Abstract
The T-cell leukemia 1 (TCL1) oncoprotein is overexpressed by chromosomal rearrangement in the majority of cases of T-cell prolymphocytic leukemia (T-PLL). In vitro, TCL1 can modulate the activity of the serine-threonine kinase AKT, a downstream effector of T-cell receptor (TCR) signaling. In a series of 86 T-PLL tumors, we show that expression of TCR, and levels of TCL1 and activated AKT are adverse prognostic markers. High-level TCL1 in TCR-expressing T-PLL is associated with higher presenting white blood cell counts, faster tumor cell doubling, and enhanced in vitro growth response to TCR engagement. In primary tumors and TCL1-transfected T-cell lines, TCR engagement leads to rapid recruitment of TCL1 and AKT to transient membrane activation complexes that include TCR-associated tyrosine kinases, including LCK. Pharmacologic inhibition of AKT activation alters the localization, stability, and levels of these transient TCL1-AKT complexes and reduces tumor cell growth. Experimental introduction and knockdown of TCL1 influence the kinetics and strength of TCR-mediated AKT activation. We propose that in T-PLL, TCL1 represents a highly regulated, targetable modulator of TCR-mediated AKT growth signaling.
Introduction
T-cell leukemia 1 (TCL1) was identified as the oncogene insertionally activated by the T-cell receptor enhancer through inv(14)(q11;q32.1) or t(14;14) (q11;q32.1) in T-cell prolymphocytic leukemia (T-PLL).1–3 TCL1 activation is seen in 70% to 80% of T-PLL but not in other T-cell tumor types.4–9 Most cases of T-PLL are highly aggressive with optimal therapy not yet established.6,10–12 Although there are few large series, several immunophenotypic tumor subsets with differing outcomes likely exist.6,8,13–17 TCL1, which is not expressed in nonneoplastic mature T cells, is one member of a family of small β-barrel proteins found predominantly in the cytoplasm whose mechanism of action remains unresolved. Rare cases of T-PLL overexpress another TCL1 homolog, MTCP1, through a X;14 translocation.18,19
When overexpressed in nonlymphoid cell line models, TCL1 has been shown to bind to the pleckstrin homology (PH) domain of AKT and to regulate its activity.20–22 This finding and subsequent structural data have suggested that TCL1 homodimers could act by promoting AKT dimerization, thus facilitating AKT transphosphorylation.23,24 Given the rarity of this tumor type, the regulation of TCL1 and AKT in primary T-PLL tumor cells has not been previously explored.
Here, we compare the clinical and immunophenotypic patterns of T-PLL and identify T-cell receptor (TCR) expression, AKT activation, and TCL1 levels as correlates of poor outcome. We show that TCR stimulation leads to rapid recruitment of TCL1, AKT, and tyrosine kinases to membrane-associated activation complexes and that introduction of TCL1 into T-cell leukemia lines or TCL1 knockdown leads to altered kinetics of AKT activation and substrate phosphorylation.
Methods
Patient samples and hematologic parameters
This study included all patients with T-PLL who were diagnosed at The University of Texas M. D. Anderson Cancer Center between 1995 and 2006 and who had available pathologic material and sufficient clinical information. The diagnosis of T-PLL was established based on the revised World Health Organization criteria25 and a previously published algorithm for diagnosis of T-cell leukemias that includes clinical, morphologic, immunophenotypic, and karyotypic features.6,8 Our routine flow cytometric panel included CD3, CD4, CD5, CD7, CD8, CD25, and CD26 and used cluster analysis to separate tumor cells from nonneoplastic T-cell populations.26,27 Tumor samples were acquired in accordance with a protocol approved by the M. D. Anderson Cancer Center institutional review board and in accordance with the Declaration of Helsinki.
Lymphocyte doubling time (LDT) was determined before treatment initiation based on at least 3 lymphocyte counts and was categorized as follows: 6 months or fewer (aggressive; score 2), 6 to 12 months (intermediate; score 1), and more than 12 months (chronic-indolent; score 0). The primary tumor cells were isolated by Ficoll Paque Plus (Amersham, Piscataway, NJ) centrifugation of peripheral blood (PB). Trypan blue staining was used to assess viability.
Primary tumor cultures and cell lines
T-PLL tumor cells were isolated from blood samples comprising 90% or more of all white blood cells (WBCs). Primary tumors from 26 cases as well as established cell lines were grown in 15% fetal calf serum in RPMI culture media with 1% penicillin-streptomycin (Gibco Laboratories, Grand Island, NY) and, in the case of cell lines, harvested in midlogarithmic phase. Stimulatory reagents included interleukin-2 (IL2) at 25 ng/mL (PeproTech, Rocky Hill, NJ), phorbol-12-myristate-13-acetate (PMA) at 50 to 100 ng/mL (Sigma-Aldrich, St Louis, MO), and ionomycin and concanavalin A (ConA) at 25 to 50 μg/mL and 1 μM, respectively (Calbiochem, La Jolla, CA). For TCR cross-linking, 50 μg/mL anti-CD3 (OKT3) and 25 μg/mL anti-CD28 (CD28.2; both eBioscience, San Diego, CA), coated onto culture plates before addition of sample, were used. Working concentrations for the PI3-kinase (PI3K) inhibitor LY294002 (Cell Signaling Technology, Beverly, MA) were 10 to 20 μM; for the integrin linked kinase (ILK) inhibitor QLT0267 (QLT, Vancouver, BC), 10 to 40 μM; and for the competitive AKT kinase inhibitor A443654 (Abbott Laboratories, Abbott Park, IL), 0.5 to 5 μM. The TCL1-negative (−) cell lines used included immature (Jurkat, CCRF-HSB2, CCRF-CEM, Molt-4) as well as mature (HH, Hut78, SKW3) T-cell tumor lines (ATCC, Manassas, VA) and TCL1 transfectants of all of these. A functional TCR pathway following CD3 engagement was present in Jurkat (derived from precursor T-lymphoblastic leukemia), HH, and Hut78 (both derived from CD4+ Sézary leukemias).
Establishment of TCL1-expressing leukemic cell lines
A retroviral TCL1 construct containing human TCL1 cDNA in a murine stem cell virus (MSCV) vector as described earlier4 was used to create stably transfected T-cell lines. The Phoenix packaging cell line 293T (courtesy of Dr G. Nolan, Stanford) was transfected with isolated vector plasmid DNA by CaCl2 coprecipitation and purified for cells with the integrated construct by 10 μg/mL puromycin (AG Scientific, San Diego, CA). After preincubation with 5 μg/mL polybrene, target cells were infected with the viral supernatants containing either MSCV-GFP-ires-puro or MSCV-TCL1-ires-puro4 in an optimized centrifugation protocol and selected for puromycin resistance. Efficiency of T-cell line transfection, as evaluated by the GFP-containing control, ranged from 40% to 90% before selection, with stably TCL1-transfected lines obtained after selection from all cell lines.
siRNA transfection experiments
A TCL1-expressing subclone of the T-cell leukemia line HH was transfected with 15 μg TCL1 (SMARTpool; Dharmacon, Lafayette, CO) or nonsense scrambled siRNA duplexes (Invitrogen, Carlsbad, CA) using nucleofection (Solution V, program O16; Amaxa, Cologne, Germany). The cultures, all with a cell viability of more than 80%, were studied at 72 hours after transfection for effects of TCR engagement. Functional readouts included TCL1 and phosphoprotein levels, AKT kinase activity, and cell proliferation.
MTT proliferation assays
For estimation of T-cell proliferation and cytotoxicity, we assessed metabolic activity and viability in the colorimetric MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra-zolium bromide) assay (CellTiter96; Promega, Madison, WI) as described earlier.28 Experiments were repeated at least twice.
Production of a monoclonal TCL1 antibody
A monoclonal antibody (designated 1-21) was produced against a specific 14–amino acid peptide epitope that maps to the outer α-loop region of TCL1, by standard immunization procedures and fusion of BALB/c mice spleen cells with the myeloma line NS1 followed by screening of hybridomas with the immunizing peptide (Antibody Shared Resource; Cold Spring Harbor Laboratory, Cold Spring, NY). Western blots using this antibody detected a strong single 13.5-kDa band in cells transfected with a TCL1-expressing construct. The specificity of the antibody was verified by mass spectrometry (MS)–based peptide sequencing of the band. Briefly, proteins immunoprecipitated with the 1-21 antibody were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), visualized by silver staining, excised, digested overnight with modified trypsin (Promega), and analyzed by liquid chromatography-tandem MS (LC-MS/MS) using an electrospray ionization ion-trap mass spectrometer (LTQ; Thermo Electron, San Jose, CA). Mass spectra were searched against the NCBI nonredundant human database (accessed June 2004)29 using Mascot (Matrix Sciences, Toronto, ON). Search results indicated antibody specificity for TCL1 based on 3 peptide sequences. Sequences derived from the related proteins TCL1B (TML1) and MTCP1 were not detected.
Western blot analysis
Whole-cell lysates or fractionated nuclear/cytoplasmic extracts (NE-PER; Pierce Biotechnology, Rockford, IL) were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes, and proteins were detected using the enhanced chemiluminescence (ECL) system (Amersham). Lysis buffers are described elsewhere.28,30 Primary antibodies included TCL1 (1-21, 1:1000), a polyclonal rabbit anti-AKT1/2 (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA), as well as pAKT-S473, pERK1/2-T202/Y204, pLAT-Y171, pLCK-Y394, pZAP70-Y493, pFKHR-S256, LCK, and the monoclonal anti–pan-pAKT-substrate RXRXXS/T-motif antibody (110B7; all from Cell Signaling Technology at 1:500-1:1000). Anti–β-actin (AC-15, 1:10 000; Sigma-Aldrich) and PARP1 (1:1000; BD PharMingen, Franklin Lakes, NJ) were used for loading controls, with the latter in the nuclear extraction experiments. Signal intensity levels were compared based on densitometry analysis using the ImageJ software (National Institutes of Health, Bethesda, MD). TCL1 and pAKT-S473 levels on Western blot were graded as absent (0), low (1), and high (2).
AKT kinase activity assays
In addition to pAKT-substrate RXRXXS/T-motif Western blots, in vitro kinase assays estimating AKT activity by measuring phosphorylation of its target GSK3 through purified cellular AKT were performed with slight modifications of the manufacturer's instructions (Cell Signaling Technology). Sonicated whole-cell lysates with 500 μg total protein were incubated with agarose bead–immobilized AKT (clone 1G1) antibody overnight at 4°C. After spin-down, collection of the supernatant for later analysis, and wash of antibody-AKT immunoprecipitates, samples were incubated with recombinant GSK3α/β-paramyosin fusion protein in the presence of 200 μM ATP in Tris- and Mg2+-based kinase buffer for 30 minutes at 30°C. Denatured samples were then analyzed for GSK3 phosphorylation using an anti-pGSK3α/β (S21/9) antiserum in Western blots of immunoprecipitates. Levels of coimmunoprecipitated proteins were compared with those in whole-cell lysates as well as those in the postimmunoprecipitation supernatants. Parallel negative controls included mouse IgG isotype plus agarose beads instead of immobilized AKT antibody as well as lysis buffer only plus antibody to analyze for nonspecific protein carryover. Direct coincubation with A443654 in the in vitro kinase reaction suppressed GSK3 and RXRXXS/T-motif phosphorylation 2 to 3 times more than when incubated in the cultures before cell lysis at the same concentration. However, as a control, restriction of A443654 coincubation to the kinase reaction did not, in contrast to cellular preinhibition (“Results”), affect the amount of detected TCL1 in the AKT immunoprecipitates.
Immunohistochemistry
Sections of formalin-fixed, paraffin-embedded bone marrow, consisting of whole core biopsy specimens or tissue microarrays containing triplicate cores, were used for peroxidase-based immunohistochemistry (LSAB+; Dakocytomation, Carpinteria, CA) with detection using diaminobenzidine. Primary antibodies included TCL1 (1-21; 1:500), pAKT-S473 (1:200), pLCK-Y394 (1:100), pLAT-Y171 (1:200), and pZAP70-Y493 (1:250) (all from Cell Signaling Technology); CD38 (SPC32; Novocastra, Newcastle upon Tyne, United Kingdom); CD45RA (4KB5, prediluted; Biomeda, Foster City, CA); CD45RO (UCHL1, 1:2000; Dakocytomation); CD69 (CH11, 1:25; Novocastra); and SHP1/2 (NL213, 1:500; Upstate, Lake Placid, NY). Antigen retrieval was done as published.28,30 Immunohistochemical results were scored semiquantitatively based on the same 3-tier scoring system (0 indicates absent; 1, dim; 2, strong staining). Confirmatory TCL1 assessment, with concordant results to immunostaining except in 2 cases, was done by Western blot or flow cytometry with a standard permeabilization protocol and AlexaF488-labeled TCL1 antibody (1-21). The specificity of each phosphoprotein antiserum was confirmed by detection of appropriate-sized bands on Western blots using 30 T-PLL lysates.
Confocal microscopy
Preparation and fixation of cytospins from single-cell suspensions as well as staining procedures were done as described.28 The primary antibodies detected TCL1 (clone 1-21, dilution: 1:50; and a polyclonal TCL1 rabbit antiserum at 1:60031), AKT1/2 (1:200; Santa Cruz Biotechnology), and pS473-AKT (clone 587F11, 1:200) and LCK (1:25; both from Cell Signaling Technology). The secondary antibodies were Cy3-conjuated AffiniPure Donkey Anti–Rabbit IgG (1:300) and fluorescein (FITC)–conjugated AffiniPure Donkey Anti–Mouse IgG (1:30), both from Jackson ImmunoResearch Laboratories (West Grove, PA).
Statistical analyses
Computations were done using the STATISTICA software (StatSoft, Tulsa, OK) based on various scoring systems for discrete data (ie, protein levels) in combination with continuous (ie, LDT) and secondarily categorized values (ie, groups of aggressiveness based on LDT). The significance level was P less than .05. The chi-square test was used for dichotomized categoric variables with Fisher exact P value indicated for 3 to 5 dependent categories. The Mann-Whitney and Kruskal-Wallis tests were used to compare continuous variables across 2 and more than 2 groups, respectively, with only 2-sided P values reported. Difference estimations in Kaplan-Meier univariate analyses were done by log-rank statistics, and Cox proportional hazards regression was used for multivariate analyses.
Results
High-level expression of TCL1 is associated with the hyperproliferative subset of T-PLL
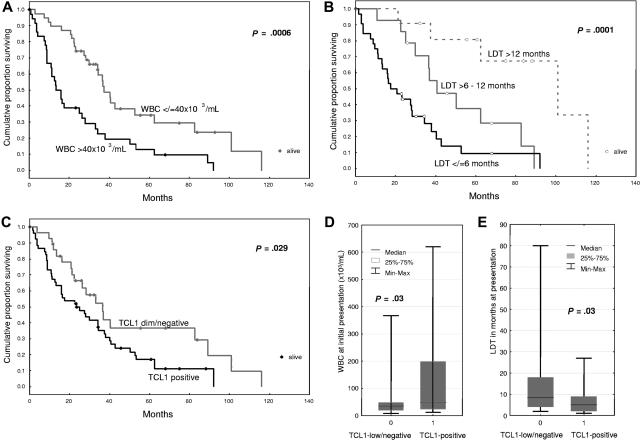
Table 1 summarizes the clinicopathologic features of the 86 cases of T-PLL (45 male and 41 female patients, median age at diagnosis of 62 years). Median WBC count at presentation was 40.0 × 109/L (range: 14.0-621.0 × 109/L) with a median peak WBC count during disease course of 179 × 109/L. After a maximum total follow up of 116.1 months (median: 25.9 months; average: 30.8 months), 20.9% (18/86) of patients were alive. Of these, only 5 patients showed, after a median of 77.8 months after diagnosis, no signs of persistent disease following therapy. The median overall survival (OS) of all patients was 27.4 months with a 5-year OS rate of 21%. There was a highly significant correlation between poor outcome and high WBC count at presentation (P < .001, Figure 1A), shorter tumor lymphocyte doubling time (LDT) (P < .001, Figure 1B) as well as with older age at presentation (Table 1). Identification of inv(14)(q11;q32.1) or t(14;14)(q11;q32.1) by conventional karyotyping was associated with accompanying deletion of chromosome 11q22–23 region and/or trisomy 8/isochromosome 8q.
Table 1.
Clinical, immunophenotypic, and molecular features of 86 cases of T-PLL
| Parameter | Distribution | Statistically significant correlations |
|---|---|---|
| Clinical features | ||
| Median age at diagnosis, y (range) | 62 (46-83) | Age > 62 y associated with shorter OS (P = .018; for >65 y P = .024) |
| Male patients, no. (%) | 45 (52.3) | — |
| Median presenting WBC count, ×109/L (range) | 40 (1.6-621) | Higher initial WBC correlated with shorter OS (P< .001); only 1 patient presented with leukopenia and 1 with normal lymphocyte counts |
| Median peak WBC count, ×109/L (range) | 179 (1.9-799) | Higher peak WBC correlated with shorter OS (P = .039, ≤/> median) |
| Median pretreatment LDT, mo (range)* | 8.57 (1->79) | Shorter LDT correlated with poor outcome; P < .001 when grouped (% surviving, median OS) as indolent (81.1%, 63.5 mo), intermediate (75.9%, 38.8 mo), and aggressive (43%, 17.5 mo) |
| Outcome | — | Median follow-up: 25.9 mo (range: 1-116.1 mo); at last follow-up, 5 patients (median age: 63 y) were in CR after therapy |
| Deaths from disease, no. (%) | 68 (79.1) | — |
| Median OS, mo† | 27.4 | — |
| 5-y survival rate, % | 21.0 | — |
| Cytogenetic features | ||
| (inv14)(q11;q32.1) or t(14;14)(q11;q32.1)/total cases analyzed (%) | 24/60 (40.0) | Detection of 14q32 alterations by conventional karyotype correlated with TCL1 protein expression (P = .027); 9/24 cases with inv14/t(14;14) had low TCL1 |
| Trisomy 8 or isochromosome 8q/total cases analyzed (%) | 21/60 (35.0) | Detection of chr 8 abnormalities by conventional karyotype correlated with inv14/t(14;14) (P < .001), del11q22–23 (P = .004), sTCR+ (P = .027) |
| −11 or deletion 11q22-23/total cases analyzed (%) | 20/60 (33.3) | Correlated with detection of 14q32 alterations (P < .001) |
| −17 or isochromosome 17q or deletion 17p/total analyzed cases | 8/60 (13.3) | — |
| Immunophenotypic features | ||
| TCL1 protein | — | Higher TCL1 correlated with shorter OS (P = .029), higher presenting WBC counts (P = .03), shorter pretreatment LDT (P = .03) |
| Absent, no. (%) | 19/82 (23.2) | — |
| Low positive, no. (%) | 15/82 (18.3) | — |
| Strong positive, no. (%) | 48/82 (58.5) | — |
| sTCR/sCD3+ (%) | 67/82 (81.7) | sTCR expression correlated with shorter OS (P < .001) and CD45RAnegative phenotype (P = .016); no sTCRγ/δ+ cases were detected |
| pAKT, no. with score 0 (%), 1 (%), 2 (%) | 12 (40), 15 (50), 3 (10) | pAKT correlated with inferior OS (P = .02), high presenting WBC (P = .002), and short pretreatment LDT (P = .013) |
| CD4+CD8− (%) | 50/81 (61.7) | Correlated with higher age (P = .008), CD26negative/dim (P = .021) |
| CD4+CD8+ (%) | 28/81 (34.6) | Correlated with younger age (P = .008), better OS (P = .027) in sTCR+ group |
| CD4−CD8+ (%) | 3/81 (3.7) | — |
| CD4−CD8− (%) | 0/81 (0) | 5 cases CD4dimCD8−; all 8 CD4dim/negative cases have absent pLAT (P = .04) |
| CD45RA+RO− (%); CD45RA+RO+ (%) | 15 (20.5); 8 (11.0) | CD45RA+ tumors had lower pAKT (P = .02), lower presenting WBC (P = .006), and better OS (P = .06; for RA+RO+ subset P = .04) |
| CD45RA− RO+ (%); CD45RA− RO− (%) | 44 (60.3); 6 (8.2) | CD45RA−CD45RO+ showed a trend toward inferior OS (P = .08); CD45RA−CD45RO− phenotype correlated with low CD25 (P = .03), low CD26 (P = .007), and high SHP1/2 (P = .01) |
| SHP1/2, no. with score 0 (%), 1 (%), 2 (%) | 9 (20), 10 (22), 26 (58) | SHP1/2 expression (IHC) correlated with lower pLCK expression (IHC, P = .05); the 9 cases negative for SHP1/2 had better OS (P = .01) |
| pLAT, no. with score 0 (%), 1 (%), 2 (%)‡ | 4 (17), 10 (44), 9 (39) | pLAT detection (IHC) correlated with pLCK (P = .005), low pAKT (P = .04), and higher peak WBC count (P = .03) |
| pLCK, no. with score 0 (%), 1 (%), 2 (%)‡ | 15 (48), 9 {29), 7 (23) | pLCK detection (IHC) correlated with younger age (P = .02) and pLAT (P = .005) |
| pZAP70, no. with score 0 (%), 1 (%), 2 (%) | 13 (41), 8 (25), 11 (34) | pZAP70 (IHC) correlated with CD25dim/negative (FACS, P = .02) and younger age (P = .012); correlated with better OS (P = .026) in the sTCR+ group |
| CD25, no. with score 0 (%), 1 (%), 2 (%)§ | 44 (58), 14 (18), 18 (24) | FACS level correlated with lower peak WBC count (P = .012) and pAKT absence (P < .001) |
| CD26, no. with score 0 (%), 1 (%), 2 (%)§ | 10 (24), 6 (14), 26 (62) | CD26 level (FACS) correlated with CD25 (P = .01) and CD38 (P = .017) |
| CD38, no. with score 0 (%), 1 (%), 2 (%)§ | 20 (35), 13 (23), 24 (42) | As above (CD26) |
| CD69, no. with score 0 (%), 1 (%), 2 (%) | 6 (24), 11 (44), 8 (32) | CD69 expression (IHC) correlated with pAKT (P = .04), lower pLAT (P = .02) |
sTCR indicates surface T-cell receptor alpha/beta detected by flow cytometry; —, not applicable; IHC, detected by paraffin immunohistochemical staining; and FACS, detected by flow cytometry.
LDT scoring as described in “Methods.”
OS is disease-specific survival from the time of diagnosis with censoring of unrelated deaths (1 case) or losses to follow-up (1 case).
pLCK+ and pLAT+ tumor cells were often found as clusters in bone marrow sections.
These scores use the following cutoffs: 0 (≤10% cells), 1 (>10%-50% cells), and 2 (>50% cells), as determined by flow cytometry.
Figure 1.
The molecular feature of TCL1 expression is associated with a hyperproliferative clinical subgroup of T-PLL. Kaplan-Meier analysis of overall survival in T-PLL patients showed an inverse association with presenting white blood cell (WBC) count (A), pretreatment lymphocyte doubling time (LDT, B), and TCL1 immunohistochemical expression (C). The TCL1+ subset of T-PLL showed higher presenting WBC counts (D) and shorter pretreatment LDT (E).
TCL1 protein expression was seen in 63 (77%) of 82 eval-uable cases by immunohistochemistry (n = 79), Western blot analysis (n = 30), and/or flow cytometry (n = 5) using a newly developed TCL1 monoclonal antibody. TCL1 levels varied greatly, with 48 cases strongly expressing the protein and 15 showing only low expression. Strong TCL1 expression correlated with poor outcome (P = .029, Figure 1C) in that the 5-year survival rate of patients with high TCL1 expression was 18.9% as opposed to 39.0% for those with TCL1-low/negative tumors (cumulative proportion surviving was 15% vs 35%). Strong TCL1 expression was also associated with higher WBC levels at diagnosis (P = .03, Figure 1D) and shorter pretreatment LDT (P = .03, Figure 1E).
Functional response to TCR engagement in T-PLL is associated with high TCL1 expression
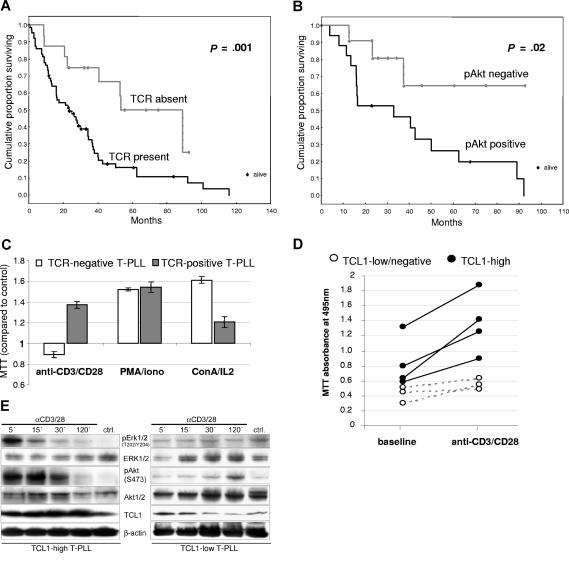
To identify correlates of increased proliferation in TCL1+ T-PLL, we performed immunohistochemical and flow cytometric profiling of components of the TCR pathway such as coreceptors, activation markers, kinases, and phosphatases. We identified respective subsets of T-PLL in relationship to T-cell development and TCR signaling activity (summarized in Table 1). Expression of sTCR, as found in 67 (81.7%) of 82 cases (including 4 with low expression), was correlated with poor outcome (P = .001, Figure 2A), compared with those 15 (18.3%) cases lacking sTCR. Overexpression of the Ser473-phosphorylated activated form of AKT by Western blot analysis with normalization to overall levels of AKT was also correlated with inferior OS (P = .02, Figure 2B). Tumors expressing sTCR tended to have higher intrinsic pAKT-S473 levels than those lacking sTCR (P = .29). Correlations of strong TCL1 expression with OS (P = .018), presenting WBC (P = .017), and pretreatment LDT (P = .01) were also more significant when analyzed in the sTCR+ subset compared with all tumors.
Figure 2.
The T-cell receptor signaling pathway is functional in T-PLL and responses are influenced by TCL1 levels. (A) Detection of surface T-cell receptor (sTCR) in T-PLL cells by flow cytometry for sCD3 and TCRα/β (67/82 sTCR+ versus 15/82 sTCR− cases) correlated with shorter overall survival (OS). (B) Expression of the Ser473-phosphorylated activated form of AKT, as analyzed by Western blot, also significantly correlated with poor OS. (C) TCR engagement stimulated growth in T-PLL tumor cells only in cases expressing sTCR, whereas ConA/IL2 or PMA/ionomycin stimulated growth in nearly all cases (MTT assay values normalized to unstimulated control). (D) In sTCR+ T-PLL, the degree of growth induction by TCR engagement (MTT assay) was higher in those tumors that strongly expressed TCL1 (solid dots) compared with TCL1-low/negative tumors (white circles). (E) Western blot analysis reveals that T-PLL with higher TCL1 levels was associated with a faster induction of pERK1/2 and activated AKT (left versus right panels).
Correlation of aggressive clinical features with levels of other components of the TCR assembly, including CD4; the phosphatases CD45RA/RO and SHP1/2; (phospho) activation epitopes of the linker of activation in T cells (LAT) protein; and the tyrosine kinases LCK and ZAP70; as well as surface T-cell activation markers CD25, CD26, CD38, and CD69 were generally less significant than for sTCR, pAKT, and TCL1 (Table 1). In a multivariate analysis of the tumor-associated phenotypic markers listed in Table 1, high TCL1 expression, sTCR, and pAKT-S473 all retained their significance as prognostic factors, even after inclusion of age into the model (Table 2 “Model including tumor features and age”). TCR expression was, in addition to presenting WBC count and pretreatment LDT, also an independent predictor of poor outcome in a further regression analysis that included clinical parameters (Table 2 “Model with inclusion of clinical parameters”).
Table 2.
Multivariate analysis of overall survival in 86 T-PLL patients based on Cox proportional hazards model integrating molecular and clinical features
| Prognostic factor | Risk of disease-related death |
||
|---|---|---|---|
| Relative risk | Standard error | P | |
| Model including tumor features and age | |||
| Age over 65 y | 1.9 | 0.26 | .015 |
| High TCL1 | 1.7 | 0.28 | .049 |
| pAKT-S473+ | 2.9 | 0.45 | .03 |
| sTCR+ | 2.2 | 0.37 | .02 |
| Model with inclusion of clinical parameters | |||
| Age over 65 y | 2.4 | 0.27 | .001 |
| WBC count over 40 ×109/L* | 2.2 | 0.28 | .006 |
| LDT, 3-tier* | 2.2 | 0.22 | <.001 |
| sTCR+ | 2.6 | 0.37 | .01 |
Both TCL1 and pAKT levels showed a strong positive correlation with each of these clinical parameters and were therefore not retained in this regression analysis.
Freshly isolated T-PLL tumor cells showed responsiveness to TCR engagement and other growth stimuli in relation to TCL1 expression. Among 19 primary T-PLL tumors, in vitro growth stimulation in response to anti-CD3/CD28 cross-linking was restricted to the presence of sTCR expression, whereas nearly all cases showed growth stimulation in response to ConA/IL2 and PMA/ionomycin (Figure 2C). Among the subset of sTCR expressing T-PLL, the proliferative response to TCR engagement in cases that were strongly positive for TCL1 surpassed that of TCL1-low/negative tumors (Figure 2D).
Stimulated T-PLL growth in culture was associated with increased levels of activated pAKT-S473 and mitogen-activated protein kinase (p-ERK1/2, p42/44) as detected by Western blot in the 26 cases studied. However, the kinetics of maximal phosphoactivation of AKT and ERK1/2 revealed a relationship to tumor TCL1 levels. In T-PLL that highly expressed TCL1, AKT activation was seen as early as 5 to 15 minutes after stimulation (Figure 2E left panel), whereas in tumors with lower TCL1 levels this often occurred at much later time points (Figure 2E right panel).
TCR engagement in T-PLL leads to rapid recruitment of TCL1 and AKT to the T-cell receptor complex
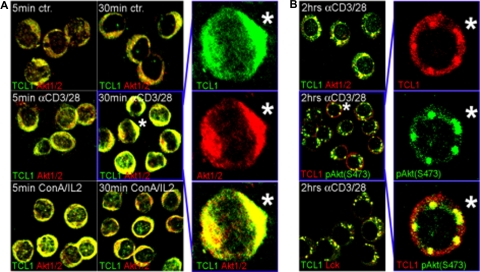
Prior to a growth stimulus, TCL1 and AKT in freshly isolated T-PLL tumors showed predominantly cytoplasmic distribution as determined by cell fractionation and were not colocalized by confocal microscopy (Figure 3A top; and not shown). Upon TCR engagement, there was rapid recruitment of TCL1 and AKT to perimembraneous sites within 5 minutes of exposure. By 30 minutes of stimulation, TCL1 and activated pAKT-S473 were colocalized to discrete membrane foci also containing TCR-associated kinases such as LCK (Figure 3B). The appearance of these complexes was consistent with the supramolecular activation clusters associated with the immunologic synapse in nonneoplastic T cells following TCR engagement.32
Figure 3.
TCL1 and AKT are recruited to membrane complexes following TCR engagement. (A) Laser confocal microscopy on unstimulated primary T-PLL cultures showed a predominantly cytoplasmic distribution of TCL1 and AKT without apparent colocalization (ctr, top panel); only rare “activated” cells show TCL1-AKT colocalization. TCR engagement using anti-CD3/28 precoated plates leads to rapid focal recruitment along with colocalization of TCL1 and AKT in a uniform perimembraneous pattern (middle panel), which was also seen with ConA/IL2 stimulation (bottom panel). (B) Continuous TCR engagement in sTCR+/sCD3+ T-PLL shifted localization of TCL1 to discrete membrane complexes that also included pAKT-S473 and LCK. Staining was done using anti-TCL1 (FITC: green) in combination with anti-AKT1/2 or anti-LCK (Cy3: red), or a TCL1 antiserum (Cy3: red) with a monoclonal anti–pAKT-S473 (FITC: green). Merged pictures are shown for low-power fields; asterisk indicates location of cell shown in enlarged panels for individual fluorochromes and merged image.
The pattern of TCL1-AKT colocalization following initial membrane recruitment was highly dependent on the type and duration of the activation stimuli. With PMA/ionomycin or ConA/IL2, TCL1 and AKT showed recruitment to perimembraneous locations but not to TCR foci (Figure 3A bottom panels; and not shown). TCL1-AKT activation complexes were also reversible, with loss of perimembraneous TCL1-AKT colocalization within 2 hours after washout of the activation stimuli in the majority of cells (not shown). Continuous ConA/IL2 or TCR engagement for up to 4 days led to the appearance of nuclear TCL1- and AKT-containing foci and correlated with the degree of proliferation observed (not shown).
Inhibitors of AKT activation block TCL1-AKT complex formation and reduce growth of T-PLL
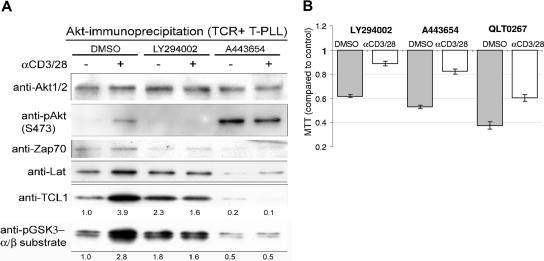
Maximal AKT activation in T-PLL cultures, as assessed by pAKT-S473 Western blot, was seen at 15 to 30 minutes following TCR engagement (Figure 4A left), at a time that coincided with the first appearance of the discrete TCR-associated foci noted by confocal microscopy. Using an immobilized AKT antibody for immunoprecipitation of activated AKT complexes in 6 T-PLL samples, we noted that maximal association between TCL1 and AKT also correlated with peak AKT activation (Figure 4A). Maximal phosphorylation of the AKT target peptide GSK3α/β by immunoprecipitated AKT also correlated with the timing of maximal TCR-stimulated TCL1-AKT complexes.
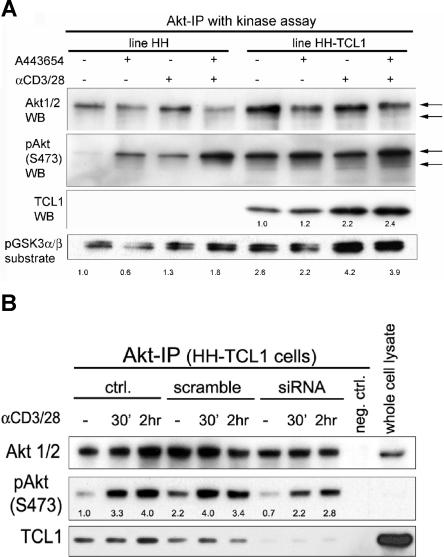
Figure 4.
Inhibitors of AKT signaling alter AKT-TCL1 complex formation and T-PLL growth in culture. (A) AKT complexes were isolated from primary T-PLL lysates by immunoprecipitation using an immobilized anti-AKT antibody, and showed increased phosphoactivated (pS473) AKT and TCL1 as well as increased amounts of ZAP70 and LAT following 30 minutes of TCR engagement. Increased AKT kinase activity following TCR stimulation was found by elevated phospho-GSK3α/β substrate levels (numbers indicate fold changes following stimulation compared with DMSO control). Preincubation of T-PLL tumor cells with the PI3K inhibitor LY294002 or the AKT inhibitor A443654 abolished the TCR-stimulated phosphorylation of AKT and GSK3α/β target and decreased the amount of TCL1 complexed with immunoprecipitated AKT. A443654 also led to an overall increase in pAKT-S473. (B) LY294002 at 20 μM, A443654 at 0.5μM, and QLT0267 at 40 μM reduced the level of proliferation in unstimulated T-PLL cultures at baseline and following TCR cross-linking compared with the DMSO vehicle control (MTT assay at 48 hours).
We assessed the effects of inhibitors that differentially affect AKT activation in 6 T-PLL cultures, including the PI3K inhibitor LY294002, the ILK inhibitor QLT0267, and the competitive AKT inhibitor A443654. All 3 drugs abolished the effects of TCR cross-linking on activation of AKT, and led to reductions in coimmunoprecipitated TCL1 along with decreased GSK substrate and pan-RXRXXS/T target motif phosphorylation (Figure 4A; and not shown). A443654 treatment of tumor cells led to increased baseline pAKT-S473 but decreased baseline levels of coimmunoprecipi-tated TCL1 consistent with inhibition of TCL1-pAKT-substrate complex formation. In contrast, incubation of the kinase reaction directly with A443654 also suppressed GSK3 and RXRXXS/T-motif phosphorylation but did not block TCL1 association with AKT (not shown).
By confocal microscopy, we observed a delayed and reduced colocalization of AKT and TCL1 after LY294002 inhibition (not shown). In A443654-treated T-PLL cells, we noted striking accumulations of TCL1 and AKT in the cytoplasm, especially following TCR engagement, but virtual absence of AKT-TCL1 membrane–associated complexes. All 3 inhibitors directed against AKT activation also reduced cell proliferation in T-PLL cultures (Figure 4B). Among them, A443654 and QLT0267 induced the most marked apoptosis, as evidenced by cell blebbing and nuclear fragmentation. This apoptotic effect was partially overcome by CD3/CD28 cross-linking.
Introduction of TCL1 into T-cell leukemia augments basal and stimulated AKT activity and renders tumor cells more resistant to inhibition of AKT
Cell lines stably expressing full-length TCL1 protein were created from 7 T-cell leukemia lines lacking TCL1. In comparison with their TCL1− parent cell lines, these 7 TCL1-transfected subclones had variably but overall slightly higher levels of baseline activated pAKT (Figure 5A) and a 1.2- to 1.8-fold increased proliferative rate in the sTCR-expressing lines (HH, Hut78, and Jurkat) but not in sTCR− lines (Molt4, CEM, HSB2, SKW3; not shown). In the TCL1-expressing subclones, activated AKT levels were also higher after TCR stimulation and the lines were more resistant to apoptosis following treatment with the inhibitor A443654 (Figure 5A; and not shown). Immunoprecipitated AKT complexes also showed higher kinase activity on exogenous GSK substrate at baseline and following TCR engagement in the TCR+ TCL1-expressing lines (Figure 5A lower panel).
Figure 5.
TCL1 introduction into leukemic T cells influences AKT phosphorylation and kinase activity. (A) Transfection of TCL1 into the sTCR+ mature T-cell leukemia line HH augmented baseline and TCR-induced AKT phosphoactivation, and increased phosphorylation of the AKT target GSK3α/β (2.6-fold at baseline and 1.3- to 4.2-fold TCR induced). Similar to primary T-PLL tumor cells, TCR cross-linking also increased the amount of TCL1 complexed with AKT by 2.2-fold. Preincubation of tumor cells with the AKT inhibitor A443654 at 0.5 μM leads to slightly decreased GSK3α/β phosphorylation, and a compensatory pAKT increase (kinase assay and Western blot following AKT immunoprecipitation). Indicated are fold changes over the unstimulated DMSO vehicle control. (B) Transient knockdown of TCL1 in the HH-TCL1 cell line reduced baseline levels of activated pAKT in immunoprecipitated complexes compared with control HH-TCL1 (down to 0.7-fold) or cells transfected with scrambled siRNA (assayed at 72 hours). TCL1 knockdown also reduced levels of activated pAKT following TCR engagement for 30 minutes and 2 hours at these time points (ie, 3.3- to 2.2-fold after 30 minutes of simulation).
Transient knockdown of TCL1 in the HH-TCL1 cell line by inhibitory RNA (siRNA) resulted in lower baseline levels of pAKT and mildly decreased levels of phosphoactivated AKT following optimal TCR cross-linking, as analyzed by Western blot. TCL1 knockdown also resulted in modest reductions in the level of baseline and TCR-stimulated activated AKT in immunoprecipitated complexes (Figure 5B), consistent with a role for TCL1 in fully activating or stabilizing AKT complexes.
Discussion
We show that coexpression of surface TCR and high TCL1 are functionally linked with AKT activation in T-PLL and associated with a highly aggressive disease subset. TCL1+ T-PLL shows hyperresponsiveness to TCR engagement in vitro and proliferates more rapidly in vivo. Upon antigen receptor cross-linking in culture, TCL1 and AKT are rapidly recruited to discrete membrane foci where they colocalize with tyrosine kinases of the TCR scaffold. Inhibition of full AKT activation in T-PLL blocks TCL1-AKT complex formation, and altered TCL1 levels modulate the kinase activity of AKT.
The role of TCL1 and MTCP1 as oncogenes in T-PLL is supported by transgenic mouse models,33,34 however these tumors develop at long latency suggesting that secondary oncogenic events are critical for full transformation. In earlier work using yeast 2-hybrid systems and cotransfection of nonlymphoid cell lines, TCL1 has been shown to physically associate with AKT through its PH domain and to modulate AKT kinase activity.20–22 An association of native TCL1 and AKT and the effects of TCL1 on AKT in T-PLL, the only known tumor type with TCL1-activating translocations, have not been previously studied given the rarity of this tumor. We had noted earlier that there is a poor correlation between overall TCL1 and baseline AKT and pAKT levels in primary B-cell tumors and lymphoid cell lines.5,35 We show here that TCL1 and AKT interactions in T-PLL are apparent only following growth and activation stimuli and that these complexes are dynamically regulated depending on the type and duration of the activation signal, and the presence or absence of TCR pathway components in a given tumor.
In T-PLL, TCL1-AKT complexes were transient, in that they could be rapidly dissolved following removal of activation stimuli and were influenced by AKT activation state. Reagents blocking full AKT activation such as the PI3K inhibitor LY29400236 and A443654, an ATP competitive inhibitor of AKT substrate binding,37 largely blocked TCR-mediated AKT stimulation, altered the kinetics of the TCL1-AKT interaction, and reduced growth in primary T-PLL cultures. Introduction of TCL1 into T-cell leukemia lines and TCL1 knockdown also influenced the level of AKT activation and target phosphorylation following TCR engagement. The magnitude of TCL1-mediated basal kinase increase was similar to published data in nonlymphoid cells.20–22,38
Our results suggest that the inconsistent relationship of TCL1 expression with AKT activation is likely related to the activation-dependent and transient nature of such complexes, making the timing and duration of exogenous growth stimuli important factors in driving transformation. This is also consistent with the effects of transgenic human TCL1 in mouse B cells and T cells. In these systems, ectopic expression of TCL1 results in only modest effects on overall AKT kinase activity in unstimulated lymphocytes but enhances cytokine secretion and proliferation of mature transgenic splenocytes following a variety of activation stimuli.4,35 These studies and our current data support the concept of TCL1 as a variable modulator of AKT activity and the dependence of the oncogenic cooperation of both molecules on activating signals.
Overall, T-PLL cases that were negative or low for TCL1, including some carrying a chromosome 14q32 rearrangement, showed lower pretreatment WBC counts and LDT. The association of TCL1 level with aggressiveness and adverse outcome in T-PLL was strongest when only sTCR+ cases were studied (P = .018) compared with inclusion of all tumors, further indicative of TCR-TCL1 cooperativity. In T-PLL, we propose that TCL1 augments TCR pathway responsiveness in the early stages of leukemia development and is sometimes downmodulated with subsequent tumor progression when its growth-promoting effects are less critical for tumor survival. The strong association of TCR pathway responsiveness with high TCL1, in our series, extends to some coreceptors, protein kinases, and phosphatases that are downstream of TCR activation (Table 1). This apparent TCR and TCL1 cooperativity may also be related to the previously identified association of outcome in T-PLL with CD45RA versus CD45RO expression or to coexpression of the CD4 and CD8 coreceptors.8,13,39
The ability of TCL1 to modulate AKT signaling through a variety of stimuli also has important implications for its role in lymphocyte biology.40 Dysregulated TCL1 as a result of specific chromosomal aberrations has not been shown in other lymphatic tumors.28,30,41 In nonneoplastic lymphoid cells, TCL1 is expressed in a small subset of thymocytes, in plasmacytoid dendritic cells, and in B cells up to the germinal center (GC) stage of maturation.5,31,35,42–47 In nonneoplastic T cells, TCL1 is expressed only at the earliest stages of thymocyte development before the TCR signaling pathway is assembled.4,42 Similarly, in nonneoplastic B cells, TCL1 expression is lost at the GC stage of maturation when B-cell receptor (BCR) signaling through appropriately presented antigen occurs.30,31,42–48 This developmentally regulated silencing of TCL1 at the onset of antigen receptor signaling suggests that TCL1 may substitute for the effects of TCR and BCR engagement on AKT activation and lower the threshold for AKT signaling by other growth stimuli (eg, cytokines). The phenotype of TCL1-deficient mice, which is characterized by mildly impaired B-cell and T-cell response to growth and differentiation stimuli, also supports this model.49
Overall, our findings support a role of TCL1 in driving aberrant or tonic TCR pathway signaling in T-PLL. Since TCL1 activation by chromosomal translocation appears to be the initiating event in T-PLL leukemogenesis, dysregulated TCL1 may be most important in supporting early clonal expansion and providing a sustained growth advantage through hyperresponsiveness to TCR and cytokine-mediated stimuli converging on AKT. This model provides several possible functional targets for T-PLL therapy and suggests that inhibition of TCL1 may synergize with blockade of AKT- or TCR-associated src family kinases. Strategies designed to alter TCL1-AKT complex stabilization could shift the activation threshold in T-PLL and inhibit tumor growth. The association of ectopic TCL1 expression only with mature T-cell transformation may thus be related to this ability to modulate the T-cell receptor pathway in a cell-specific manner.
Acknowledgments
This work was supported by a grant from the CLL Global Research Foundation (D.J. and M.H.); NCI grants CA16672 (D.J.), CA90571, and CA107300 (M.A.T.); an Odyssey Special Fellowship Award and a DFG stipend HE3553/2–1 (M.H.).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.H. designed and performed experiments, analyzed data, and drafted the paper; K.A.P. designed and performed experiments; M.A.T. provided vital reagents, reviewed experimental design, and performed data interpretation; M.K. provided vital reagents, and reviewed experimental design; F.R. assisted in patient accrual, collection of case material, and data analysis; R.K. produced reagents and analyzed data; D.J. designed experiments, analyzed data, and drafted the paper.
Conflict-of-interest disclosure: M.H., R.K., and D.J. have commercial interests in the TCL1 antibodies reported here. All other authors declare no competing financial interests.
Correspondence: Dan Jones, Department of Hematopathology, Box 72, 1515 Holcombe Blvd, Houston TX 77030; e-mail: dajones@mdanderson.org.
References
- 1.Isobe M, Russo G, Haluska FG, Croce CM. Cloning of the gene encoding the delta subunit of the human T-cell receptor reveals its physical organization within the alpha-subunit locus and its involvement in chromosome translocations in T-cell malignancy. Proc Natl Acad Sci U S A. 1988;85:3933–3937. doi: 10.1073/pnas.85.11.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russo G, Isobe M, Gatti R, et al. Molecular analysis of a t(14;14) translocation in leukemic T-cells of an ataxia telangiectasia patient. Proc Natl Acad Sci U S A. 1989;86:602–606. doi: 10.1073/pnas.86.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brito-Babapulle V, Catovsky D. Inversions and tandem translocations involving chromosome 14q11 and 14q32 in T-prolymphocytic leukemia and T-cell leukemias in patients with ataxia telangiectasia. Cancer Genet Cytogenet. 1991;55:1–9. doi: 10.1016/0165-4608(91)90228-m. [DOI] [PubMed] [Google Scholar]
- 4.Hoyer KK, Herling M, Bagrintseva K, et al. T cell leukemia-1 modulates TCR signal strength and IFN-gamma levels through phosphatidylinositol 3-kinase and protein kinase C pathway activation. J Immunol. 2005;175:864–873. doi: 10.4049/jimmunol.175.2.864. [DOI] [PubMed] [Google Scholar]
- 5.Herling M, Teitell MA, Shen RR, Medeiros LJ, Jones D. TCL1 expression in plasmacytoid dendritic cells (DC2s) and the related CD4+ CD56+ blastic tumors of skin. Blood. 2003;101:5007–5009. doi: 10.1182/blood-2002-10-3297. [DOI] [PubMed] [Google Scholar]
- 6.Herling M, Khoury JD, Washington LT, Duvic M, Keating MJ, Jones D. A systematic approach to diagnosis of mature T-cell leukemias reveals heterogeneity among WHO categories. Blood. 2004;104:328–335. doi: 10.1182/blood-2004-01-0002. [DOI] [PubMed] [Google Scholar]
- 7.Soulier J, Pierron G, Vecchione D, et al. A complex pattern of recurrent chromosomal losses and gains in T-cell prolymphocytic leukemia. Genes Chromosomes Cancer. 2001;31:248–254. doi: 10.1002/gcc.1141. [DOI] [PubMed] [Google Scholar]
- 8.Ravandi F, O'Brien S, Jones D, et al. T-cell prolymphocytic leukemia: a single-institution experience. Clin Lymphoma Myeloma. 2005;6:234–239. doi: 10.3816/CLM.2005.n.051. [DOI] [PubMed] [Google Scholar]
- 9.Maljaei SH, Brito-Babapulle V, Hiorns LR, Catovsky D. Abnormalities of chromosomes 8, 11, 14, and X in T-prolymphocytic leukemia studied by fluorescence in situ hybridization. Cancer Genet Cytogenet. 1998;103:110–116. doi: 10.1016/s0165-4608(97)00410-x. [DOI] [PubMed] [Google Scholar]
- 10.Matutes E, Brito-Babapulle V, Swansbury J, et al. Clinical and laboratory features of 78 cases of T-prolymphocytic leukemia. Blood. 1991;78:3269–3274. [PubMed] [Google Scholar]
- 11.Keating MJ, Cazin B, Coutre S, et al. Campath-1H treatment of T-cell prolymphocytic leukemia in patients for whom at least one prior chemotherapy regimen has failed. J Clin Oncol. 2002;20:205–213. doi: 10.1200/JCO.2002.20.1.205. [DOI] [PubMed] [Google Scholar]
- 12.Dearden CE, Matutes E, Cazin B, et al. High remission rate in T-cell prolymphocytic leukemia with CAMPATH-1H. Blood. 2001;98:1721–1726. doi: 10.1182/blood.v98.6.1721. [DOI] [PubMed] [Google Scholar]
- 13.Garand R, Goasguen J, Brizard A, et al. Indolent course as a relatively frequent presentation in T-prolymphocytic leukaemia: Groupe Francais d'Hematologie Cellulaire. Br J Haematol. 1998;103:488–494. doi: 10.1046/j.1365-2141.1998.00977.x. [DOI] [PubMed] [Google Scholar]
- 14.Brito-Babapulle V, Maljaie SH, Matutes E, Hedges M, Yuille M, Catovsky D. Relationship of T leukaemias with cerebriform nuclei to T-prolymphocytic leukaemia: a cytogenetic analysis with in situ hybridization. Br J Haematol. 1997;96:724–732. doi: 10.1046/j.1365-2141.1997.9702605.x. [DOI] [PubMed] [Google Scholar]
- 15.Catovsky D, Matutes E, Brito-Babapulle V. Is T-cell CLL a disease entity [letter]? Br J Haematol. 1996;94:580. [PubMed] [Google Scholar]
- 16.Domingo-Domenech E, Gonzalez-Barca E, Salar A, Claros AD, Granena A. Indolent course as presentation in t-prolymphocytic leukaemia [letter]. Br J Haematol. 1999;105:840. doi: 10.1046/j.1365-2141.1999.01485.x. [DOI] [PubMed] [Google Scholar]
- 17.Hoyer JD, Ross CW, Li CY, et al. True T-cell chronic lymphocytic leukemia: a morphologic and immunophenotypic study of 25 cases. Blood. 1995;86:1163–1169. [PubMed] [Google Scholar]
- 18.Madani A, Choukroun V, Soulier J, et al. Expression of p13MTCP1 is restricted to mature T-cell proliferations with t(X;14) translocations. Blood. 1996;87:1923–1927. [PubMed] [Google Scholar]
- 19.Stern MH, Soulier J, Rosenzwajg M, et al. MTCP-1: a novel gene on the human chromosome Xq28 translocated to the T cell receptor alpha/delta locus in mature T cell proliferations. Oncogene. 1993;8:2475–2483. [PubMed] [Google Scholar]
- 20.Laine J, Kunstle G, Obata T, Sha M, Noguchi M. The protooncogene TCL1 is an Akt kinase coactivator. Mol Cell. 2000;6:395–407. doi: 10.1016/s1097-2765(00)00039-3. [DOI] [PubMed] [Google Scholar]
- 21.Kunstle G, Laine J, Pierron G, et al. Identification of Akt association and oligomerization domains of the Akt kinase coactivator TCL1. Mol Cell Biol. 2002;22:1513–1525. doi: 10.1128/mcb.22.5.1513-1525.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pekarsky Y, Koval A, Hallas C, et al. Tcl1 enhances Akt kinase activity and mediates its nuclear translocation. Proc Natl Acad Sci U S A. 2000;97:3028–3033. doi: 10.1073/pnas.040557697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Auguin D, Barthe P, Royer C, et al. Structural basis for the co-activation of protein kinase B by T-cell leukemia-1 (TCL1) family proto-oncoproteins. J Biol Chem. 2004;279:35890–35902. doi: 10.1074/jbc.M400364200. [DOI] [PubMed] [Google Scholar]
- 24.French SW, Shen RR, Koh PJ, Malone CS, Mallick P, Teitell MA. A modeled hydrophobic domain on the TCL1 oncoprotein mediates association with AKT at the cytoplasmic membrane. Biochemistry. 2002;41:6376–6382. doi: 10.1021/bi016068o. [DOI] [PubMed] [Google Scholar]
- 25.Jaffe ES, Harris N, Stein H, Vardiman JW. Tumours of Hematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2001. [Google Scholar]
- 26.Jones D, Dang NH, Duvic M, Washington LT, Huh YO. Absence of CD26 expression is a useful marker for diagnosis of T-cell lymphoma in peripheral blood. Am J Clin Pathol. 2001;115:885–892. doi: 10.1309/U1Y6-J4AG-5M4M-7AYV. [DOI] [PubMed] [Google Scholar]
- 27.Washington LT, Huh YO, Powers LC, Duvic M, Jones D. A stable aberrant immunophenotype characterizes nearly all cases of cutaneous T-cell lymphoma in blood and can be used to monitor response to therapy. BMC Clin Pathol. 2002;2:5. doi: 10.1186/1472-6890-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herling M, Patel KA, Khalili J, et al. TCL1 shows a regulated expression pattern in chronic lymphocytic leukemia that correlates with molecular subtypes and proliferative state. Leukemia. 2006;20:280–285. doi: 10.1038/sj.leu.2404017. [DOI] [PubMed] [Google Scholar]
- 29.National Center for Biotechnology Information. RefSeq. http://www.ncbi.nlm.nih.gov/RefSeq/
- 30.Herling M, Patel KA, Hsi ED, et al. TCL1 in B-cell tumors retains its normal B-cell pattern of regulation and is a marker of differentiation stage. Am J Surg Pathol. 2007;31:1123–1129. doi: 10.1097/PAS.0b013e31802e2201. [DOI] [PubMed] [Google Scholar]
- 31.Said JW, Hoyer KK, French SW, et al. TCL1 oncogene expression in B cell subsets from lymphoid hyperplasia and distinct classes of B cell lymphoma. Lab Invest. 2001;81:555–564. doi: 10.1038/labinvest.3780264. [DOI] [PubMed] [Google Scholar]
- 32.Vega F, Luthra R, Medeiros LJ, et al. Clonal heterogeneity in mycosis fungoides and its relationship to clinical course. Blood. 2002;100:3369–3373. doi: 10.1182/blood.V100.9.3369. [DOI] [PubMed] [Google Scholar]
- 33.Fu ZQ, Du Bois GC, Song SP, et al. Crystal structure of MTCP-1: implications for role of TCL-1 and MTCP-1 in T cell malignancies. Proc Natl Acad Sci U S A. 1998;95:3413–3418. doi: 10.1073/pnas.95.7.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gritti C, Dastot H, Soulier J, et al. Transgenic mice for MTCP1 develop T-cell prolymphocytic leukemia. Blood. 1998;92:368–373. [PubMed] [Google Scholar]
- 35.Hoyer KK, French SW, Turner DE, et al. Dysregulated TCL1 promotes multiple classes of mature B cell lymphoma. Proc Natl Acad Sci U S A. 2002;99:14392–14397. doi: 10.1073/pnas.212410199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 37.Luo Y, Shoemaker AR, Liu X, et al. Potent and selective inhibitors of Akt kinases slow the progress of tumors in vivo. Mol Cancer Ther. 2005;4:977–986. doi: 10.1158/1535-7163.MCT-05-0005. [DOI] [PubMed] [Google Scholar]
- 38.Laine J, Kunstle G, Obata T, Noguchi M. Differential regulation of Akt kinase isoforms by the members of the TCL1 oncogene family. J Biol Chem. 2002;277:3743–3751. doi: 10.1074/jbc.M107069200. [DOI] [PubMed] [Google Scholar]
- 39.Ascani S, Leoni P, Fraternali Orcioni G, et al. T-cell prolymphocytic leukaemia: does the expression of CD8+ phenotype justify the identification of a new subtype? description of two cases and review of the literature. Ann Oncol. 1999;10:649–653. doi: 10.1023/a:1008349422735. [DOI] [PubMed] [Google Scholar]
- 40.Noguchi M, Ropars V, Roumestand C, Suizu F. Proto-oncogene TCL1: more than just as a coactivator for Akt. Faseb J. 2007;21:2273–2284. doi: 10.1096/fj.06-7684com. [DOI] [PubMed] [Google Scholar]
- 41.Fink SR, Paternoster SF, Smoley SA, et al. Fluorescent-labeled DNA probes applied to novel biological aspects of B-cell chronic lymphocytic leukemia. Leuk Res. 2005;29:253–262. doi: 10.1016/j.leukres.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 42.Virgilio L, Narducci MG, Isobe M, et al. Identification of the TCL1 gene involved in T-cell malignancies. Proc Natl Acad Sci U S A. 1994;91:12530–12534. doi: 10.1073/pnas.91.26.12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roos J, Hennig I, Schwaller J, et al. Expression of TCL1 in hematologic disorders. Pathobiology. 2001;69:59–66. doi: 10.1159/000048758. [DOI] [PubMed] [Google Scholar]
- 44.Narducci MG, Pescarmona E, Lazzeri C, et al. Regulation of TCL1 expression in B- and T-cell lymphomas and reactive lymphoid tissues. Cancer Res. 2000;60:2095–2100. [PubMed] [Google Scholar]
- 45.Takizawa J, Suzuki R, Kuroda H, et al. Expression of the TCL1 gene at 14q32 in B-cell malignancies but not in adult T-cell leukemia. Jpn J Cancer Res. 1998;89:712–718. doi: 10.1111/j.1349-7006.1998.tb03275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakayama I, Murao S, Kitazawa S, Azumi A, Yamamoto M, Maeda S. Activation of the TCL1 protein in B cell lymphomas. Pathol Int. 2000;50:191–199. doi: 10.1046/j.1440-1827.2000.01023.x. [DOI] [PubMed] [Google Scholar]
- 47.Teitell M, Damore MA, Sulur GG, et al. TCL1 oncogene expression in AIDS-related lymphomas and lymphoid tissues. Proc Natl Acad Sci U S A. 1999;96:9809–9814. doi: 10.1073/pnas.96.17.9809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuraishy AI, French SW, Sherman M, et al. TORC2 regulates germinal center repression of the TCL1 oncoprotein to promote B cell development and inhibit transformation. Proc Natl Acad Sci U S A. 2007;104:10175–10180. doi: 10.1073/pnas.0704170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kang SM, Narducci MG, Lazzeri C, et al. Impaired T- and B-cell development in Tcl1-deficient mice. Blood. 2005;105:1288–1294. doi: 10.1182/blood-2004-04-1453. [DOI] [PubMed] [Google Scholar]