Abstract
Activated factor VIII (FVIIIa) forms a procoagulant complex with factor IXa on negatively charged membranes, including activated platelet surfaces. Membrane attachment involves the FVIII C2 domain; involvement of the adjacent C1 domain has not been established. Binding of recombinant FVIII C1C2 and C2 proteins to platelets was detected by flow cytometry using (1) anti-C2 monoclonal antibody ESH8 followed by a phycoerythrin-labeled secondary antibody; (2) biotinylated C1C2 detected by phycoerythrin-labeled streptavidin, and (3) C1C2 and C2 site-specifically labeled with fluorescein. Highest binding and lowest background were obtained using fluorescein-conjugated proteins. More than 90% of activated platelets bound C1C2, compared with approximately 50% for equimolar C2. Estimates using fluorescent microbeads indicated approximately 7000 C1C2-binding sites per platelet, approximately 1400 for C2, and approximately 3000 for fluorescein-labeled FVIIIa. Unlike C2 or FVIII(a), C1C2 bound to approximately 700 sites/platelet before activation. C1C2 binding to activated platelets appeared independent of von Willebrand factor and was competed effectively by FVIII(a), but only partially by excess C2. Fluorescein-labeled FVIIIa was competed much more effectively by C1C2 than C2 for binding to activated platelets. Two monoclonal antibodies that inhibit C2 binding to membranes competed platelet binding of C2 more effectively than C1C2. Thus, the C1 domain of FVIII contributes to platelet-binding affinity.
Introduction
Factor VIII (FVIII) circulates in plasma in a noncovalent complex with von Willebrand factor (VWF); this interaction is mediated by the FVIII C2 domain and an acidic sequence prior to the A3 domain.1–3 Upon proteolytic activation, FVIIIa is released from VWF as a heterotrimer composed of the A1 and A2 domains plus the FVIIIa light chain, A3-C1-C2. Activated platelet membranes expose negatively charged phosphatidylserine (PS), which increases from 2% to 10% or more of the surface phospholipids upon activation.4,5 FVIIIa forms a complex with FIXa and calcium on negatively charged phospholipid membranes, enhancing FIXa catalysis 100 000- to 200 000-fold.6–8 Although FVIII can bind to FIXa on phospholipids,9 or directly to activated platelets,10 FVIIIa is required for procoagulant activity.9 A hydrophobic surface on the FVIII(a) C2 domain11 becomes buried in the phospholipid membrane upon binding,12,13 and basic C2 residues make favorable charge-charge interactions with negatively charged PS head groups. Although FVIIIa and the light chain bind to PS-containing vesicles and activated platelets with similar affinities, the affinity of the recombinant C2 domain is 5- to 100-fold lower,10,14,15 suggesting possible roles for the C1 and/or A3 domains
To address the potential role of the C1 domain in FVIII(a) attachment to platelets, a recombinant human FVIII C1C2 protein (residues 2020-2332) was produced in Escherichia coli, refolded, and characterized. The binding of C1C2 to platelet surfaces both before and after platelet activation was compared with C211,15 binding using flow cytometry. A point mutation in C2, S2296C, was generated for both C2 and C1C2. This solvent-exposed residue is distal to the C2 membrane-binding surface11 and was used to label both proteins with sulfhydryl-linked fluorescent probes. This allowed stoichiometric labeling and produced lower background signals than detection systems using labeled anti-C2 antibodies or biotinylation at residue C2296 followed by labeled streptavidin detection. The numbers of binding sites on platelets for C1C2 and C2 were estimated using near-saturating amounts of the labeled proteins.
Methods
Recombinant C1C2 and C2
Wild-type C2 (residues 2169-2332, with an N-terminal extension of 5 residues and substitution C2169V to avoid improper disulfide formation) and C2-2296C were expressed in Pichia pastoris and purified as described.11 The presence of a single reactive Cys under the mild reduction conditions used for labeling was confirmed using Ellman assay.16 Protein concentrations were determined using calculated extinction coefficients17 of 1.8 for C2 and 1.85 for C1C2. C1C2 cDNA was generated from hFVIII cDNA18 (provided by Randal Kaufman, University of Michigan) by polymerase chain reaction (PCR) and inserted into the BamHI site of the pET-28b vector (Novagen, Madison, WI). Primers were as follows: forward, CGGGATCCGAAGTGTCAGACTCCCCTGG, and reverse, CGGGATCCTCAGTAGAGGTCCTGTGCC. This vector introduces an N-terminal His-Tag/thrombin/T7-Tag. The BL21-CodonPlus E coli strain (Stratagene, La Jolla, CA) was the expression host. LB broth (20 mL; Becton Dickinson, Sparks, MD) containing 50 μg/mL each of chloramphenicol and kanamycin was inoculated and shaken at 37°C overnight. LB (1 L) was inoculated with this overnight culture and shaken at 37°C until the A600nm reached 0.6. Expression was induced with 1 mM IPTG (isopropyl-beta-D-thiogalactopyranoside; Invitrogen, Carlsbad, CA). The culture was shaken for 15 to 20 minutes at 37°C and stored at 4°C overnight. The cells were centrifuged, frozen rapidly, and stored at − 70°C. Cell pellets were resuspended in 20 mL room-temperature BugBuster Protein Extraction Reagent (Novagen) with one protease inhibitor cocktail tablet/50 mL (Complete Protease Inhibitor; Roche, Indianapolis, IN), 1 μL (25 units) benzonase nuclease (Novagen)/mL, and 1 KU rLysozyme Solution (Novagen)/mL, then incubated on a rotating mixer for 10 to 20 minutes at room temperature. Insoluble cell debris containing inclusion bodies was concentrated by centrifugation at 16 000g for 20 minutes at 4°C. This pellet was resuspended in 10 mL BugBuster reagent plus additives as described in this paragraph, vortexed, and left at room temperature for 5 minutes. BugBuster reagent (10 mL) plus 2 protease inhibitor tablets were added to 90 mL deionized water; 25 mL of this solution was added to the suspension, which was centrifuged at 5000g for 15 minutes at 4°C. The pellet was washed 3 more times by vortexing with 25 mL of the same solution, then centrifuging as described.
The washed pellet was suspended in 10 mL of 8 M urea and dialyzed against 1 L of 6 M urea in a 4-L beaker at 4°C. Every 3 hours, 250 mL of 25 mM Tris-HCl (pH 8.5) was added until the volume reached 3 L. Restriction grade thrombin approximately 10 U (Novagen) was added and the solution was left at room temperature for 2 hours. A protease inhibitor minitablet (Roche) was then added to the sample along with solubilization buffer 1 × IB (Novagen) diluted to a final concentration of 10 mM reduced glutathione and 2 mM oxidized glutathione. This sample was dialyzed against 1 L of 2 M urea. The sample was diluted sequentially as above to 3 L, dialyzed against 25 mM Tris-HCl (pH 8.5), and then concentrated to 1 to 2 mg/mL using centrifugal concentrators (Centricon-10 000; Millipore, Bedford, MA).
Codon 2296 in C1C2 was changed from Ser (TCT) to Cys (TGT) using the QuickChange XL Site-Directed Mutagenesis Kit (Stratagene). Primers were as follows: forward, CCTGTGGTGAACTGTCTAGACCCACCGTTACTG, and reverse, CAGTAACGGTGGGTCTAGACAGTTCACCACAGG. The mutation was confirmed by DNA sequencing. C1C2-2296C was expressed and purified in the same manner as the wild-type protein. The mutant C1C2 and C2 proteins were indistinguishable from their unlabeled counterparts on SDS–polyacrylamide gel electrophoresis. Western blotting with the anti-C2 monoclonal antibody ESH8 (American Diagnostica, Stamford CT) followed by HRP-linked sheep anti–mouse IgG (Amersham-GE, Amersham, United Kingdom) and detection on films using enhanced chemiluminescence (ECL) Western Blotting Detection Reagents (Amersham-GE) yielded a single luminescent band for each purified C1C2 and C2 protein.
Freshly purified C1C2–2296C was mildly reduced as above. Iodoacetyl-biotin solution (30 μL, 2.2 mg PEO-iodoacetyl biotin [Pierce, Rockford, IL] in 1 mL 50 mM Tris-HCl [pH 8.0] including 5 mM EDTA) was added to 2 mL C1C2-2296C (2 mg/mL), and the mixture was incubated for 90 minutes in the dark at room temperature. The biotin-labeled C1C2-2296C was dialyzed against 25 mM Tris-HCl (pH 8.5). C1C2-2296C, C2-2296C,11 and FVIII were also directly labeled with a sulfhydryl-linked fluorescein probe after mild reduction and desalting as above. Briefly, for C1C2 and C2, 50 μL 5-iodoacetamidofluorescein (5-IAF [Pierce]; 1 mg 5-IAF in 100 μL dimethylformamide) was added to the reduced proteins at a 10-fold excess, and the solutions were incubated for 2 hours at room temperature, then dialyzed against 25 mM Tris-HCl (pH 8.5). FVIII (0.5 mg in 3 mL) was labeled with fluorescein-5-maleimide (Pierce) at a molar ratio of 25:1 (label:FVIII) overnight at 4°C, under mild reducing conditions, and dialyzed against 25 mM Tris-HCl (pH 7.5). All fluorescein labeling procedures were carried out in the dark. These proteins are hence referred to as “fluorescein labeled.”
Platelet preparations
Healthy blood donors, 2 unrelated subjects with severe type 3 von Willebrand disease (VWF:Ag undetectable; no VWF treatment for > 2 weeks), and 1 severe hemophilia A subject (inversion positive, no FVIII treatment for > 72 hours) provided samples with informed consent under protocols approved by the University of Washington's Human Subjects Review Committee and obtained in accordance with the Declaration of Helsinki.
Freshly collected whole blood (15 mL) was added to a polypropylene tube containing approximately 2.1 mL acid citrate dextrose (ACD, 2.5% wt/vol trisodium citrate, 1.5% wt/vol citric acid, and 2.0% wt/vol dextrose). PGE1 (Sigma, St Louis, MO) was added to 50 ng/mL and apyrase (Sigma) to approximately 2.5 U/mL. Platelet preparations and analyses were all performed at room temperature unless otherwise indicated. The sample was mixed by inversion and centrifuged at approximately 200g for 20 minutes. Platelet-rich plasma (PRP) was transferred with a polypropylene pipette to a fresh 50-mL tube and centrifuged at approximately 200g for 5 minutes. The nonpelleted PRP was transferred into another tube.
The amount of platelet-bound plasma proteins was reduced using an albumin gradient procedure modified from Ahmad and Walsh.10 Briefly, 400 μL albumin solution (Path-o-Cyte 5; ICN Biomedicals, Aurora, OH) and 400 μL Tyrode buffer (HEPES-Tyrode buffer: 126 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 0.38 mM NaH2PO4, 5.6 mM dextrose, 6.22 mM sodium HEPES, 8.78 mM HEPES-free acid, and 0.1% bovine serum albumin [BSA]; pH adjusted to 6.5 with ACD) were added to a 15-mL polystyrene tube to which apyrase was then added (final concentration: 5 U/mL). The solution was vortexed and warmed to approximately 37°C, and room temperature PRP was then layered on top of the albumin/Tyrode/apyrase solution and Tyrode buffer was added to 15 mL. This solution was centrifuged for 15 minutes at approximately 900g (at room temperature). The platelet-poor plasma and the upper portion of the albumin solution were removed. Tyrode buffer containing 2.5 U/mL apyrase was added to approximately 14 mL, and the tube was warmed to 37°C. This procedure was repeated twice. The final pellet was resuspended in 3.5 mL Tyrode/apyrase buffer. PRP (1 mL) was loaded onto a 30 × 1-cm Sepharose 2B column (K30; Pharmacia, Piscataway, NJ) equilibrated with Tyrode buffer (pH 7.4). Gel-filtered platelets were collected and counted on a COULTER T-890 (Beckman-Coulter, Fullerton, CA). For initial screening, and where indicated in the results, platelets from healthy subjects were washed but not filtered prior to activation.
Factor VIIIa generation and activity
Recombinant FVIII (Advate) was used for competition studies with fluorescein-labeled C1C2 and C2 and was purchased from Baxter Biosciences (Westlake Village, CA) and reconstituted in double-distilled H2O to 1666 Units/mL or a final concentration of approximately 1 μM. FVIIIa was prepared by addition of 0.1 μM human α-thrombin (Haematologic Technologies, Essex Junction, VT) for 5 minutes at 37°C followed by 10 μM PPACK (EMD Biosciences, La Jolla, CA).19 For direct fluorescein labeling, a second recombinant FVIII (Kogenate FS) was purchased from Bayer Pharmaceuticals (Elkhart, IN), labeled with fluorescein (above) and thrombin activated as with the other FVIII preparation. FVIII clotting activity was assessed using a 1-stage, semiautomated assay system essentially as described for factor IX activity,20 but using commercial factor VIII–deficient plasma. This assay was also carried out substituting a commercial platelet lysate (Precision BioLogic, Dartmouth, NS) for the phospholipid preparation.
Flow cytometry to quantitate platelet binding
The binding of C1C2 and C2 proteins to platelets was detected by flow cytometry using a Becton Dickinson FACSCalibur with all photomultipliers in log mode. The proteins were labeled by 3 methods: (1) immunologic detection of C2 and C1C2 using the anti-C2 monoclonal antibody ESH8 (American Diagnostica) followed by PE-secondary antibody (phycoerythrin-labeled rat anti–mouse IgG2a+b; BD Bioscience, San Diego, CA); (2) biotinylation of C1C2-2296C at the free cysteine using iodoacetyl-biotin (Pierce) followed by detection with streptavidin-PE (BD Bioscience); and (3) covalent labeling of C2-2296C and C1C2-2296C using the fluorescein probe 5-IAF. The numbers of fluorescein-conjugated C1C2-2296C or C2-2296C proteins bound per platelet were estimated from standard curves generated from standardized, similar-sized FITC-labeled microspheres (no. 824B; Bangs Laboratories, Fishers, IN).
Each flow cytometry measurement required 100 μL suspended platelets (2.5 × 108 platelets/mL). Platelets were activated by addition of the thrombin receptor peptide SFLLRN-amide (final concentration: 20 μM; Peptides International, Louisville, KY) for 10 minutes. The platelet activation status was assessed by measuring surface-bound P-selectin with fluorescein isothiocyanate (FITC)–labeled antihuman P-selectin antibody, 5 μL (stock: 0.5 mg/mL; BD Bioscience). Various amounts of C1C2 or C2 were added before or after platelet activation. The suspensions were incubated for one hour, then centrifuged at approximately 900g for 5 minutes. The supernatant was then removed and the platelets were resuspended in 100 μL CWB buffer (11 mM glucose, 128 mM NaCl, 4.3 mM NaH2PO4, 7.5 mM Na2HPO4, 4.8 mM sodium citrate, 2.4 mM citric acid, 0.35% [wt/vol] BSA, adjusted to pH 6.5). This wash procedure was performed twice. For the buffer used in all binding or competition experiments with FVIII or FVIIIa, 5 mM CaCl2 was added to 10 mM HEPES with 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, and 5 mM dextrose at pH 7.4.
For immunologic detection, negative controls to detect nonspecific binding to platelets before or after activation were as follows: (1) platelets + ESH8 + PE-secondary antibody, and (2) platelets + PE-secondary antibody. Of an ESH8 stock solution, 1 μL (1 mg/mL) was added to 100 μL washed platelets, and the tubes were incubated for one hour; then the platelets were washed again as above. Of a PE-secondary antibody stock solution, 5 μL (1.5 μg/mL) was added to 100 μL platelets plus or minus ESH8, and the suspensions were incubated for 30 minutes. The platelets were washed, resuspended in 100 μL CWB, and analyzed. To address the concern that residual plasma-derived FVIII might adhere to the washed platelets and bind to ESH8, platelets from a severe hemophilia A patient were analyzed. For biotin-conjugated C1C2-2296C detection, 3 μL streptavidin-PE (0.5 mg/mL stock) was added to platelets that were then washed as described above. The control experiment without C1C2 indicated the extent of nonspecific binding.
Fluorescein-labeled FVIIIa, C1C2-2296C, and C2-2296C were each added to platelets either prior to or after activation with SFLLRN. The suspensions were incubated for one hour at room temperature, then washed and analyzed by flow cytometry. The protein concentrations yielding almost-saturated binding to platelets, both before and after activation, were determined by measuring the signals after adding increasing amounts of the labeled proteins until saturation was reached.
For competition experiments, fluorescein-labeled proteins at a single subsaturation concentration for each experiment were incubated for one hour with platelets at room temperature and washed as above. Unlabeled proteins from equimolar to as much as 500-fold molar excess were then added and incubated for another hour before washing and analysis by flow cytometry. For immunologic inhibition of binding, increasing concentrations of antibodies ESH4 (American Diagnostica) or NMC-VIII/6 (kindly provided by M. Shima, Nara, Japan) were preincubated with fluorescein-labeled C1C2 or C2 for 30 minutes, and these mixtures were incubated with activated platelets for 1 hour. The platelets were washed and residual binding of labeled proteins to platelets was determined as described under “Platelet preparations.”
Results
Expression, purification, and characterization of FVIII C1C2
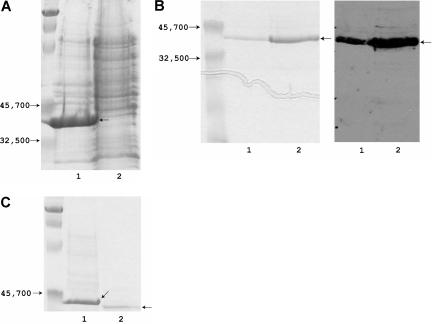
C1C2 remained in the insoluble fraction of the cell lysate (Figure 1), but the refolded protein was soluble and at least 95% pure. Within 1 month of storage at 4°C, concentrated samples of C1C2 (1-2 mg/mL) tended to develop visible precipitates. Therefore, all experiments were performed using protein stored less than one week that showed no sign of precipitation. The correct amino acid sequence and proper disulfide formation were confirmed unambiguously by mass spectroscopy of tryptic digests, and there was negligible contamination with non–FVIII-derived peptides (not shown).
Figure 1.
Expression and purification of the FVIII C1C2 domain. (A) Lanes 1 and 2 show the insoluble and soluble fractions of an E coli cell lysate (15% SDS-PAGE, Coomassie Blue staining; marker proteins on far left). (B) Refolded C1C2 run on a 15% acrylamide gel, with 5 μg protein in lane 1 and 10 μg in lane 2; the left panel shows Coomassie blue staining (marker proteins on far left), and the right panel shows a Western blot using ESH8. (C) Removal of the amino-terminal peptide: lanes 1 and 2 show C1C2 before and after thrombin cleavage (10% SDS-PAGE, Coomassie blue staining; marker proteins on far left). The image was cropped below the C1C2 band because the lower percentage of acrylamide resulted in the loading dye running much closer to the C1C2 band than in the 15% gels; additional Coomassie-stained gels and Western blots showed no evidence of proteolytic degradation or lower molecular weight contaminants (not shown). The thrombin-cleaved C1C2 (containing a 16–amino acid amino-terminal extension) was used for all binding and inhibition studies.
The ability of C1C2 and C2 to compete FVIII procoagulant activity was determined using a one-stage FVIII activity assay. A dose response was seen for C2 and C1C2 (data not shown); 50% inhibition occurred at 50 μM for C2 and 12.5 μM for C1C2. Using the commercial platelet lysate as a phospholipid source, 50% inhibition was observed at 25 μM C1C2, whereas there was no detectable inhibition with 25 μM C2.
Platelet activation
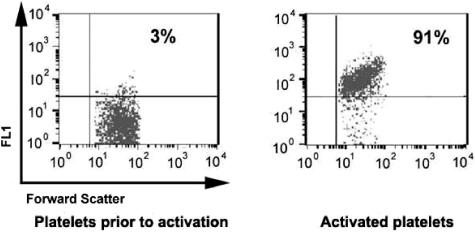
Prior to platelet activation by SFLLRN-amide, binding of anti–P-selectin to the platelet surface was essentially background. For 5 platelet preparations, the percentage of labeled (activated) platelets after addition of SFFLRN-amide averaged 92% (range, 90%-94%; Figure 2).
Figure 2.
Platelet activation by SFLLRN amide. Binding of FITC-labeled anti–P-selectin (FL1 on the Y-axis is relative fluorescence using the channel for fluorescein) to platelets was near background prior to activation with the thrombin receptor peptide but increased to 90% of cells stained following activation of fresh platelets.
Binding of FVIII, FVIIIa, C1C2, and C2 proteins to platelets
The direct labeling of C2 and C1C2 gave both the lowest background binding and the highest level of staining. Binding results were qualitatively similar using all 3 labeling systems (Table 1), which served as internal controls for several potentially confounding variables including nonspecific binding, variations in antibody affinities, and possible perturbations due to the 2296C substitution. Control assays indicated no difference in binding of C1C2 or C2 to platelets with or without CaCl2 (data not shown). Interestingly, prior to platelet activation, C1C2 bound in all 3 assay systems, whereas C2 showed little binding over the background level (Table 1). In contrast, both C1C2 and C2 bound to activated platelets in a concentration-dependent manner.
Table 1.
Binding of C domain proteins by flow cytometry
| Labeling method | C2 or C1C2 final concentration, μM | % platelets labeled with C2 |
% platelets labeled with C1C2 |
||
|---|---|---|---|---|---|
| Before activation | Activated | Before activation | Activated | ||
| ESH8 | 1.7 | <4 | 26 | 18 | 47 |
| 3.4 | 4.5 | 31 | 24 | 58 | |
| 6.8 | 5.6 | 42 | 31 | 66 | |
| Biotin | 0.3 | ND | ND | 19 | 39 |
| 0.6 | ND | ND | 37 | 63 | |
| 1.2 | ND | ND | 52 | 81 | |
| Direct | 0.025 | 0.1 | 7 | 9 | 32 |
| 0.05 | 0.2 | 22 | 15 | 73 | |
| 0.125 | 0.5 | 33 | 25 | 83 | |
| 0.25 | 1.1 | 55 | 35 | 91 | |
Results are representative of determinations carried out for at least 4 different platelet preparations. Labeling methods were (a) ESH8 followed by PE-anti–murine IgG, (b) biotinylated C1C2-2296C labeled with PE-streptavidin, and (c) C2-2296C or C1C2-2296C labeled directly with a sulfhydryl-fluorescein probe. C2 binding did not increase when higher concentrations were added (not shown). The percentage of cells labeled for ESH8- and biotin-labeling methods was calculated from flow cytometer output after subtracting background binding and gating as described in “Flow cytometry to quantitate platelet binding.” C2 and C1C2 binding were compared at equimolar concentrations using the same platelet preparation for each set of measurements for ESH8- and fluorescein-labeled experiments.
Activated indicates platelets following SFLLRN activation; ND, not determined.
Immunologic detection.
Binding of C1C2 and C2 was compared using 4 different preparations of activated platelets; representative results for one preparation are shown in Table 1. At 6.8 μM final protein concentration, the percentage of activated platelets binding C1C2 averaged 66% (range, 63%-73%), whereas the percentage of platelets that bound C2 averaged 46% (range, 36%-60%). A similar trend was seen for all 4 platelet preparations. Preincubation of 6.8 μM C1C2 or C2 with a 5-fold molar excess of ESH4 reduced the percentage of platelets labeled from 66% to 19% for C1C2 and from 42% to 9% for C2. Background binding, determined from concurrent controls run without addition of C1C2 or C2, showed 10% to 15% of the platelets became labeled by immunoglobulin, both before and after activation; this background binding was subtracted from all results for C2 and C1C2 binding presented in Table 1 (ESH8).
Platelets from a subject with severe hemophilia A showed no difference in C1C2 binding detected immunologically, prior to or after activation, compared with platelets from a healthy control donor that were assayed concurrently (not shown).
Detection using biotinylated C1C2-2296C.
For biotin-conjugated C1C2-2296C (C1C2b), lower concentrations of both ligand and platelets produced significant binding of C1C2b (Figure 3A; Table 1) compared with immunologic detection. C1C2b (1.2 μM) was added to 6 platelet preparations, and the percentage of platelets that produced a PE-streptavidin signal averaged 76% (range, 57%-84%). Binding of C1C2b to platelets prior to activation was again observed, but at a much lower level than after activation (Table 1). Control experiments showed background PE-streptavidin signals of up to 4%, which was subtracted from results for C1C2b binding presented in Table 1. The binding of C1C2b to platelets from 2 unrelated subjects with severe type 3 von Willebrand disease showed no difference compared with activated platelets from a healthy control donor that were assayed concurrently (not shown).
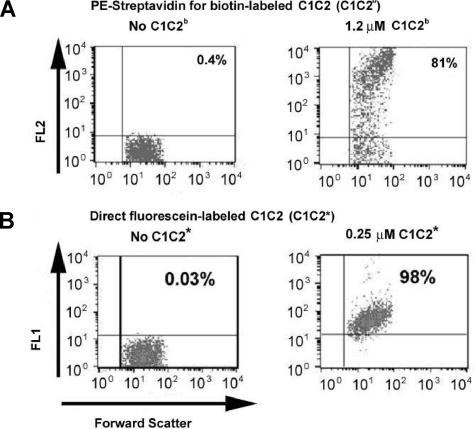
Figure 3.
Binding of biotinylated C1C2–2296C (C1C2b) and fluorescein-labeled C1C2-2296C (C1C2*) to platelets. (A) Binding of C1C2b to platelets was detected by PE-streptavidin (FL2 on the y-axis is relative fluorescence using the channel for phycoerythrin, PE). The left panel shows platelets with PE-streptavidin added as a control. The right panel shows platelets after addition of 1.2 μM C1C2b followed by PE-streptavidin. (B) Binding of fluorescein-labeled C1C2-2296C. The left panel shows the platelet-only control. The right panel shows platelets after addition of 0.25 μM C1C2* (FL1, y-axis). Note the reduced scatter and higher percentage of platelets binding compared with panel A, although only one fifth as much labeled C1C2 was used as in panel A. Shown are percentages of platelets binding C1C2b-PE-streptavidin or C1C2* above the background fluorescence signal from platelets without the fluorescent probe.
Detection with fluorescein-labeled C2 and C1C2.
Binding of C2-2296C and C1C2-2296C labeled covalently with a fluorescein probe was evaluated for 14 platelet preparations; a typical result is shown in Figure 3B and a representative dose-response result is shown in Table 1 and Figure 4. Upon adding 0.25 μM fluorescein-labeled C1C2 (C1C2*) to 5 activated platelet preparations, 98% of the platelets became labeled (range, 96%-99%); 5 additional platelet preparations were then assayed using a second preparation of C1C2*, and 91% became labeled (range, 83%-93%) at 0.25 μM. In this second set of activated platelets, fluorescein-labeled C2 (C2*) was tested and dose-dependent binding was observed. After adding 0.25 μM C2*, the average percentage of labeled, activated platelets was 55% (range, 47%-57%); all other concentrations of C2* showed similarly reduced staining compared with equimolar amounts of C1C2*.
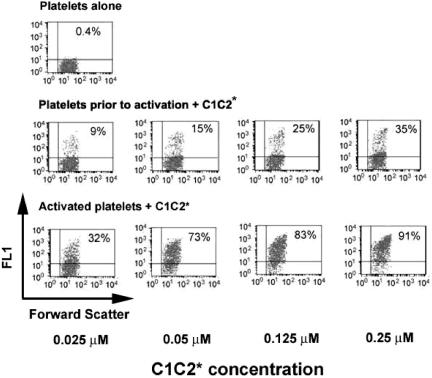
Figure 4.
Binding of fluorescein-labeled C1C2-2296C (C1C2*) to platelets before (center panel) and after (bottom panel) activation. The results are consistent with a smaller population of lower-affinity C1C2-binding sites on platelets prior to activation, whereas more than 90% of activated platelets became labeled after addition of 0.25 μM C1C2*. The upper-left panel shows the platelet control without added C1C2. Percentages are as in previous figures.
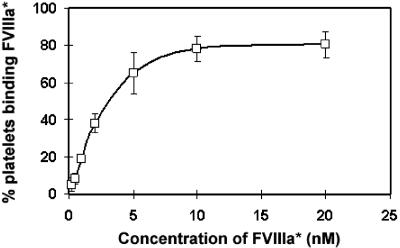
Binding to platelets before activation was tested by adding 0.025 μM to 0.25 μM C1C2*; the percentage of labeled platelets increased from 9% to 35% (Table 1). For C2*, only 1% of platelets before activation became labeled at 0.25 μM, compared with 55% average labeling after activation. At all protein concentrations tested, binding of C2* to activated platelets was considerably lower than that of equimolar C1C2*. Fluorescein-labeled FVIII or FVIIIa (FVIII* or FVIIIa*) bound to activated platelets; the latter is shown in Figure 5. Binding of fluorescein-labeled FVIII or FVIIIa was not detectable prior to platelet activation.
Figure 5.
Binding of fluorescein-labeled FVIIIa (FVIIIa*) to activated platelets. From 0.2 to 20 nM FVIIIa* was added to activated platelets. Maximal binding occurred at approximately 10 nM FVIIIa, and half-maximal binding gave an estimated Kd of approximately 2.5 nM. Similar results were obtained for binding of fluorescein-labeled FVIII to activated platelets (not shown). Error bars represent SD.
Competition experiments
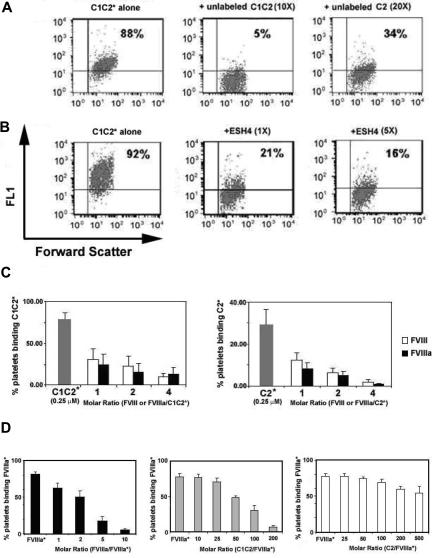
Further experiments were carried out using the fluorescein-labeled proteins. A 10-fold molar excess of unlabeled C1C2 reduced almost-saturation binding of fluorescein-labeled C1C2 to activated platelets from 88% to 5%. In contrast, a 20-fold molar excess of unlabeled C2 reduced binding of labeled C1C2 only to 34% (Figure 6A). When 0.25 μM fluorescein-labeled C2 was added to activated platelets, a 5-fold molar excess of unlabeled C2 reduced the percentage of stained, activated platelets from 54% to 14%; similar results were seen using the immunologic detection method (not shown).
Figure 6.
Competition assays. Fluorescein-labeled C1C2 (C1C2*), C2 (C2*), or FVIIIa (FVIIIa*) were added to activated platelets. The bound, labeled proteins were competed by adding increasing amounts of unlabeled C2, C1C2, FVIII, or FVIIIa; alternatively, platelet binding was examined after preincubation with a monoclonal antibody, ESH4. (A) C1C2* competition by C1C2 or C2: 0.125 μM C1C2* binding was reduced from 88% (left) to 5% after a 10-fold molar excess of unlabeled C1C2 (center), but adding a 20-fold molar excess of unlabeled C2 reduced the percentage of labeled platelets to only 34% (right). (B) C1C2* competition by ESH4: 0.125 μM C1C2* binding was reduced from 92% to 21% by adding equimolar ESH4 and to 16% with a 5-fold molar excess of ESH4. (C) C1C2* or C2* competition by FVIII or FVIIIa: 0.25 μM C1C2* (left) or C2* (right) was competed by adding unlabeled FVIII ( ) or FVIIIa (
) or FVIIIa ( ); controls are shown as (
); controls are shown as ( ). Before competition, binding was near saturation for C1C2* and for C2*; however, note the different scales for the left and right panels, which indicate that binding of C2* to activated platelets was lower than for C1C2*. Data represent the mean and standard deviations (upper error bars shown) for measurements using 6 different platelet preparations. Representative data for one of the C1C2* competition experiments plotted in histogram and scattergram format are available in Figure S1A,B, available on the Blood website; see the Supplemental Figure link at the top of the online article. (D) FVIIIa* competition by FVIIIa, C1C2, or C2: 10 nM FVIIIa* (near-saturation binding, Figure 5) was competed by FVIIIa (left panel,
). Before competition, binding was near saturation for C1C2* and for C2*; however, note the different scales for the left and right panels, which indicate that binding of C2* to activated platelets was lower than for C1C2*. Data represent the mean and standard deviations (upper error bars shown) for measurements using 6 different platelet preparations. Representative data for one of the C1C2* competition experiments plotted in histogram and scattergram format are available in Figure S1A,B, available on the Blood website; see the Supplemental Figure link at the top of the online article. (D) FVIIIa* competition by FVIIIa, C1C2, or C2: 10 nM FVIIIa* (near-saturation binding, Figure 5) was competed by FVIIIa (left panel,  ), C1C2 (center panel,
), C1C2 (center panel,  ), or C2 (right panel,
), or C2 (right panel,  ) at molar ratios as indicated. Data represent the mean and standard deviations (upper error bars shown) for measurements using 5 (for right and center panels) and 4 (for left panel) different platelet preparations. Representative data from one of the FVIIIa*-C1C2 competition experiments plotted in histogram and scattergram format are available in Figure S1C,D.
) at molar ratios as indicated. Data represent the mean and standard deviations (upper error bars shown) for measurements using 5 (for right and center panels) and 4 (for left panel) different platelet preparations. Representative data from one of the FVIIIa*-C1C2 competition experiments plotted in histogram and scattergram format are available in Figure S1C,D.
When equimolar ESH4 was preincubated with 0.25 μM fluorescein-labeled C1C2, labeling of activated platelets was reduced from 92% to 21%, and with a 5-fold molar excess of antibody the percentage of labeled platelets was 16% (Figure 6B). Preincubation of ESH4 with fluorescein-labeled C2 (not shown) reduced labeling of activated platelets from 50% to 16% with an equimolar ratio and to 6% with 5-fold molar ESH4 excess. Similar experiments using NMC-VIII/6 (not shown) found that the percentage of platelets binding fluorescein-labeled C1C2 was reduced from 91% to 19% and 7%, and the percentage of platelets binding fluorescein-labeled C2 was reduced from 59% to 2% and 1%, by equimolar or 5-fold molar excess IgG, respectively.
As shown in Figure 6C, FVIII or FVIIIa competed C1C2* and C2* for binding to activated platelets. At a 4-fold molar excess of either FVIII species, C2 binding was negligible (1%), whereas 11% of platelets still bound C1C2. The reciprocal experiment was also performed in which fluorescein-labeled FVIIIa was added to activated platelets followed by variable amounts of unlabeled FVIIIa, C1C2, or C2. C1C2 competed 50% of the labeled FVIIIa at approximately 80-fold molar excess (as did a ∼ 3-fold molar excess of unlabeled FVIIIa), whereas C2 competed only approximately 30% at a 500-fold molar excess (Figure 6D). Similar results were seen with FVIII (data not shown).
Estimates of platelet-binding sites
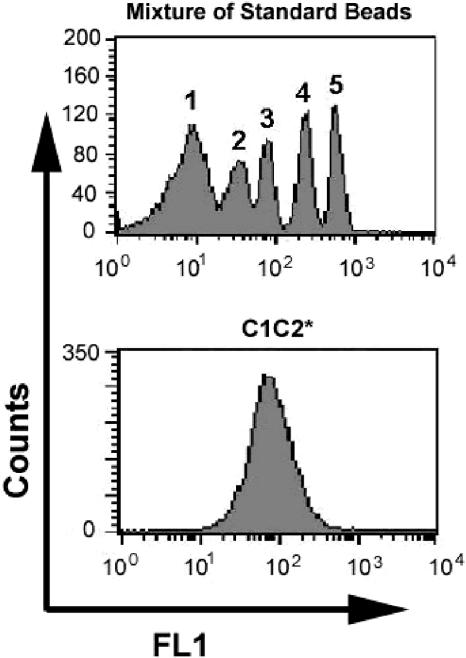
The numbers of C1C2-, C2-, and FVIIIa-binding sites per platelet were estimated from curves generated by measuring fluorescent intensities of standardized beads. Figure 7 shows a histogram of fluorescent intensities of a standard, FITC-labeled bead mixture, and a second histogram showing saturation binding of fluorescein-labeled C1C2. Saturation binding of fluorescein-labeled C1C2, C2, and FVIIIa to platelets before or after activation was carried out using 3 platelet preparations per protein. From the standard curve, the number of C1C2-binding sites was estimated to be approximately 7000 per activated platelet (range, 6100-7600), and approximately 700 per platelet prior to activation (range, 650-750). There were approximately 1400 binding sites per activated platelet for fluorescein-labeled C2 (range, 1330-1450) and, as seen earlier with immunologic detection of C2 binding, there was no significant binding of C2 to platelets prior to activation. Fluorescein-labeled FVIIIa did not bind to platelets before activation, and it bound to approximately 3000 sites per activated platelet.
Figure 7.
Estimated number of fluorescein-labeled C1C2 (C1C2*)–binding sites per platelet. The top histogram shows fluorescent signals from a mixture of 7.6 μm beads, where 1 is the peak for unlabeled beads and 2 through 5 are signals from the same beads containing increasing, defined amounts of FITC label. The bottom panel shows a histogram representation of near-saturation binding of 0.25 μM C1C2* to activated platelets. From 3 sets of similar measurements using different platelet preparations, the estimate ranged from 6100 to 7600 C1C2 molecules bound per activated platelet.
Discussion
The FVIIIa light chain binds to phospholipid vesicles14,15 and activated platelets10,14 with a higher affinity than C2, suggesting the C1 domain may contribute to membrane binding. A molecular model of the FVIII C1 domain,21 constructed from its homology with C2, indicated that C1 lacks the loops that expose adjacent hydrophobic residues (M2199-F2200 and L2251-L2252) in the analogous region of C2. Nevertheless, its strongly basic character and the conserved discoidin-type folding motif22 suggest that C1 may indeed play a role in membrane binding. The hemophilic FVIII C1 mutation R2150H reduces FVIII cofactor activity and is associated with neutralizing antibodies.23,24 FVIII-R2150H has lower affinity for VWF.25 Antibodies from an inhibitor patient with this mutation bound to A3-C1 fragments of wild-type FVIII and either blocked or potentiated FVIII-VWF binding24; furthermore, a patient-derived monoclonal antibody reduced the binding of FVIIIa to phosphatidylserine.26 A FVIII light chain–specific single-chain variable domain fragment (scFv) localized to the A3-C1 region and interfered with the binding of FVIIIa to phospholipid vesicles.27 These observations suggest a possible role for C1 and/or A3 in FVIIIa binding to membranes.
A model of FVIIIa bound to a phospholipid surface was obtained by fitting the C2 crystal structure and homology-based models of the other domains into density from 2D electron crystallography.28,29 This model indicates that C2 contacts the membrane, while C1 forms almost a right angle with C2, suggesting that C1 might not directly participate in membrane binding. In contrast, in a crystal structure of the homologous factor Vai protein (FVa, missing its A2 domain), C1 and C2 are oriented side by side,30 such that both domains could interact with phospholipid membranes. As in FVIII, the C2 domain contains the primary membrane-binding site of FV.31,32 The FV A333 and C134 domains also appear to interact with membranes. In FV and/or FVIII, the C domains may reorient during membrane attachment. Alternatively, conformational changes within the C2 domain(s) could increase the affinity of FVa and FVIIIa for membranes.14
In the current study, the FVIII C1C2 protein blocked FVIII clotting activity at a 4-fold lower concentration than C2. In this 1-stage assay, 50% inhibition was achieved at 105 molar excess of C2 over FVIII, essentially the same ratio that produced 50% inhibition of FVIII in an intrinsic X-ase assay.15 In addition to the functional assay, Takeshima et al15 also found that competition of FVIIIa binding to phospholipids by the FVIII light chain was achieved at equimolar FVIIIa/light chain ratios, but 50- to 100-fold excess C2 was required, suggesting that other regions besides C2 were required for optimal phospholipid binding. Both of these assay systems used phospholipid preparations. Binding to activated platelet surfaces, as in the present study, is a more physiologic assay system. Clotting assays were carried out with standard phospholipids or substituting a platelet lysate for the phospholipids. Inhibition by C1C2 was seen in both assays but C2, at the same concentration as C1C2, failed to inhibit when the platelet lysate was used.
Binding of C1C2 and C2 proteins to platelets before and after platelet activation was compared using 3 techniques. Labeling with ESH8, which does not interfere with membrane binding,35 followed by a fluorescent secondary antibody had the advantage that the C proteins themselves were unmodified. However, there was a high background signal due to antibody adsorption to platelets, and high concentrations of proteins were required to approach saturation of the fluorescent signal. Immunologic detection experiments carried out using platelets from a severe hemophilia A donor showed similar background binding, indicating that gel-filtered and albumin-washed normal platelet preparations had no detectable surface-bound FVIII. The binding of biotin-conjugated C1C2 to platelets from 2 unrelated subjects with severe type 3 von Willebrand disease was also similar to results for healthy controls, consistent with VWF binding to FVIII having little or no role in binding of FVIIIa to activated platelets.
The nonimmunologic labeling strategies used C2 and C1C2 mutant proteins labeled with biotin or fluorescein at an introduced free cysteine residue. Ser2296 is distant from the membrane-binding site of C2; the crystal structure of C2-S2296C11 confirmed it is folded properly. Fluorescein-labeled C2-2296C and C1C2-2296C readily bound to activated platelets. These proteins reached saturation binding at lower concentrations than the immunologic method required, possibly reflecting relatively lower affinities of the antibodies. Results of assays using the 3 methods were qualitatively similar, however (Table 1). C1C2 consistently bound to activated platelets at higher levels than C2 (Table 1; Figure 4), and it was more effective than C2 at competing with fluorescein-labeled FVIIIa, C1C2, and C2 for platelet-binding sites.
Only C1C2 showed significant, dose-dependent binding to platelets prior to activation. P-selectin was not detectable on platelet surfaces before activation, so this residual approximately 10% C1C2 binding did not appear to reflect a subpopulation of activated platelets. This C1C2 binding was only partially competed by FVIII or FVIIIa. Fluorescein-labeled FVIII and FVIIIa did not bind to platelets before activation, as reported by others,19,36,37 so this observed C1C2 binding is unlikely to be physiologically relevant. However, Nesheim et al38 reported 450 binding sites per activated platelet for 35S-Met–labeled FVIII and approximately 20 FVIII molecules bound per nonactivated platelet. Rosing et al4 measured a low level of FXa activity in assays using washed, nonactivated platelets that was abolished by phospholipase A2, suggesting FVIIIa could enhance the “tenase” reaction at the resting platelet surface, but significant generation of FXa by FVIIIa/FIXa does not occur on resting platelets.39 Binding of procoagulant proteins FV, FVa, and FXa to nonactivated platelet surfaces has also been demonstrated.40 The concentrations of C1C2 required for saturation binding to activated and nonactivated platelets were well above the Kd determinations for FVIII and FVIIIa.14 The high-affinity VWF-FVIII interaction precludes significant transfer of FVIII to the surface of a resting platelet41; thus binding of FVIII to resting platelets is unlikely to have physiological significance. However, our results are consistent with previous observations that some FVIIIa may bind to sites on the platelet surface prior to platelet activation.42
The estimate of 7000 C1C2-binding sites per activated platelet (Figure 7) was 4- to 5-fold greater than the 1400 sites estimated for C2. Previous estimates of FVIIIa molecules bound to activated platelets have ranged from 200 to 2000 molecules bound per platelet,10,19,37,38,43 although higher estimates of up to approximately 25 000 sites per activated platelet have also been reported.42–45 Different methods of activating the platelets might account for some of this variation, and heterogeneity in the activated platelet population can also complicate analysis of binding.43 However, the observed differences in binding of C2 and C1C2 to platelets compared with the present study under the same conditions indicate that C1 confers affinity for the platelet surface both before and after activation. It is important to emphasize that the use of flow techniques provides data relevant to saturation binding, and thus the current study should yield fairly accurate estimates of the maximum number of binding sites per platelet.
ESH4 interferes with the binding of C2 to negatively charged phospholipid surfaces46 as does NMC-VIII/6 (K.P.P., unpublished data, January 10, 2006), which is similar to NMC-VIII/5.35 Binding of C1C2 to platelets was partially blocked by both ESH4 and NMC-VIII/6. In contrast, binding of C2 to the activated platelet membrane was almost entirely blocked by ESH4 or NMC-VIII/6, indicating that C1C2 contributes additional binding sites or, alternatively, confers higher affinity through conformational changes.
Our results support relative affinities for activated platelet surfaces of FVIIIa > C1C2 > C2. Further studies are needed to distinguish direct versus indirect effects of the C1 domain in binding of FVIII and FVIIIa in contributing to the binding interactions. The physiological attachment of FVIIIa and FIXa to the platelet surface likely involves interactions with other membrane components besides phospholipids19,38 and is influenced by various agonists (eg, fibrin).37 The orientation of C1 with respect to the membrane surface and the roles of specific residues in membrane binding are the subjects of current investigations.
Supplementary Material
Acknowledgments
We thank Midori Shima for providing NMC/VIII-6, and Randal Kaufman for FVIII cDNA. We also thank Xiaoping Wu and Noel Blake for flow cytometry assistance, Gayle Teramura for help preparing platelets, and Laura Stewart for performing FVIII clotting activity assays.
This work was supported in part by NIH R01-HL 071093-01 (A.R.T.) and a Judith Graham Pool fellowship from the National Hemophilia Foundation for T.-C.H.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: T.-C.H. performed the research; K.P.P. provided critical materials, design, and analysis; A.R.T. contributed to the design and analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Arthur R. Thompson, Puget Sound Blood Center, 921 Terry Ave, Seattle, WA 98104-1256; e-mail: arthomps@u.washington.edu.
References
- 1.Leyte A, Verbeet MP, Brodniewicz-Proba T, Van Mourik JA, Mertens K. The interaction between human blood-coagulation factor VIII and von Willebrand factor: characterization of a high-affinity binding site on factor VIII. Biochem J. 1989;257:679–683. doi: 10.1042/bj2570679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foster PA, Fulcher CA, Houghten RA, Zimmerman TS. A synthetic factor VIII peptide of eight amino acid residues (1677–1684) contains the binding region of an anti-factor VIII antibody which inhibits the binding of factor VIII to von Willebrand factor. Thromb Haemost. 1990;63:403–406. [PubMed] [Google Scholar]
- 3.Saenko EL, Scandella D. The acidic region of the factor VIII light chain and the C2 domain together form the high affinity binding site for von willebrand factor. J Biol Chem. 1997;272:18007–18014. doi: 10.1074/jbc.272.29.18007. [DOI] [PubMed] [Google Scholar]
- 4.Rosing J, van Rijn JL, Bevers EM, van Dieijen G, Comfurius P, Zwaal RF. The role of activated human platelets in prothrombin and factor X activation. Blood. 1985;65:319–332. [PubMed] [Google Scholar]
- 5.Jurk K, Kehrel BE. Platelets: physiology and biochemistry. Semin Thromb Hemost. 2005;31:381–392. doi: 10.1055/s-2005-916671. [DOI] [PubMed] [Google Scholar]
- 6.van Dieijen G, Tans G, Rosing J, Hemker HC. The role of phospholipid and factor VIIIa in the activation of bovine factor X. J Biol Chem. 1981;256:3433–3442. [PubMed] [Google Scholar]
- 7.Duffy EJ, Lollar P. Intrinsic pathway activation of factor X and its activation peptide-deficient derivative, factor Xdes-143–191. J Biol Chem. 1992;267:7821–7827. [PubMed] [Google Scholar]
- 8.Gilbert GE, Arena AA. Activation of the factor VIIIa-factor IXa enzyme complex of blood coagulation by membranes containing phosphatidyl-L-serine. J Biol Chem. 1996;271:11120–11125. doi: 10.1074/jbc.271.19.11120. [DOI] [PubMed] [Google Scholar]
- 9.Duffy EJ, Parker ET, Mutucumarana VP, Johnson AE, Lollar P. Binding of factor VIIIa and factor VIII to factor IXa on phospholipid vesicles. J Biol Chem. 1992;267:17006–17011. [PubMed] [Google Scholar]
- 10.Ahmad SS, Walsh PN. Role of the C2 domain of factor VIIIa in the assembly of factor-X activating complex on the platelet membrane. Biochemistry. 2005;44:13858–13865. doi: 10.1021/bi0511033. [DOI] [PubMed] [Google Scholar]
- 11.Pratt KP, Shen BW, Takeshima K, Davie EW, Fujikawa K, Stoddard BL. Structure of the C2 domain of human factor VIII at 1.5 A resolution. Nature. 1999;402:439–442. doi: 10.1038/46601. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert GE, Kaufman RJ, Arena AA, Miao H, Pipe SW. Four hydrophobic amino acids of the factor VIII C2 domain are constituents of both the membrane-binding and von Willebrand factor-binding motifs. J Biol Chem. 2002;277:6374–6381. doi: 10.1074/jbc.M104732200. [DOI] [PubMed] [Google Scholar]
- 13.Lewis DA, Pound ML, Ortel TL. Contributions of Asn2198, Met2199, and Phe2200 in the factor VIII C2 domain to cofactor activity, phospholipid-binding, and von Willebrand factor-binding. Thromb Haemost. 2003;89:795–802. [PubMed] [Google Scholar]
- 14.Saenko EL, Scandella D, Yakhyaev AV, Greco NJ. Activation of factor VIII by thrombin increases its affinity for binding to synthetic phospholipid membranes and activated platelets. J Biol Chem. 1998;273:27918–27926. doi: 10.1074/jbc.273.43.27918. [DOI] [PubMed] [Google Scholar]
- 15.Takeshima K, Smith C, Tait J, Fujikawa K. The preparation and phospholipid binding property of the C2 domain of human factor VIII. Thromb Haemost. 2003;89:788–794. [PubMed] [Google Scholar]
- 16.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 17.Pace CN, Vajdos F, Fee L, Grimsley G, Gray T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995;4:2411–2423. doi: 10.1002/pro.5560041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vehar GA, Keyt B, Eaton D, et al. Structure of human factor VIII. Nature. 1984;312:337–342. doi: 10.1038/312337a0. [DOI] [PubMed] [Google Scholar]
- 19.Ahmad SS, Scandura JM, Walsh PN. Structural and functional characterization of platelet receptor-mediated factor VIII binding. J Biol Chem. 2000;275:13071–13081. doi: 10.1074/jbc.275.17.13071. [DOI] [PubMed] [Google Scholar]
- 20.Thompson AR, Chen SH. Characterization of factor IX defects in hemophilia B patients. Methods Enzymol. 1993;222:143–169. doi: 10.1016/0076-6879(93)22011-4. [DOI] [PubMed] [Google Scholar]
- 21.Liu ML, Shen BW, Nakaya S, et al. Hemophilic factor VIII C1- and C2-domain missense mutations and their modeling to the 1.5-angstrom human C2-domain crystal structure. Blood. 2000;96:979–987. [PubMed] [Google Scholar]
- 22.Fuentes-Prior P, Fujikawa K, Pratt KP. New insights into binding interfaces of coagulation factors V and VIII and their homologues lessons from high resolution crystal structures. Curr Protein Pept Sci. 2002;3:313–339. doi: 10.2174/1389203023380639. [DOI] [PubMed] [Google Scholar]
- 23.Hay CR. Factor VIII inhibitors in mild and moderate-severity haemophilia A. Haemophilia. 1998;4:558–563. doi: 10.1046/j.1365-2516.1998.440558.x. [DOI] [PubMed] [Google Scholar]
- 24.Peerlinck K, Jacquemin MG, Arnout J, et al. Antifactor VIII antibody inhibiting allogeneic but not autologous factor VIII in patients with mild hemophilia A. Blood. 1999;93:2267–2273. [PubMed] [Google Scholar]
- 25.Gilles JG, Lavend'homme R, Peerlinck K, et al. Some factor VIII (FVIII) inhibitors recognise a FVIII epitope(s) that is present only on FVIII-vWF complexes. Thromb Haemost. 1999;82:40–45. [PubMed] [Google Scholar]
- 26.Jacquemin M, Benhida A, Peerlinck K, et al. A human antibody directed to the factor VIII C1 domain inhibits factor VIII cofactor activity and binding to von Willebrand factor. Blood. 2000;95:156–163. [PubMed] [Google Scholar]
- 27.Lewis DA, Bovenschen N, Mertens K, Voorberg J, Ortel TL. Phospholipid vesicles interfere with the binding of antibody fragments to the light chain of factor VIII. Thromb Haemost. 2005;93:833–841. doi: 10.1160/TH04-11-0729. [DOI] [PubMed] [Google Scholar]
- 28.Stoylova SS, Lenting PJ, Kemball-Cook G, Holzenburg A. Electron crystallography of human blood coagulation factor VIII bound to phospholipid monolayers. J Biol Chem. 1999;274:36573–36578. doi: 10.1074/jbc.274.51.36573. [DOI] [PubMed] [Google Scholar]
- 29.Stoilova-McPhie S, Villoutreix BO, Mertens K, Kemball-Cook G, Holzenburg A. 3-Dimensional structure of membrane-bound coagulation factor VIII: modeling of the factor VIII heterodimer within a 3-dimensional density map derived by electron crystallography. Blood. 2002;99:1215–1223. doi: 10.1182/blood.v99.4.1215. [DOI] [PubMed] [Google Scholar]
- 30.Adams TE, Hockin MF, Mann KG, Everse SJ. The crystal structure of activated protein C-inactivated bovine factor Va: Implications for cofactor function. Proc Natl Acad Sci U S A. 2004;101:8918–8923. doi: 10.1073/pnas.0403072101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ortel TL, Devore-Carter D, Quinn-Allen M, Kane WH. Deletion analysis of recombinant human factor V: evidence for a phosphatidylserine binding site in the second C-type domain. J Biol Chem. 1992;267:4189–4198. [PubMed] [Google Scholar]
- 32.Majumder R, Quinn-Allen MA, Kane WH, Lentz BR. The phosphatidylserine binding site of the factor Va C2 domain accounts for membrane binding but does not contribute to the assembly or activity of a human factor Xa-factor Va complex. Biochemistry. 2005;44:711–718. doi: 10.1021/bi047962t. [DOI] [PubMed] [Google Scholar]
- 33.Kalafatis M, Rand MD, Mann KG. Factor Va-membrane interaction is mediated by two regions located on the light chain of the cofactor. Biochemistry. 1994;33:486–493. doi: 10.1021/bi00168a013. [DOI] [PubMed] [Google Scholar]
- 34.Saleh M, Peng W, Quinn-Allen MA, et al. The factor V C1 domain is involved in membrane binding: identification of functionally important amino acid residues within the C1 domain of factor V using alanine scanning mutagenesis. Thromb Haemost. 2004;91:16–27. doi: 10.1160/TH03-04-0222. [DOI] [PubMed] [Google Scholar]
- 35.Scandella D, Gilbert GE, Shima M, et al. Some factor VIII inhibitor antibodies recognize a common epitope corresponding to C2 domain amino acids 2248 through 2312, which overlap a phospholipid-binding site. Blood. 1995;86:1811–1819. [PubMed] [Google Scholar]
- 36.Li X, Gabriel DA. The physical exchange of factor VIII (FVIII) between von Willebrand factor and activated platelets and the effect of the FVIII B-domain on platelet binding. Biochemistry. 1997;36:10760–10767. doi: 10.1021/bi970052+. [DOI] [PubMed] [Google Scholar]
- 37.Phillips JE, Lord ST, Gilbert GE. Fibrin stimulates platelets to increase factor VIIIa binding site expression. J Thromb Haemost. 2004;2:1806–1815. doi: 10.1111/j.1538-7836.2004.00919.x. [DOI] [PubMed] [Google Scholar]
- 38.Nesheim ME, Pittman DD, Wang JH, Slonosky D, Giles AR, Kaufman RJ. The binding of 35S-labeled recombinant factor VIII to activated and unactivated human platelets. J Biol Chem. 1988;263:16467–16470. [PubMed] [Google Scholar]
- 39.Rawala-Sheikh R, Ahmad SS, Ashby B, Walsh PN. Kinetics of coagulation factor X activation by platelet-bound factor IXa. Biochemistry. 1990;29:2606–2611. doi: 10.1021/bi00462a025. [DOI] [PubMed] [Google Scholar]
- 40.Tracy PB, Nesheim ME, Mann KG. Coordinate binding of factor Va and factor Xa to the unstimulated platelet. J Biol Chem. 1981;256:743–751. [PubMed] [Google Scholar]
- 41.Nesheim M, Pittman DD, Giles AR, et al. The effect of plasma von Willebrand factor on the binding of human factor VIII to thrombin-activated human platelets. J Biol Chem. 1991;266:17815–17820. [PubMed] [Google Scholar]
- 42.Soberano ME, Clarke D, Zucker MB. Binding of thrombin-activated human factor VIII to platelets. Br J Haematol. 1986;64:571–585. doi: 10.1111/j.1365-2141.1986.tb02213.x. [DOI] [PubMed] [Google Scholar]
- 43.Panteleev MA, Ananyeva NM, Greco NJ, Ataullakhanov FI, Saenko EL. Two subpopulations of thrombin-activated platelets differ in their binding of the components of the intrinsic factor X-activating complex. J Thromb Haemost. 2005;3:2545–2553. doi: 10.1111/j.1538-7836.2005.01616.x. [DOI] [PubMed] [Google Scholar]
- 44.Perret BA, Furlan M, Beck EA. Binding of bovine factor VIII-coated colloidal gold particles to receptors on platelet membranes. Agents Actions. 1981;11:657–659. doi: 10.1007/BF01978784. [DOI] [PubMed] [Google Scholar]
- 45.Kempton CL, Hoffman M, Roberts HR, Monroe DM. Platelet heterogeneity: variation in coagulation complexes on platelet subpopulations. Arterioscler Thromb Vasc Biol. 2005;25:861–866. doi: 10.1161/01.ATV.0000155987.26583.9b. [DOI] [PubMed] [Google Scholar]
- 46.Brinkman HJ, Mertens K, van Mourik JA. Phospholipid-binding domain of factor VIII is involved in endothelial cell-mediated activation of factor X by factor IXa. Arterioscler Thromb Vasc Biol. 2002;22:511–516. doi: 10.1161/hq0302.105359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.