Abstract
The high risk of sustaining subsequent vertebral fractures after an initial fracture cannot be explained solely by low bone mass. Extra-osseous factors, such as neuromuscular characteristics may help to explain this clinical dilemma. Elderly women with (n = 11) and without (n = 14) osteoporotic vertebral fractures performed rapid shoulder flexion to perturb the trunk while standing on a flat and short base. Neuromuscular postural responses of the paraspinal muscles at T6 and T12, and deep lumbar multifidus at L4 were recorded using intramuscular electromyography (EMG). Both groups demonstrated bursts of EMG that were initiated either before or shortly after the onset of shoulder flexion (P < 0.05). Paraspinal and multifidus onset occurred earlier in the non-fracture group (50–0 ms before deltoid onset) compared to the fracture group (25 ms before and 25 ms after deltoid onset) in the flat base condition. In the short base condition, EMG amplitude increased significantly above baseline earlier in the non-fracture group (75–25 ms before deltoid onset) compared to the fracture group (25–0 ms before deltoid onset) at T6 and T12; yet multifidus EMG increased above baseline earlier in the fracture group (50–25 ms before deltoid) compared to the non-fracture group (25–0 ms before deltoid). Time to reach maximum amplitude was shorter in the fracture group. Hypothetically, the longer time to initiate a postural response and shorter time to reach maximum amplitude in the fracture group may indicate a neuromuscular contribution towards subsequent fracture aetiology. This response could also be an adaptive characteristic of the central nervous system to minimise vertebral loading time.
Keywords: Osteoporosis, Vertebral fracture, Paraspinal muscle, Electromyography, Neuromuscular control
Introduction
Vertebral fractures are associated with a number of physical impairments and psychosocial morbidities and pose a significant burden on the public health system [12, 32]. Furthermore, these sequelae become more pronounced with each subsequent vertebral fracture sustained [26]. Once an individual sustains a vertebral fracture, the risk of subsequent vertebral fracture increases by up to four to sevenfold and then exponentially for each fracture sustained thereafter [37, 38]—known as the ‘vertebral fracture cascade’. However, despite the large volume of research dedicated to examining fracture risk and the efficacy of pharmacologic agents, little is understood surrounding the aetiology of vertebral fractures, particularly mechanisms underlying subsequent fractures.
Although bone mineral density (BMD) accounts for a large proportion of the variance observed in vertebral bone strength in the thoracic and lumbar spine [43], areal BMD still remains an inadequate predictor of vertebral fracture risk. The strong relationship between BMD and bone strength underlines the rationale for the use of densitometry in assessing fracture risk and skeletal status. However, evidence from epidemiologic studies suggests a lack of discriminant power of standard BMD measures [28] and a poor sensitivity of areal BMD T-scores in identifying fracture cases [10]. This suggests that factors other than BMD may influence the aetiology of first-time and subsequent vertebral fracture [4]. Local factors such as cortical bone structure [33], bone quality [1] and bone geometry [13] appear to have an influential role. However, extra-osseous factors may also influence vertebral fracture aetiology. Previous research has found the presence of an osteoporotic vertebral fracture to be associated with reduced back extensor strength [41], decreased spinal mobility [30], altered balance characteristics and changes in trunk muscle control [14] compared to individuals with osteoporosis and no history of vertebral fracture. However, muscle control characteristics specifically around common fracture sites and in muscles that provide intersegmental stability remain unexplored
Osteoporotic vertebral fractures occur most frequently in the mid-thoracic spine and thoracolumbar junction [7, 11]. The paraspinal muscles attach to the vertebrae or in close proximity, and are responsible for generating large compressive forces. Therefore quantification of muscle force at the mid-thoracic spine and thoracolumbar junction would be advantageous. Notably, vertebral fractures have been associated with increased segmental spinal loads and trunk muscle forces predicted through biomechanical models using optimisation routines [6]. A limitation of optimisation models is that differences in neuromuscular strategies between individuals cannot be explored. Neuromuscular strategies are best explored using electromyography (EMG). However, deriving muscle force from muscle activity data collected through EMG usually requires normalisation of EMG data to a maximum voluntary contraction. This procedure would not be appropriate in a population with compromised vertebral strength. An alternative approach is to examine temporal characteristics of muscle activity during a postural response. Rapid, voluntary arm movement is a suitable paradigm in which to measure a muscular postural response and has been used for this purpose in previous research [18–20, 23, 31]. Rapid flexion of the upper limb moves the centre of mass of the body anteriorly and causes resultant flexion motion between trunk segments of up to 8°, and to maintain equilibrium, the central nervous system initiates appropriate responses in the paraspinal muscles [18]. The nature of these responses may be different between individuals with and without fractures and therefore help to explain a potential mechanism underlying the aetiology of subsequent vertebral fracture.
The anatomy of the lumbar multifidus muscle suggests that it has an important role in maintaining stability of the lumbar spine [45]. Previous research has identified that individuals with low back pain have atrophy of the multifidus muscle [16] and changes in recruitment [27]. Recent studies suggest that impaired activity of multifidus in low back pain may be associated with a greater response of more superficial paraspinal muscles [17]. Maladaptive multifidus recruitment patterns may compromise intersegmental stability of the lumbar spine and therefore reduce the ability of the spine to resist shear loading. In a population with underlying vertebral fragility, either of these changes may be sufficient to increase fracture risk.
To specifically measure paraspinal muscle activity at the mid-thoracic and thoracolumbar levels and deep lumbar multifidus, intra-muscular EMG is required [31]. This study examined the association between vertebral fracture and paraspinal muscle recruitment characteristics in a population with osteoporosis using intra-muscular EMG.
Materials and methods
Participants
Twenty-five elderly, community dwelling females with osteoporosis were recruited and divided into two groups—those with an osteoporotic vertebral fracture in the thoracic spine (n = 11) and those without fracture (n = 14). Osteoporosis was confirmed on the basis of bone densitometry tests according to the classification system proposed by the World Health Organization. Vertebral fractures were identified from standing, lateral radiographs of the thoracic and lumbar spine based on a conservative morphometric deformity criteria. Vertebrae were classified as wedge-fractured when anterior vertebral height was reduced ≥30% compared with posterior height in that and the adjacent superior or inferior vertebra, measured using digital image processing software [29]. Qualitative review by a radiologist ensured that compression fractures were not overlooked. Fifteen thoracic wedge fractures were identified in the fracture group at vertebrae T4 (20%), T5 (13.3%), T6 (26.6%), T7 (6.7%), T8 (20%), T9 (6.7%), T12 (6.7%). Radiographs were also used to measure thoracic curvature using the regional vertebral centroid angle [5, 15]. Physical activity was assessed using the Physical Activity Scale for the Elderly (PASE), which has been validated previously [44]. Pain prior to and during testing was measured using a visual analogue scale (VAS). There were no significant differences in the physical characteristics between the groups (all: P > 0.05, Table 1). Pain prior to, and during testing ranged from 0 to 2/10 on the VAS and was not different between the groups (P > 0.05, Table 1).
Table 1.
Physical characteristics and subjective reporting of pain using the VAS in each group, presented as the mean (SD)
| Group | n | Age (years) | Mass (kg) | Height (m) | BMI | Kyphosis (°) | PASE | VAS score prior to test | VAS score during test |
|---|---|---|---|---|---|---|---|---|---|
| Fracture | 11 | 68.4 (6.7) | 67.6 (10.7) | 1.61 (0.06) | 26.1 (4.0) | 33.5 (8.6) | 161.1 (48.8) | 0.6 (0.7) | 1.1 (0.8) |
| No fracture | 14 | 64.0 (8.9) | 59.8 (9.2) | 1.58 (0.04) | 24.0 (3.3) | 32.5 (7.6) | 156.7 (54.5) | 0.7 (0.8) | 1.1 (0.6) |
BMI body mass index, PASE physical activity scale for the elderly, VAS visual analogue scale for pain (scored between 0 and 10)
All participants provided written, informed consent, and approval to conduct the study was granted by institutional Human Research Ethics Committees. Participants described in this study have also been involved in other projects conducted by our group.
Electromyography
Electromyographic activity of the longissimus thoracis at T6 and T12, and deep lumbar multifidus at L4 were recorded using intra-muscular electrodes. We did not record EMG from participant-specific fracture sites, but rather vertebral levels that commonly fracture in osteoporosis, namely T6 and T12. Bipolar fine-wire electrodes were made from two equal lengths of Teflon coated stainless steel wire (140 μm diameter, A-M systems Inc., Carlsborg, WA, USA) and were inserted into a hypodermic needle (longissimus: 0.65 mm × 32 mm; multifidus: 0.65 mm × 70 mm). Teflon coating (1–2 mm) was removed from the end of the wires and the tips were bent back for form a hook that would embed in the muscle tissue. Electrodes were inserted with ultrasound guidance into the left longissimus thoracis muscle at the T6 and T12 levels ∼1 cm lateral to the spinous process and into the deep multifidus muscle at L4, ∼4 cm lateral to the L4 spinous process in an antero-medial direction [30]. A pair of Ag/AgCl adhesive EMG electrodes with a 10 mm diameter and 20 mm inter-electrode distance were placed over the right anterior deltoid muscle. A ground electrode was placed over the iliac crest. EMG data were amplified with a gain of 1,000, band pass filtered between 20 and 1,000 Hz using a second order Butterworth 12 dB/octave filter, including a notch filter at 50 Hz, and sampled at 2,000 Hz. Data were recorded and stored using Spike 2, version 4.10 software (Cambridge Electronic Design Limited, UK), and exported for analysis with Matlab 7.5 (The Mathworks Inc., Natick, MA, USA).
Task protocol
Participants stood in a frame surrounded on three sides by rails, which they were encouraged to only use for balance when required. Two standing surfaces were used in the frame, presented in a random order—a flat base and a short base. The short base consisted of a beam of wood (40 mm × 120 mm × 1,000 mm) on which participants stood with feet shoulder width apart and equal weight through both feet. When standing on the short base, the participants’ toes and heels were placed over the edges of the beam but were unable to touch the floor. The short base was used to challenge balance by reducing the contribution of the ankle muscles to postural control, thus increasing the demand for the hip strategy involving hip and trunk movement [24]. Participants performed rapid right arm movements (shoulder flexion to ∼60°) in response to a light positioned at eye level, during which EMG data were collected. Between each arm movement trial, participants were instructed to relax their back muscles, and this was confirmed from the real-time EMG recording. An accelerometer was attached to the right hand to provide information regarding the onset of arm movement. EMG data for the back muscles were collected over ten trials of rapid arm movements.
Data analysis
For each participant and each base, EMG data for the deltoid were displayed and time of muscle onset was identified visually as the point where EMG activity increased above baseline (Fig. 1, top). EMG data were displayed without reference to activity of other muscles, other biomechanical data and without knowledge of the participant group from which the data were derived. EMG amplitude was then calculated for the back muscles during 25 ms epochs before and after the time of onset of deltoid EMG (10 epochs in total). As performance of a maximum voluntary contraction was not appropriate in this population due to vertebral fragility it was not possible to normalise EMG data in this manner. Instead, EMG data were normalised to the peak activity recorded for each muscle across epochs in the flat base condition to allow comparison between epochs and bases. This type of normalisation increases the sensitivity to detect differences between epochs and bases but does not permit comparison between muscles or subject groups. Where EMG traces were overtly affected by motion artefact, these data were removed from statistical analysis. This occurred for 1 participant in the flat base and three participants in the short base condition.
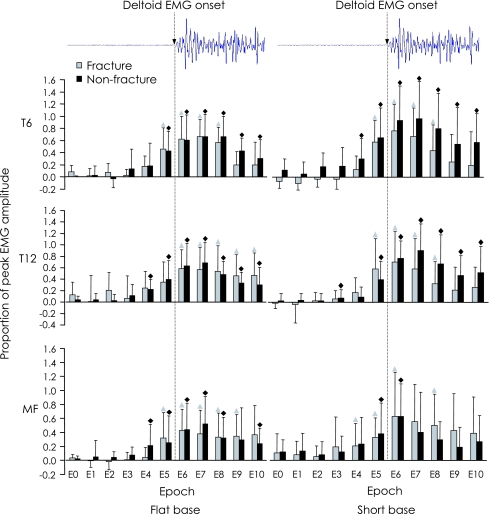
Fig. 1.
Normalised mean EMG (as a proportion of peak amplitude in the flat surface condition) for epochs before (125–0 ms) and after (0–125 ms) onset of deltoid for fracture (grey bars) and non-fracture (black bars) groups in the flat base (left panel) and short base (right panel) conditions for longissimus thoracis at T6 and T12, and deep multifidus (MF) at L4. Epochs in which EMG amplitude rose significantly above baseline EMG (epoch 0) are indicated with symbols (fracture group = triangle, non-fracture group = diamond, P < 0.05). The EMG normalisation approach precludes statistical comparisons of EMG amplitude within epochs between subject groups and between muscles; however qualitative differences between groups in response times are identified in this figure. Error bars represent 1 SD
Statistical analysis
Descriptive characteristics were compared between subject groups with independent t tests and Mann–Whitney U tests. For each group, a one-way repeated measures ANOVA was used to compare normalised EMG amplitude between epochs for a given base. Separate ANOVA analyses were conducted for each base. Paired t tests were used to compare normalised EMG between bases at three epochs selected a priori for each muscle: baseline, maximum, and earliest response (defined as the first epoch in which amplitude rose significantly above baseline). Given the exploratory nature of the study, we considered Bonferroni adjustments for post hoc comparisons to be too conservative; therefore Sharpened Bonferroni adjustments were used for multiple post hoc comparisons. The level of statistical significance was set at α = 0.05. All statistical analyses were conducted using SPSS for Windows, version 11.0 (SPSS Inc, Chicago, IL, USA).
Results
Both groups demonstrated bursts of EMG activity before and after rapid shoulder flexion. For each muscle, normalised EMG amplitude increased above the baseline amplitude recorded during epoch 0 (125 ms before deltoid onset) (P < 0.05, Fig. 1) at some point during the trial as determined by the ANOVA. The time to initiate a postural response, defined as the epoch in which EMG amplitude increased significantly above baseline (P < 0.05), identified with post hoc comparisons, differed between the groups.
Flat base condition
At T6, EMG amplitude increased significantly above baseline during epoch 5 (25–0 ms before deltoid onset) in both groups. At T12, EMG amplitude in the non-fracture group increased in epoch 4 (50–25 ms before deltoid onset), whereas EMG amplitude in the fracture group did not increase until epoch 6 (0–25 ms after deltoid onset). Similarly for multifidus, EMG amplitude in the non-fracture group increased in epoch 4 (50–25 ms before deltoid onset), whereas EMG amplitude in the fracture group did not increase until epoch 5 (25–0 ms before deltoid onset).
Short base condition
At T6, EMG amplitude in the non-fracture group increased in epoch 4 (50–25 ms before deltoid onset), whereas EMG amplitude in the fracture group did not increase until epoch 5 (25–0 ms before deltoid onset). At T12, EMG amplitude in the non-fracture group increased in epoch 3 (75–50 ms before deltoid onset), whereas amplitude in the fracture group did not increase until epoch 5 (25–0 ms before deltoid onset). The EMG amplitude increase above baseline in multifidus showed an opposite pattern; EMG activity in the fracture group increased earlier (epoch 4: 50–25 ms before deltoid onset) than in the non-fracture group (epoch 5: 25–0 ms before deltoid onset).
Time to reach maximum EMG amplitude
The time to reach maximum amplitude differed between groups in the short base condition for each muscle, and only at T12 for the flat base condition (Table 2). In the short base condition, participants with fractures reached maximum amplitude in epoch 6, compared to the non-fracture group who reached maximum in epoch 7. In the flat base condition both groups reached maximum amplitude at in epoch 7 for T6 and epoch 6 for multifidus. At the T12 level, the fracture group reached maximum amplitude during epoch 6, while the non-fracture group reached maximum in epoch 7 during the flat base condition. See Table 2 for a summary of these results.
Table 2.
Summary of epoch data for both groups at longissimus thoracis (LT) at T6 and T12, and deep lumbar multifidus (MF) at L4
| Time to increase above baseline | Time to reach max amplitude | |||||||
|---|---|---|---|---|---|---|---|---|
| Flat base | Short base | Flat base | Short base | |||||
| Fracture | Non-fracture | Fracture | Non-fracture | Fracture | Non-fracture | Fracture | Non-fracture | |
| LT-T6 | 5 | 5 | 5 | 4 | 7 | 7 | 6 | 7 |
| LT-T12 | 6 | 4 | 5 | 3 | 6 | 7 | 6 | 7 |
| MF-L4 | 5 | 4 | 4 | 5 | 6 | 6 | 6 | 7 |
Comparison between bases
For both groups there was no difference in normalised maximum EMG amplitude or earliest response EMG amplitude between bases for any muscle. Baseline EMG was significantly lower in the short base condition for the fracture group at T6 (P = 0.029). No other differences were apparent for baseline EMG between bases at T12 or multifidus for both groups.
Discussion
This study provides evidence that there is a differential pattern of paraspinal muscle recruitment between individuals with and without osteoporotic vertebral fractures and these changes are present at commonly fractured levels in the mid-thoracic spine and thoracolumbar junction. This finding may partly help to explain the complex and worrying clinical problem of the ‘vertebral fracture cascade’. Most notably, individuals who had sustained a vertebral fracture demonstrated delayed activation and a shorter time to reach maximum amplitude of the paraspinal muscles compared to individuals with no history of vertebral fracture.
The rapid arm movement paradigm used in this study provides an opportunity to investigate the strategy implemented by the central nervous system (CNS) to manage a sudden change in trunk equilibrium [3, 22]. The differential neuromuscular responses observed may be associated with greater vertebral loading in the fracture group given that muscle force is delivered over a shorter time, and thus point to a mechanism underlying the vertebral fracture cascade. However, the decision by the CNS to adopt this neuromuscular response may also be an adaptive/protective strategy. The longer time to initiate a response and shorter time to reach maximum amplitude may highlight a mechanism aimed at minimising the duration of vertebral loading. Further studies using a detailed anatomic model driven by EMG would be required to clarify the nature of these loading strategies.
A consistent pattern of activity was observed in longissimus thoracis during the arm movement task; onsets of T6 and T12 EMG activity occurred 25–50 ms after those of the non-fracture group, except in the flat base condition for T6 in which both groups demonstrated a significant rise in EMG activity above baseline at epoch 5. Results of this study are consistent with those reported previously using surface EMG in individuals with osteoporotic vertebral fractures [14]. That study demonstrated a delay in activation of the erector spinae muscle at T7 in individuals with vertebral fractures by 50 ms, and a reliance on trunk muscle co-contraction to maintain equilibrium. Co-contraction contrasts to the alternating trunk muscle activity patterns reported in younger populations during similar tasks [2].
Trunk muscle activity may be regarded as ‘feed-forward’ if onsets occur between 100 ms before to 50 ms after the onset of deltoid [21]. Thus, results of the present study agree with previous reports that suggest a feed-forward pattern for erector spinae and multifidus activation relative to deltoid onset [3, 17, 21, 23, 47]. A major element of the present study is that EMG recordings were made at commonly fractured sites, and from the paraspinal muscles, which are known to contribute significantly to compressive vertebral loading due to their short moment arm, particularly in individuals with vertebral fractures [13]. Although EMG was not collected from participant-specific fracture levels, the majority of fractures sustained by participants in this study occurred at T6, in agreement with previous reports [7, 11].
For multifidus, the onset of EMG activity in the non-fracture group preceded that of the fracture group in the flat base condition; however the opposite pattern was noticed in the short base condition. Consistent with previous research, the deep multifidus was active prior to deltoid [31]. The reason for earlier activation of the multifidus in the fracture group of 25 ms during the short base condition is uncertain; however we propose three possible explanations. First, it may be that individuals with osteoporotic vertebral fractures experience greater spinal instability therefore requiring a more rapid activation of the multifidus muscle compared to those without fractures. Second, the earlier response of multifidus may be necessary to accommodate for the delayed response of the more superficial long erector spinae muscles in the thoracic spine. Third, EMG of the lumbar multifidus was collected at L4 and vertebral fractures rarely occur at this level. Findings presented in this study may indicate that neuromuscular changes in the trunk extensors occur specifically at commonly fractured levels or that a CNS adaptation has occurred in the fracture group to increase lumbar intersegmental stability by recruiting multifidus relatively earlier.
As expected, muscle responses varied according to the task. In general, paraspinal muscles were recruited earlier in the short base condition (75–50 ms) compared to the flat base condition (50–25 ms), although little difference was observed in EMG amplitude between bases. This may reflect a greater demand placed on the CNS in the short base condition that required more rapid activation of the paraspinal muscles. On a flat base, the body rotates as a rigid mass about the ankle joints to maintain equilibrium in response to sagittal plane perturbations [24]. The short base decreases the ability for individuals to use an ankle strategy (ankle torques) to maintain postural control, and equilibrium is maintained by generation of horizontal shear forces from hip and trunk movement [24]. Muscular responses in the trunk therefore become more pronounced. Indeed, difficulty in executing postural tasks, particularly involving balance, has been reported previously in the elderly population [46].
The delay in recruitment of the paraspinal muscles and its likely consequence, a shorter time to reach maximum amplitude, may have several implications. A previous study showed greater segmental loading in upright stance in individuals who had sustained a vertebral fracture [6]. Combining higher static vertebral loads with a higher loading rate may be sufficient to cause vertebral failure by increasing trabecular strains [25]. Alternatively, cyclic repetitions of this neuromuscular response may fatigue trabecular bone and accelerate disc degeneration, thereby increasing subsequent fracture risk [8, 36]. However, the neuromuscular contribution to these degenerative mechanisms may only be viewed as speculative at this time given the current knowledge in the literature. Importantly, the generally shorter time to reach maximum amplitude displayed by the fracture group may represent a compensatory strategy employed by the CNS to overcome the delay in activation and maintain trunk equilibrium, or minimise the duration of muscle loading.
The mechanisms explaining the delayed paraspinal muscle activity in the fracture group are uncertain. Inhibition of muscle function due to pain may be attributable to symptomatic fractures, while subtle changes in thoracic kyphosis may have altered the mechanical properties of the muscles [39]. Previous research has confirmed changes in muscle recruitment as a consequence of pain [19, 21, 23, 48]. Other factors related to vertebral fractures such as decreased mobility and fear of falling could also influence muscle activation characteristics [35]. Furthermore, individuals with vertebral fractures demonstrate lower back-extensor and systemic strength compared to individuals without fractures [9, 42]. In the presence of weakened musculature a more rapid response to reach maximum amplitude may be required to in order to satisfy the equilibrium requirements. Indeed, this hypothesis may help to explain the reduced risk of subsequent vertebral fracture seen after a programme of back-extensor strengthening [40].
Previous studies have established that back-extensor strengthening, orthoses and proprioceptive re-education are beneficial in reducing the risk of osteoporotic vertebral fractures [39]. However, care should be taken when prescribing paraspinal-strengthening exercises in order to minimise compression forces through already weakened vertebrae, and orthoses should not replace the role of active muscles in the long-term to avoid muscle deconditioning. The findings presented in this study have clinical significance and may help to optimise musculoskeletal rehabilitation for this population. This study provides evidence of the existence of altered neuromuscular patterns in individuals who have sustained vertebral fractures compared to those who have no history of vertebral fracture and this may be interpreted as one of the sequelae of vertebral fractures. Future research examining the efficacy of interventions directed towards modifying this neuromuscular response and the longitudinal efficacy in reducing fracture risk is therefore warranted. Neuromuscular retraining in individuals with low back pain has proved to be effective in reducing pain and improving function [34], thus benefits, particularly a reduction in the vertebral fracture cascade, may be seen in the population of individuals with osteoporotic vertebral fractures. However, we cannot be sure whether changing the response will decrease fracture risk as it not yet known whether the altered neuromuscular responses are an adaptive strategy employed by the CNS. The cross-sectional design of the study precludes a cause-effect inference between an altered neuromuscular strategy and vertebral fracture, thus future research should adopt a longitudinal design to overcome this limitation. Future research should also utilise biomechanical trunk models driven by EMG to elucidate the influence of neuromuscular strategies on vertebral loading in this population. Temporal activation in this study was limited to specific epochs, thus more specific information might be obtained from identifying accurate onset/offset times.
Acknowledgments
We thank the Medical Imaging Department, St Vincent’s Hospital, Melbourne; Mr Tim Wrigley and Dr Sallie Cowan for technical assistance; and funding provided from the Physiotherapy Research Foundation (Australia) grant (013/05). Paul Hodges is supported by the National Health and Medical Research Council of Australia.
Contributor Information
Andrew M. Briggs, Email: abriggs@cabrini.com.au
Kim L. Bennell, Phone: +61-3-83444171, FAX: +61-3-83444188, Email: k.bennell@unimelb.edu.au
References
- 1.Aaron JE, Shore PA, Shore RC, et al. Trabecular architecture in women and men of similar bone mass with and without vertebral fracture: II. Three-dimensional histology. Bone. 2000;27:277–282. doi: 10.1016/S8756-3282(00)00328-8. [DOI] [PubMed] [Google Scholar]
- 2.Aruin AS, Latash ML. Directional specificity of postural muscles in feed-forward postural reactions during fast voluntary arm movements. Exp Brain Res. 1995;103:323–332. doi: 10.1007/BF00231718. [DOI] [PubMed] [Google Scholar]
- 3.Belen’kii VY, Gurfinkel VS, Pal’tsev YI. Elements of control of voluntary movements. Biofizika. 1967;12:142–147. [PubMed] [Google Scholar]
- 4.Briggs AM, Greig AM, Wark JD, et al. A review of anatomical and mechanical factors affecting vertebral body integrity. Int J Med Sci. 2004;1:170–180. doi: 10.7150/ijms.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briggs AM, Tully EA, Adams PE, et al. Vertebral centroid and Cobb angle measures of thoracic kyphosis. Inter Med J. 2005;35:A96. doi: 10.1007/s00256-007-0284-8. [DOI] [PubMed] [Google Scholar]
- 6.Briggs AM, Wrigley TV, Dieën JHv. The effect of osteoporotic vertebral fracture on predicted spinal loads in vivo. Eur Spine J. 2006;15:1785–1795. doi: 10.1007/s00586-006-0158-0. [DOI] [PubMed] [Google Scholar]
- 7.Davies KM, Stegman MR, Heaney RP, et al. Prevalence and severity of vertebral fracture: the Saunders county bone quality study. Osteoporos Int. 1996;6:160–165. doi: 10.1007/BF01623941. [DOI] [PubMed] [Google Scholar]
- 8.Dieën JHv, Kingma I, Meijer R, et al. Stress distribution changes in bovine vertebrae just below the endplate after sustained loading. Clin Biomech. 2001;16:S135–S142. doi: 10.1016/S0268-0033(00)00081-4. [DOI] [PubMed] [Google Scholar]
- 9.Dixon WG, Lunt M, Pye SR, et al. Low grip strength is associated with bone mineral density and vertebral fracture in women. Rheumatology. 2005;44:642–646. doi: 10.1093/rheumatology/keh569. [DOI] [PubMed] [Google Scholar]
- 10.Duan YB, Duboeuf F, Munoz F, et al. The fracture risk index and bone mineral density as predictors of vertebral structural failure. Osteoporos Int. 2006;17:54–60. doi: 10.1007/s00198-005-1893-5. [DOI] [PubMed] [Google Scholar]
- 11.Eastell R, Cedel SL, Wahner HW, et al. Classification of vertebral fractures. J Bone Miner Res. 1991;6:207–215. doi: 10.1002/jbmr.5650060302. [DOI] [PubMed] [Google Scholar]
- 12.Finnern HW, Sykes DP. The hospital cost of vertebral fractures in the EU: estimates using national datasets. Osteoporos Int. 2003;14:429–436. doi: 10.1007/s00198-003-1395-2. [DOI] [PubMed] [Google Scholar]
- 13.Gilsanz V, Loro LM, Roe TF, et al. Vertebral size in elderly women with osteoporosis: mechanical implications and relationships to fractures. J Clin Invest. 1995;95:2332–2337. doi: 10.1172/JCI117925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greig AM (2006) Trunk neuromuscular control and balance characteristics in individuals with osteoporotic vertebral fracture. PhD Thesis, University of Melbourne
- 15.Harrison DE, Cailliet R, Harrison DD, et al. Reliability of centroid, Cobb, and Harrison posterior tangent methods: Which to choose for analysis of thoracic kyphosis. Spine. 2001;26:E227–E234. doi: 10.1097/00007632-200106010-00002. [DOI] [PubMed] [Google Scholar]
- 16.Hides JA, Stokes MJ, Saide M, et al. Evidence of lumbar multifidus muscle wasting ipsilateral to symptoms in patients with acute subacute low back pain. Spine. 1994;19:165–172. doi: 10.1097/00007632-199401001-00009. [DOI] [PubMed] [Google Scholar]
- 17.Hodges P, Cholewicki J, Coppieters M et al (2006) Trunk muscle activity is increased during experimental back pain, but the pattern varies between individuals. International Society for Electrophysiology and Kinesiology Annual Scientific Meeting, Turin, Italy
- 18.Hodges P, Cresswell A, Thorstensson A. Preparatory trunk motion accompanies rapid upper limb movement. Exp Brain Res. 1999;124:69–79. doi: 10.1007/s002210050601. [DOI] [PubMed] [Google Scholar]
- 19.Hodges PW. Changes in motor planning of feedforward postural responses of the trunk muscles in low back pain. Exp Brain Res. 2001;141:261–266. doi: 10.1007/s002210100873. [DOI] [PubMed] [Google Scholar]
- 20.Hodges PW, Cresswell AG, Thorstensson A. Perturbed upper limb movements cause short-latency postural responses in trunk muscles. Exp Brain Res. 2001;138:243–250. doi: 10.1007/s002210100693. [DOI] [PubMed] [Google Scholar]
- 21.Hodges PW, Richardson CA. Altered trunk muscle recruitment in people with low back pain with upper limb movement at different speeds. Arch Phys Med Rehab. 1999;80:1005–1112. doi: 10.1016/S0003-9993(99)90052-7. [DOI] [PubMed] [Google Scholar]
- 22.Hodges PW, Richardson CA. Feedforward contraction of transversus abdominis is not influenced by the direction of arm movement. Exp Brain Res. 1997;114:362–370. doi: 10.1007/PL00005644. [DOI] [PubMed] [Google Scholar]
- 23.Hodges PW, Richardson CA. Inefficient muscular stabilization of the lumbar spine associated with low back pain: a motor control evaluation of transversus abdominis. Spine. 1996;21:2640–2650. doi: 10.1097/00007632-199611150-00014. [DOI] [PubMed] [Google Scholar]
- 24.Horak FB, Nashner LM. Central programming of postural movements: adaptations to altered support-surface configurations. J Neurophysiol. 1986;55:1369–1381. doi: 10.1152/jn.1986.55.6.1369. [DOI] [PubMed] [Google Scholar]
- 25.Kopperdahl DL, Pearlman JL, Keaveny TM. Biomechanical consequences of an isolated overload on the human vertebral body. J Orthop Res. 2000;18:685–690. doi: 10.1002/jor.1100180502. [DOI] [PubMed] [Google Scholar]
- 26.Lindsay R, Burge RT, Strauss DM. One year outcomes and costs following a vertebral fracture. Osteoporos Int. 2005;16:78–85. doi: 10.1007/s00198-004-1646-x. [DOI] [PubMed] [Google Scholar]
- 27.MacDonald D, Moseley GL, Hodges P (2004) The function of the lumbar multifidus in unilateral low back pain. 5th Interdisciplinary world congress on low back and pelvic pain, Melbourne
- 28.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. Br Med J. 1996;312:1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCloskey EV, Spector TD, Eyres KS, et al. The assessment of vertebral deformity: a method for use in population studies and clinical trials. Osteoporos Int. 1993;3:138–147. doi: 10.1007/BF01623275. [DOI] [PubMed] [Google Scholar]
- 30.Miyakoshi N, Hongo M, Maekawa S, et al. Factors related to spinal mobility in patients with postmenopausal osteoporosis. Osteoporos Int. 2005;16:1871–1874. doi: 10.1007/s00198-005-1953-x. [DOI] [PubMed] [Google Scholar]
- 31.Moseley GL, Hodges PW, Gandevia SC. Deep and superficial fibers of the lumbar multifidus muscle are differentially active during voluntary arm movements. Spine. 2002;27:E29–E36. doi: 10.1097/00007632-200201150-00013. [DOI] [PubMed] [Google Scholar]
- 32.Nevitt MC, Ettinger B, Black DM, et al. The association of radiographically detected vertebral fractures with back pain and function: a prospective study. Ann Intern Med. 1998;128:793–800. doi: 10.7326/0003-4819-128-10-199805150-00001. [DOI] [PubMed] [Google Scholar]
- 33.Oleksik A, Ott SM, Vedi S, et al. Bone structure in patients with low bone mineral density with or without vertebral fractures. J Bone Miner Res. 2000;15:1368–1375. doi: 10.1359/jbmr.2000.15.7.1368. [DOI] [PubMed] [Google Scholar]
- 34.O’Sullivan PB, Twomey LT, Allison GT. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine. 1997;22:2959–2967. doi: 10.1097/00007632-199712150-00020. [DOI] [PubMed] [Google Scholar]
- 35.Pluijm SFM, Tromp AM, Smit JH, et al. Consequences of vertebral deformities in older men and women. J Bone Miner Res. 2000;15:1564–1572. doi: 10.1359/jbmr.2000.15.8.1564. [DOI] [PubMed] [Google Scholar]
- 36.Pollintine P, Dolan P, Tobias JH, et al. Intervertebral disc degeneration can lead to “stress-shielding” of the anterior vertebral body—a cause of osteoporotic vertebral fracture? Spine. 2004;29:774–782. doi: 10.1097/01.BRS.0000119401.23006.D2. [DOI] [PubMed] [Google Scholar]
- 37.Ross PD, Davis JW, Epstein RS, et al. Pre-existing fractures and bone mass predict vertebral fracture incidence in women. Ann Intern Med. 1991;114:919–923. doi: 10.7326/0003-4819-114-11-919. [DOI] [PubMed] [Google Scholar]
- 38.Ross PD, Genant HK, Davis JW, et al. Predicting vertebral fracture incidence from prevalent fractures and bone density among non-black, osteoporotic women. Osteoporos Int. 1993;3:120–126. doi: 10.1007/BF01623272. [DOI] [PubMed] [Google Scholar]
- 39.Sinaki M. Critical appraisal of physical rehabilitation measures after osteoporotic vertebral fracture. Osteoporos Int. 2003;14:774–779. doi: 10.1007/s00198-003-1446-8. [DOI] [PubMed] [Google Scholar]
- 40.Sinaki M, Itoi E, Wahner HW, et al. Stronger back muscles reduce the incidence of vertebral fractures: a prospective 10 year follow-up of postmenopausal women. Bone. 2002;30:836–841. doi: 10.1016/S8756-3282(02)00739-1. [DOI] [PubMed] [Google Scholar]
- 41.Sinaki M, Khosla S, Limburg PJ, et al. Muscle strength in osteoporotic versus normal women. Osteoporos Int. 1993;3:8–12. doi: 10.1007/BF01623170. [DOI] [PubMed] [Google Scholar]
- 42.Sinaki M, Wollan PC, Scott RW, et al. Can strong back extensors prevent vertebral fractures in women with osteoporosis? Mayo Clin Proc. 1996;71:951–956. doi: 10.4065/71.10.951. [DOI] [PubMed] [Google Scholar]
- 43.Singer K, Edmondston S, Day R, et al. Prediction of thoracic and lumbar vertebral body compressive strength. Correlations with bone mineral density and vertebral region. Bone. 1995;17:167–174. doi: 10.1016/S8756-3282(95)00165-4. [DOI] [PubMed] [Google Scholar]
- 44.Washburn RA, McAuley E, Katula J, et al. The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52:643–651. doi: 10.1016/S0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 45.Wilke HJ, Wolf S, Claes LE, et al. Stability increase in the lumbar spine with different muscle groups. Spine. 1995;20:192–198. doi: 10.1097/00007632-199501150-00011. [DOI] [PubMed] [Google Scholar]
- 46.Woollacott MH, Shumway-Cook A, Nashner LM. Aging and posture control: changes in sensory organization and muscular co-ordination. Int J Aging Hum Dev. 1986;23:97–114. doi: 10.2190/VXN3-N3RT-54JB-X16X. [DOI] [PubMed] [Google Scholar]
- 47.Zattara M, Bouisset S. Posturo-kinetic organisation during the early phase of voluntary upper limb movement. I. Normal subjects. J Neurol Neurosurg Psychiatry. 1988;51:956–965. doi: 10.1136/jnnp.51.7.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zedka M, Prochazka A, Knight B, et al. Voluntary and reflex control of human back muscles during induced pain. J Physiol Lond. 1999;520:591–604. doi: 10.1111/j.1469-7793.1999.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]