up. We excluded some papers that described a BKP technique that might be considered as non-routine.
Implications for further research
This review has identified the need for long-term prospective studies in patients with neoplastic VCFs directly comparing BKP with both conventional medical care and also with vertebroplasty. Three randomised controlled trials of BKP are registered and currently underway (see Table 9). There is an increasing need to identify those patients who could gain most from BKP and, therefore, represent the most cost effective use of healthcare resources. Potential subgroups that deserve particular consideration include the age of the fracture, multiple compared to single fractures, and degree of morphological dysfunction.
Table 9.
Registered ongoing randomised controlled trials of BKP
| Trial name trial registration # | ‘FREE’ NCT00211211 | ‘CAFÉ’ NCT00211237 | ‘CEEP’ NCT00279877 | KAVIAR NCT00323609 |
|---|---|---|---|---|
| Intervention | Balloon kyphoplasty | Balloon kyphoplasty | Balloon kyphoplasty | Balloon kyphoplasty |
| Comparator | Medical therapy | Medical therapy | Vertebroplasty | Vertebroplasty |
| Indication | Vertebral body compression fractures (VCF) due to primary or secondary osteoporosis, multiple myeloma or osteolytic metastatic tumours | Painful vertebral body compression fractures (VCF) in cancer patients including multiple myeloma, metastatic breast and lung cancer | Painful osteoporotic compression fractures | Painful osteoporotic compression fractures |
| Primary outcome(s) | Quality of life (SF-36) | Pain (VAS), disability (Roland–Morris) and safety | Pain (Roland scale) | Proportion with subsequent fracture |
| Secondary outcomes | Pain, functional capacity, vertebral height, spinal deformity, healthcare resources, safety, cost effectiveness | Disability, quality of life, back pain, ambulatory status, vertebral height | Quality of life, functional capacity, healthcare care resources, safety, cost effectiveness | Change in back pain; back function; quality of life; rate of serious adverse events; change in vertebral body height and angular deformity; VCF-related health care utilization |
| Sample size* | 300 | 200 | 112 | 1,234 |
| Follow up | Up to 2-years | Up to 1-year | Up to 2-years | Up to 2-years |
| Setting | Europe Multicentre |
US/Europe Multicentre |
US Multicenter |
Worldwide Multicenter |
| Recruitment Start date Expected end date |
February 2003 December 2005 |
May 2005 Not known |
31st May 2006 31st May 2007 |
August 2006 August 2011 |
| Principal investigator | Late Professor Oloff Johnell UMAS University hospital Dept. of Orthopedics 20502 Malmö, Sweden |
Prof. M. Hussein Cleveland Clinical Myeloma Research Center 9500 Euclid-A, Cleveland OH 44195, USA |
Dr. Avery Evans Mayo Clinic 200 1str Street SW, Rochester MN 55905, USA |
Dr Jacques Dion, Emory University-Department of Interventional Neuroradiology, 1364 Clifton Road, NE Atlanta, G |
| Funder | Kyphon | Kyphon | Mayo Clinic, Cardinal, ArthroCare Corporation, Cook | Kyphon |
* Total number of intervention and control patients
Conclusions
There are now prospective studies of low bias, with follow-up of 12 months or more, which demonstrate balloon kyphoplasty to be more effective than medical management of osteoporotic vertebral compression fractures and as least as effective as vertebroplasty. Results from ongoing RCTs will provide further information in the near future.
Appendix
1. Included studies
Comparative
i. Fourney DR, Schomer DF, Nader R et al (2003) Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg (Spine 1) 98:21–30E2
ii. Grohs JG, Matzner M, Trieb K, Krepler P (2005) Minimal invasive stabilization of osteoporotic vertebral fractures: a prospective nonrandomised comparison of vertebroplasty and balloon kyphoplasty. J Spinal Disord Tech 18:238–242
iii. (i) Kasperk C, Hillmeier J, Noldge G, Grafe IA, Dafonseca K, Raupp D, Bardenheuer H, Libicher M, Liegibel UM, Sommer U, Hilscher U, Pyerin W, Vetter M, Meinzer HP, Meeder PJ, Nawroth P, Taylor RS (2005) Treatment of painful vertebral fractures by kyphoplasty in patients with primary osteoporosis: a prospective nonrandomised controlled study. J Bone Miner Res 20:604–612
(ii) Grafe IA, Da Fonseca K, Hillmeier J, Meeder PJ, Libicher M, Noldge G, Bardenheuer H, Pyerin W, Basler L, Weiss C, Taylor RS, Nawroth P, Kasperk C (2005) Reduction of pain and fracture incidence after kyphoplasty: 1-year outcomes of a prospective controlled trial of patients with primary osteoporosis. Osteoporos Int 16:2005–2012
(iii) Grafe I, DeFonseca K, Hillmeier J, Meeder P-J, Libicher M, Nodge G, ommer M, Hilscher U, Naworth P, Kasperk C. Kyphoplasty persistently reduces pain in patients with osteoporotic vertebral compression fractures—3 year outcome of a prospectively controlled cohort study. IOF World Congress of Osteoporosis. 2nd June 2006, Toronto, Canada
iv. Komp M, Ruetten S, Godolias G (2004) Minimally invasive therapy for functionally unstable osteoporotic vertebral fracture by means of kyphoplasty: prospective comparative study of 19 surgically and 17 conservatively treated patients. J Miner Stoffwechs 11(Suppl 1):13–15
v. Masala S et al (2004) Vertebroplasty and kyphoplasty in treatment of malignant vertebral fractures. J Chemotherapy 16(Suppl 5):30–33
vi. Nussbaum DA, Gailloud P, Murphy K (2004) A review of complications associated with vertebroplasty and kyphoplasty as reported to the Food and Drug Administration medical device related web site. J Vas Interven Radiol 15:1185–1192
vii. Pflugmacher R, Kandziora K, Schröder R, Schleicher P, Scholz M, Schnake Haas K, Khodadadyan-Klostermann C (2005) Vertebroplasty and kyphoplasty in osteoporotic fractures of vertebral bodies—a prospective 1-year follow-up analysis. Fortschr Röntgenstr 177:1670–1676
viii. Weisskopf M, Herlein S, Birnbaum K et al (2003) Kyphoplasty—a new minimal invasive treatment for repositioning and stabilising vertebral bodies. Zeit fur Orthop und Inre Grenz 141:406–411
Case series
ix. Atalay B, Caner H, Gokce C, Altinors N (2005) Kyphoplasty: 2 years of experience in a neurosurgery department. Surg Neurol 64(Suppl 2):S72–S76
x. Buisson T, Beaudic Y, Godard J, Czorny A, Grumblat A (2005) Fractures du rachis traitees par cyphoplastie avec ballonets. Etude preliminary sur 6 cas. La Revue de l’ADPHSO 30:79–83
xi. Coumans JV, Reinhardt M-K, Lieberman IH (2003) Kyphoplasty for vertebral compression fractures: 1-year clinical outcomes from a prospective study. J Neurosurg 99(Suppl 1): 44–50
xii. Crandall D, Slaughter D, Hankins PJ, Moore C, Jerman J (2004) Acute versus chronic vertebral compression fractures treated with kyphoplasty: early results. Spine J 4:418–424
xiii. Darius T, Vanderschot P, Broos P (2003) Balloon kyphoplasty: a new treatment option for painful osteoporotic vertebral body compression fractures. Tijdschrift voor Geneekuunde 59:1141–1152
xiv. Dudeney S, Lieberman IH, Reinhardt M-K et al (2002) Kyphoplasty in the treatment of osteolytic vertebral compression fractures as a result of multiple myeloma. J Clin Oncol 20:2382–2387
xv. Feltes C, Fountas KN, Machinis T, Nikolakakos LG, Dimopoulos V, Davydov R, Kassam M, Johnston KW, Robinson JS (2005) Immediate and early postoperative pain relief after kyphoplasty without significant restoration of vertebral body height in acute osteoporotic vertebral fractures. Neurosurg Focus 18:e5
xvi. Fribourg D, Tang C, Sra P, Delamarter R, Bae H (2004) Incidence of subsequent vertebral fracture after kyphoplasty. Spine 29:2270–2276
xvii. Gaitanis IN, Hadjipavlou AG, Katonis PG, Tzermiadianos MN, Pasku DS, Patwardhan AG (2005) Balloon kyphoplasty for the treatment of pathological vertebral compressive fractures. Eur Spine J 14:250–256
xviii. (i) Garfin SR. A multi-center postmarketing registry to assess outcomes of treatment of vertebral body compression fractures with an inflatable bone tamp. Final report 2003 (Kyphon registry report—unpublished)
(ii) Garfin SR, Buckley RA, Ledlie J (2006) Balloon kyphoplasty for symptomatic vertebral body compression fractures results in rapid, significant, and sustained improvements in back pain, function, and quality of life for elderly patients. Spine (in press)
xix. Grohs JG, Krepler P (2004) Minimal-invasive stabilisierung osteoporotischer wirbelkorpereinbruche. Radiologe 44:254–259
xx. Harrop JS, Prpa B, Reinhardt MK, Lieberman I (2004) Primary and secondary osteoporosis’ incidence of subsequent vertebral compression fractures after kyphoplasty. Spine 29:2120–2125
xxi. Hillmeier J, Meeder PJ, Noldge G et al (2003) Minimal invasive reduction and internal stabilisation of osteoporotic vertebral body fracture. Oper Orthop Traumatol 4:343–362
xxii. Hillmeier J, Grafe I, Da Fonseca K, Meeder PJ, Noldge G, Libicher M, Kock HJ, Haag M, Kasperk C (2004) The evaluation of balloon kyphoplasty for osteoporotic vertebral fractures. An interdisciplinary concept. Orthopade 33:893–904
xxiii. Khanna AJ, Reinhardt MK, Togawa D, Lieberman IH (2006) Functional outcomes of kyphoplasty for the treatment of osteoporotic and osteolytic vertebral compression fractures. Osteoporos Int 17:817–826
xxiv. Kasperk C et al (2003) Kyphoplastie—Konzept zur behandlung schmerzhafter wirbelkorperbruch. Deutsches Afzteblatt 100:1748–1753
xxv. Lane JM, Hong R, Koob J, Kiechle T, Niesvizky R, Pearse R, Siegel D, Poynton AR (2004) Kyphoplasty enhances function and structural alignment in multiple myeloma. Clin Orthop Relat Res 426:49–53
xxvi. (i) Ledlie JT, Renfro MB (2005) Decreases in the number and severity of morphometrically defined vertebral deformities after kyphoplasty. Neurosurg Focus 18:e4
(ii) Ledlie JT, Renfro M (2003) Balloon kyphoplasty: one-year outcomes in vertebral body height restoration, chronic pain and activity levels. J Neurosurg (Spine 1) 98(Suppl 1):36–42
(iii) Ledlie JT, Refro MB (2006) Kyphoplasty treatment of vertebral fractures: 2-year outcomes show sustained benefit. Spine 31:57–64
xxvii. Lieberman IH, Dudeney S, Reinhardt M-K et al (2001) Initial outcome and efficacy of “kyphoplasty” in the treatment of painful osteoporotic vertebral compression fractures. Spine 26:1631–1638
xxviii. Lieberman I, Reinhardt MK (2003) Vertebroplasty and kyphoplasty for osteolytic vertebral collapse. Clin Orthop Relat Res 415S:S176–S186
xxix. Libicher M, Vetter M, Wolf I, Noeldge G, Kasperk C, Grafe I, Da Fonseca K, Hillmeier J, Meeder PJ, Meinzer HP, Kauffmann GW (2005) CT volumetry of intravertebral cement after kyphoplasty. Comparison of polymethylmethacrylate and calcium phosphate in a 12-month follow-up. Eur Radiol 13:1544–1549
xxx. Majd ME, Farley S, Holt R (2005) Preliminary outcomes and efficacy of the first 360 consecutive kyphoplasties for the treatment of painful osteoporotic vertebral compression fractures. Spine J 5:244–255
xxxi. Masala S, Cesaroni A, Sergiaconi G, Fiori R et al (2004) Percutaneous kyphoplasty: new treatment for painful vertebral body fractures. In Vivo 18:149–154
xxxii. Masala S, Fiori R, Massari F, Simonetti G (2005) Kyphoplasty: indications, contraindications and technique. La Radiologia Medica 110:97–105
xxxiii. Phillips FM, Wetzel FT, Lieberman I et al (2002) An in vivo comparison of the potential for extravertebral cement leak after vertebroplasty and kyphoplasty. Spine 27:2173–2178
xxxiv. Phillips FM, Ho E, Campbell-Hupp M et al (2003) Early radiographic and clinical results of balloon kyphoplasty for the treatment of osteoporotic vertebral compression fractures. Spine 28
xxxv. Pradhan BB, Bae HW, Kropf MA, Patel VV, Delamarter RB (2006) Kyphoplasty reduction of osteoporotic vertebral compression fractures: correction of local kyphosis versus overall sagittal alignment. Spine 31:435–441
xxxvi. Rhyne A, Banit D, Laxer E, Odum S, Nussman D (2004) Kyphoplasty: report of eighty-two thoracolumbar osteoporotic vertebral fractures. J Orthop Trauma 18:294–299
xxxvii. Tang H, Lu Y, Wang BQ, Chen H (2005) The study of treatment of osteoporotic thoracolumbar multi-vertebral compressive fractures by kyphoplasty with one balloon. Zhonghua Wai Ke Za Zhi 43:1568–7151
xxxviii. Theodorou DJ, Theodorou SJ, Duncan TD et al (2002) Percutaneous balloon kyphoplasty for the correction of spinal deformity in painful vertebral compression fractures. J Clin Imaging 26:1–5
xxxix. Villavicencio AT, Burneikiene S, Bulsara K, Thramann JT (2005) Intraoperative three-dimensional fluoroscopy-based computerized tomography guidance for percutaneous kyphoplasty. Neurosurg Focus 18:E3
xl. Voggenreiter G, Brocker K, Düber C, Obertacke U, Röhrl B, Sadik M (2004) Behandlungsergebnisse der Ballonkyphoplastik bei osteoporotischen Wirbelkörperfrakturen im höheren Lebensalter. J für Mineralstoffwechsel 11:12–14
xli. Voggenreiter G (2005) Balloon kyphoplasty is effective in deformity correction of osteoporotic vertebral compression fractures. Spine 30:2806–2812
xlii. Wilhelm K, Stoffel M, Ringel F et al (2003) Preliminary experience with balloon kyphoplasty for the treatment of painful osteoporotic fractures. Rofo Fortschritte auf dem Gebeite der Rontgenstrahlen und der Neuen Bildgebenden Verfahren 175:1690–1696
xliii. Yang HL, Niu GQ, Liang DC, Wang GL, Meng B, Chen L, Lu J, Zhou Y, Mao HQ, Zhao LJ, Liu XY, Gu XH, Ni CF, Tang TS (2004) The contrast study between single and double balloon bilateral dilatation of kyphoplasty Chin J Surg 42:1299–1302
2. Studies excluded on the basis of full paper
Acosta FL Jr. Aryan HE. Taylor WR. Ames CP (2005) Kyphoplasty-augmented short-segment pedicle screw fixation of traumatic lumbar burst fractures: initial clinical experience and literature review. Neurosurg Focus 18:e9. (burst fractures and combined treatment)
Berlemann U, Franz T, Orler R, Heini PF (2004) Kyphoplasty for treatment of osteoporotic vertebral fractures: a prospective non-randomised study. Eur Spine J 13:496–501 (open procedure and traumatic fractures)
Heini PF, Orler R (2004) Kyphoplasty for treatment of osteoporotic fractures. Eur Spine J 13:184–192 (duplicate publication)
Boszczyk BM, Bierschneider M, Hauck S, Vastmans J, Potulski M, Beisse R, Robert B, Jaksche H (2004) Conventional and semi-open kyphoplasty. Orthopede 33:13–21 (review and open procedure)
Boszczyk BM et al (2004) Microsurgical interlaminary vertebro- and kyphoplasty for severe osteoporotic fractures. J Neurosurg (Spine 1) 100:32–37 (open procedure)
de Falco R, Scarano E, Di Celmo D, Grasso U, Guarnieri L (2005) Balloon kyphoplasty in traumatic fractures of the thoracolumbar junction. Preliminary experience in 12 cases. J Neurosurg Sci 49:147–153 (traumatic fractures)
Deen HG, Nottmeier EW (2005) Balloon kyphoplasty for treatment of sacral insufficiency fractures. Report of three cases. Neurosurg Focus 18(3):e7 (type of fracture)
Deen HG, Aranda-Michel J, Reimer R, Putzke JD (2005) Preliminary results of balloon kyphoplasty for vertebral compression fractures in organ transplant recipients. Neurosurg Focus 18(3):e6 (patient population)
Gerszten PC, Germanwala A, Burton SA, Welch WC, Ozhasoglu C, Vogel WJ (2005) Combination kyphoplasty and spinal radiosurgery: a new treatment paradigm for pathological fractures. Neurosurg Focus 18(3):e8 (combination treatment)
Hentschel SJ, Burton AW, Fourney DR, Rhines LD, Mendel E (2005) Percutaneous vertebroplasty and kyphoplasty performed at a cancer center: refuting proposed contraindications. J Neurosurg Spine 2:436–440 (balloon kyphoplasty and vertebroplasty outcomes reported combined)
Ronge R (2005) Complications after vertebroplasty and kyphoplasty–cement emboli recognized on typical radiographic images. Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 17:934. (no data reported)
Tomita S, Kin A, Yazu M, Abe M (2003) Biomechanical evaluation of kyphoplasty and vertebroplasty with calcium phosphate cement in a simulated osteoporotic compression fracture. J Orthop Sci 8:192–197 (cadaver)
Palussière J, Berge J, Gangi A, Cotton A, Pasco1 A, Bertagnoli R, Jaksche1 H, Carpeggiani P, Deramond H (2005) Clinical results of an open prospective study of a bis-GMA composite in percutaneous vertebral augmentation. Eur Spine J 14:982–991 (inappropriate intervention)
Schulte BU, Brucher D, Trompeter M, Remy C, Reimer P (2006) Balloon-assisted percutaneous vertebroplasty in patients with osteoporotic vertebral body compression fractures–first results Rofo 178:207–213. (inappropriate intervention)
Verlaan J-J, Dhert WJA, Verbout AJ Oner FCS ((2005) Balloon vertebroplasty in combination with pedicle screw instrumentation: a novel technique to treat thoracic and lumbar burst fractures. Spine 30:e73–e79 (burst factures)
Wong W, Mathis JM (2005) Vertebroplasty and kyphoplasty: techniques for avoiding complications and pitfalls Neurosurg Focus 18:E2 (hypothetical cases)
Footnotes
This report has been undertaken through unrestricted funding by Kyphon Inc. The planning, conduct and conclusions of this report are made independently from the company.
References
- 1.Borgstrom F, Zethraeus N, Johnell O, Lidgren L, Ponzer S, Svensson O, Abdon P, Ornstein E, Lunsjo K, Thorngren KG, Sernbo I, Rehnberg C, Jonsson B. Costs and quality of life associated with osteoporosis-related fractures in Sweden. Osteoporos Int. 2006;17:637–650. doi: 10.1007/s00198-005-0015-8. [DOI] [PubMed] [Google Scholar]
- 2.Bouza C, Lopez T, Magro A, Navalpotro L, Amate JM. Efficacy and safety of balloon kyphoplasty in the treatment of vertebral compression fractures: a systematic review. Eur Spine J. 2006;21:1–18. doi: 10.1007/s00586-005-0048-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997;315:153–157. doi: 10.1136/bmj.315.7121.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finnern HW, Sykes DP. The hospital cost of vertebral fractures in the EU: estimates using national datasets. Osteoporos Int. 2003;14:429–436. doi: 10.1007/s00198-003-1395-2. [DOI] [PubMed] [Google Scholar]
- 6.Fribourg D, Yang C, Sra P, Delamarter R, Bae H. Incidence of subsequent vertebral fractures after kyphoplasty. Spine. 2004;29:2270–2276. doi: 10.1097/01.brs.0000142469.41565.2a. [DOI] [PubMed] [Google Scholar]
- 7.Garfin SR, Yuan HA, Reiley MA. New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine. 2001;26:1511–1515. doi: 10.1097/00007632-200107150-00002. [DOI] [PubMed] [Google Scholar]
- 8.Garfin SR, Buckley RA, Ledlie J (2006) Balloon kyphoplasty for symptomatic vertebral body compression fractures results in rapid, significant, and sustained improvements in back pain, function, and quality of life for elderly patients. Spine (in press) [DOI] [PubMed]
- 9.Hadjipavlou AG, Tzermiadianos MN, Katonis PG, Szpalski M. Percutaneous vertebroplasty and balloon kyphoplasty for the treatment of osteoporotic vertebral compression fractures and osteolytic tumours. J Bone Joint Surg Br. 2005;87:1595–1604. doi: 10.1302/0301-620X.87B12.16074. [DOI] [PubMed] [Google Scholar]
- 10.Higgins JPT, Green S (2005) Cochrane handbook for systematic reviews of interventions 4.2.5 (updated May 2005). In: The Cochrane library, issue 3. Wiley, Chichester, http://www.cochrane.org/resources/handbook/ (last accessed 2nd January 2006)
- 11.Jarvik JG, Kallmes DF, Deyo RA. Kyphoplasty: more answers or more questions? Spine. 2006;31:65–66. doi: 10.1097/01.brs.0000192686.23902.06. [DOI] [PubMed] [Google Scholar]
- 12.Lieberman IH, Phillips FM, Togawa D, Modic M, Masaryk T, Obuchowski N, Slipman CW. Vertebral augmentation and the limits of interpreting complications reported in the Food and Drug Administration Manufacturer and User Facility Device Experience Database. J Vasc Interv Radiol. 2004;15:1193–1196. doi: 10.1097/01.RVI.0000136028.86147.A4. [DOI] [PubMed] [Google Scholar]
- 13.Nussbaum DA, Gailloud P, Murphy K. A review of complications associated with vertebroplasty and kyphoplasty as reported to the Food and Drug Administration medical device related website. J Vasc Interv Radiol. 2004;15:1185–1192. doi: 10.1097/01.RVI.0000144757.14780.E0. [DOI] [PubMed] [Google Scholar]
- 14.Ray NF, Chan JK, Thamer M, Melton LJ., III Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12:24–35. doi: 10.1359/jbmr.1997.12.1.24. [DOI] [PubMed] [Google Scholar]
- 15.Roy DK, O’Neill TW, Finn JD, Lunt M, Silman AJ, Felsenberg D, Armbrecht G, Banzer D, Benevolenskaya LI, Bhalla A, Bruges Armas J, Cannata JB, Cooper C, Dequeker J, Diaz MN, Eastell R, Yershova OB, Felsch B, Gowin W, Havelka S, Hoszowski K, Ismail AA, Jajic I, Janott I, Johnell O, Kanis JA, Kragl G, Lopez Vaz A, Lorenc R, Lyritis G, Masaryk P, Matthis C, Miazgowski T, Gennari C, Pols HA, Poor G, Raspe HH, Reid DM, Reisinger W, Scheidt-Nave C, Stepan JJ, Todd CJ, Weber K, Woolf AD, Reeve J, for the European Prospective Osteoporosis Study (EPOS) (2003) Determinants of incident vertebral fracture in men and women: results from the European Prospective Osteoporosis Study (EPOS). Osteoporos Int 14:19–26 [DOI] [PubMed]
- 16.Taylor RS, Buyten JP, Buchser E. Spinal cord stimulation for chronic back and leg pain and failed back surgery syndrome: a systematic review and analysis of prognostic factors. Spine. 2005;30:152–160. doi: 10.1097/01.brs.0000152115.79236.6e. [DOI] [PubMed] [Google Scholar]
- 17.Taylor RS, Taylor RJ, Fritzell P (2007) Balloon kyphoplasty and vertebroplasty for vertebral compression fractures: a comparative systematic review of efficacy and safety. Spine (in press) [DOI] [PubMed]