Abstract
5-azacytidine (5-Aza) is a potent inducer of fetal hemoglobin (HbF) in people with β-thalassemia and sickle cell disease. Two models have been proposed to explain this activity. The first is based on the drug's ability to inhibit global DNA methylation, including the fetal globin genes, resulting in their activation. The second is based on 5-Aza's cytotoxicity and observations that HbF production is enhanced during marrow recovery. We tested these models using human primary cells in an in vitro erythroid differentiation system. We found that doses of 5-Aza that produce near maximal induction of γ-globin mRNA and HbF do not alter cell growth, differentiation kinetics, or cell cycle, but do cause a localized demethylation of the γ promoter. However, when we reduced γ promoter methylation to levels equivalent to those seen with 5-Aza or to the lower levels seen in primary fetal erythroid cells using DNMT1 siRNA and shRNA, we observed no induction of γ-globin mRNA or HbF. These results suggest that 5-Aza induction of HbF is not the result of global DNA demethylation or of changes in differentiation kinetics, but involves an alternative, previously unrecognized mechanism. Other results suggest that posttranscriptional regulation plays an important role in the 5-Aza response.
Introduction
Mutations which allow expression of the fetal globin genes in adults with sickle cell disease (SCD) or β-thalassemia can produce a significant decrease in disease severity.1,2 This has led to a search for pharmacologic agents that can activate fetal globin gene expression during adult erythropoiesis. Several agents have been shown to have this property when studied in human or primate systems.3 These include the nucleoside analog DNA methyltransferase (DNMT) inhibitors 5-azacytidine (5-Aza) and 2′deoxy-5-azacytidine (decitabine), sodium butyrate and derivatives, histone deacetylase (HDAC) inhibitors, and cytotoxic agents including hydroxyurea, cytosine arabinoside, and vinblastine. While all of these agents are active, none exhibit the optimal combination of safety, efficacy, and convenience of use that would make them applicable to most hemoglobinopathy patients, especially those who lack access to modern medical facilities. These factors provide a strong rationale for developing new pharmacologic strategies that specifically target fetal gene activation without cytotoxicity, widespread epigenetic alterations, or difficult to manage side effects.
The rational design of such targeted therapies is likely to require an in-depth knowledge of the molecular mechanisms underlying globin gene switching and pharmacologically mediated fetal gene reactivation. To this end, we have investigated the mechanism of 5-azacytidine (5-Aza). This was the first pharmacologic agent shown to have the ability to reactivate fetal gene expression in humans with β-thalassemia4 and SCD5 and that can provide long-term clinical benefits for hemoglobinopathy patients.6 5-Aza was originally brought to the clinic as a cytotoxic cancer chemotherapy agent. Only later was it found to be a potent inhibitor of DNA methyltransferase enzymes.7 These 2 properties, cytotoxicity and inhibition of DNA methylation, led to the development of 2 distinct theories to explain the ability of 5-Aza to induce fetal hemoglobin (HbF) production in adult erythroid cells. The first hypothesis was that 5-Aza's ability to inhibit global DNA methylation would result in demethylation around the fetal globin genes leading to their activation in differentiating adult erythroid cells.8 Data showing that 5-Aza causes demethylation of a specific CpG residue upstream of the transcriptional start site of the γ-globin genes provided evidence in support of this model.4 More recent data demonstrating the ability of decitabine (5-aza-2′-deoxycytidine) to cause demethylation at multiple CpGs in the promoters of baboon fetal globin genes are also consistent with this model.9 The second hypothesis proposes that 5-Aza's cytotoxic effects cause the loss of a cohort of differentiating erythroid cells. Under this model, the marrow of treated subjects then responds with rapid erythroid proliferation. These effects have been observed in baboons and humans treated with 5-Aza and other cytotoxic agents as evidenced by an initial decrease in marrow-derived CFUe and erythroid clusters, followed by an expansion of erythroid precursor cells with an increased proportion of HbF-containing cells.10 Recent reviews present these 2 models as the leading candidates to explain DNMT inhibitor induction of HbF.3,11,12 Each of the models makes specific predictions that can be experimentally tested. To determine which model best accounts for the activity of 5-Aza, we have developed an in vitro erythroid differentiation system that demonstrates induction of fetal globin gene expression and HbF production by 5-Aza. Using this system, we have determined the effects of 5-Aza on cellular growth and differentiation kinetics and on DNA methylation patterns. These results provide new insights into the regulation of the human globin genes and the mechanisms underlying fetal Hb induction.
Methods
In vitro erythroid differentiation and flow cytometry
Our in vitro erythroid differentiation system has been previously described.13 Granulocyte colony-stimulating factor (G-CSF)–stimulated peripheral blood CD34+ cells were obtained from the National Heart, Lung, and Blood Institute (NHLBI) Programs of Excellence for Gene Therapy Hematopoietic Cell Processing Core at the University of Washington, using a protocol approved by the University of Washington Institutional Review Board. Informed consent was obtained in accordance with the Declaration of Helsinki. The following antibodies (BD Immunocytochemistry, San Jose, CA) were used for flow cytometry: anti-CD34-PE (8G12), anti-CD45-PerCP-Cy5.5 (TÜ116), anti-CD71-APC (M-A712), and anti-CD235a-FITC (GA-R2). Matching isotype control antibodies were used as negative controls. Cell-cycle analysis was performed using propidium iodide staining of cells followed by flow cytometric determination of DNA content.
RNA and hemoglobin analysis
Total RNA was isolated, reverse transcribed, and analyzed by real-time polymerase chain reaction (RT-PCR) as previously described.13 Levels of globin mRNA were calculated by the method of Larionov et al14 using β-actin and GAPDH as normalizing genes. RT-PCR primers for mRNA quantification of α-, β- and γ-globin, DNMT1, and DNMT3 have been previously described.13 Primer sequences for nascent gene expression were α-globin: 5′-CACCCCTCACTCTGCTTCTC-3′ (S), 5′-CTTGCCGTGGCCCTTAAC-3′ (AS); β-globin: 5′-CACCCCTCACTCTGTGCTTCTC-3′ (S), 5′-CATCAAGCGTCCCATAGACTCAC-3′ (AS), γ-globin: 5′-GTAGGCTCTGGTGACCAGGACA-3′ (S), 5′-CTTGACTTTGGGGTTGCCC-3′ (AS); and GAPDH: 5′-ACACAGGGCCGGGATTGTCT-3′ (S) and 5′-ACCGGACTGCTCAGGCAAAG-3′ (AS). The α-globin primer has been previously published15; the others were designed for this study. PCR products for α-globin, β-globin, and γ-globin nascent RNAs crossed the intron 2/exon 2 boundaries. The GAPDH nascent RNA product is within intron 1. Hb HPLC analysis was performed as described by Ou and Rognerud.16
DNA methylation analysis
Bisulfite modification and sequencing was performed as described.13 Global LINE element methylation analysis was performed by the method of Yang, et al, using the primers 5′-TTTTGAGTTAGGTGTGGGATATA-3′ and 5′-AAAATCAAAAAATTCCCTTTC-3′.17
DNMT1 siRNA and shRNA
siRNA for DNMT1 or a fluorescein-labeled control siRNA (Santa Cruz Biotechnology, Santa Cruz, CA) were transfected into cells using HiPerFect reagent (Qiagen, Valencia, CA) following the manufacturer's suggested protocol for transfection of suspension cells on day +11 of the standard culture procedure. Untreated control cells were mock transfected without siRNA. Flow cytometry was performed at 6 hours on cells transfected with the labeled siRNA to assess transfection efficiency. Anti-DNMT1 and mismatch control shRNAs from Ting et al18 were cloned into the pLL3.7 lentiviral vector plasmid19 that also contains a green fluorescent protein (GFP) gene. Retrovirus was produced using 293T cells. Differentiating CD34+ cells were transfected on day +5 of the culture period. GFP expressing cells were selected by FACS on day +7 and then continued on the standard differentiation protocol. The following antibodies were used for Western blotting: anti-DNMT-1 (Imgenex, San Diego, CA); anti-DNMT-3a (Santa Cruz), and anti-β-actin (Sigma, St Louis, MO).
Results
In vitro differentiation of adult erythroid cells
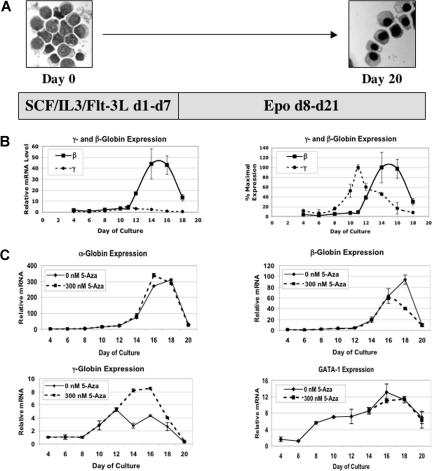
To test the 2 models of 5-Aza induced fetal globin gene expression, we used the in vitro erythroid differentiation system shown in Figure 1A.13 This system started with purified G-CSF–mobilized CD34+ peripheral blood cells from normal volunteers. These cells were cultured for 7 days in medium containing stem cell factor (SCF), IL-3, and Flt-3 ligand. The medium was then changed to one containing erythropoietin (Epo) for an additional 12 to 14 days. This system resulted in the production of a population of more than 90% end-stage erythroid cells with highly condensed nuclei.13 Figure 1B shows the expression profiles for the γ- and β-globin genes during differentiation. The first panel shows expression of the genes on the same relative scale. As expected for adult erythropoiesis, β-globin expression far exceeded that for γ-globin. In the second panel, the expression data were normalized to the maximal mRNA level for each gene. As has been previously described,20 γ-globin expression preceded β-globin expression during differentiation. This differentiation system has several qualities that make it useful for our experiments. These in-clude the relatively high purity of erythroid cells it produces, a logarithmic cell expansion of more than 2000-fold that provides sufficient cells for molecular studies, hemoglobin production similar to that seen in adult humans (99% HbA, 1% HbF),13 and a recapitulation of the transient low-level γ-globin gene expression that occurs during early differentiation of adult erythroid cells.
Figure 1.
5-azacytidine increases γ-globin and decreases β-globin mRNA levels. (A) The in vitro differentiation system used in this research. (B) γ- and β-globin steady-state mRNA levels during in vitro differentiation. The first panel shows RNA levels on the same scale. The second panel shows mRNA levels normalized to the maximal expression for each mRNA to emphasize the different time courses of expression. (C) Effects of daily 300 nM 5-Aza on steady-state mRNA levels for the α-, β-, and γ-globin and the GATA-1 genes. CD34+ cells were from different normal donors were used for experiments in panels B and C. Each RT-PCR analysis was performed in triplicate. Error bars represent 1 SD. Microscopy was performed using an Olympus model CHT light microscope (Olympus Optical, Tokyo, Japan). Magnification of Figure 1A was 100× (10×/10×; numeric aperture of the 10× objective was 0.25). Images were acquired with a FujiFilm FinePix A345 digital camera (Fujifilm USA, Valhalla, NY). Images were processed using iPhoto version 6.0.6 (Apple Computer, Cupertino, CA).
5-Aza induction of fetal globin gene expression
We next tested the ability of 5-Aza to induce fetal globin gene expression during differentiation. Because 5-Aza has a relatively short half-life of approximately 5 hours in aqueous solution at neutral pH21 and to mimic the daily administration schedules used in most clinical applications of 5-Aza, we applied the drug on a daily basis from days 10 to 20 of differentiation. We chose an initial drug concentration of 300 nM based on preliminary experiments in K562 cells which showed that this dose was the maximum that did not alter cell expansion rate (data not shown). Figure 1C shows the expression profiles of the α, β, and γ-globin genes and the GATA-1 gene with and without 5-Aza as determined by quantitative RT-PCR. These results show that while 5-Aza did not alter the expression of the α-globin or GATA-1 genes, it produced an increase in γ-globin mRNA and a decrease in β-globin mRNA. Note that γ-globin mRNA remained high beyond the point where levels in untreated cells begin to decline.
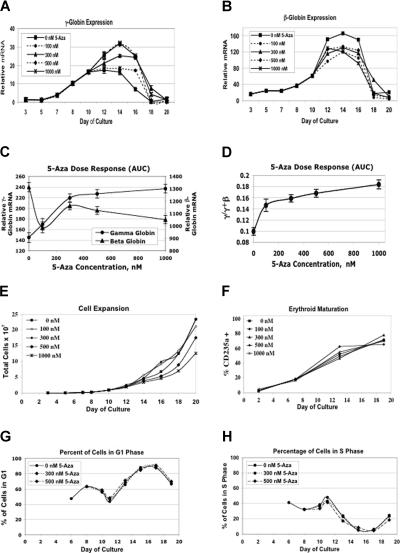
We next sought to determine the dose-response for 5-Aza induction of γ-globin gene expression. This was important to the goal of our study. If we could show, for example, that γ-globin induction occurred at doses lower than those associated with growth inhibition, then this would argue against the cytotoxicity model of 5-Aza action. Similarly, if induction of gene expression occurred at doses where DNA methylation was not changed, then this would argue against the methylation hypo-thesis. The effects of 5-Aza concentrations ranging from 0 to 1000 nM on γ and β-globin gene expression are shown in Figure 2A,B. All concentrations of 5-Aza produced higher peak levels and more prolonged expression of γ-globin mRNA. β-globin mRNA levels were suppressed by all 5-Aza doses. Dose-response curves for these data are shown in Figure 2C,D. Here, levels of expression refer to the areas under the expression curves for each gene at each dose, providing a measure of total mRNA production throughout differentiation. Figure 2C shows that a dose of 300 nM provided a near-maximal increase in γ-globin gene expression and that even the lowest dose tested (100 nM) stimulated significant γ-globin mRNA accumulation compared with the untreated control. Figure 2D shows a similar relationship for the proportion of γ-globin mRNA over total γ plus β-globin mRNA. Figure 2E shows that cell growth rates declined at doses of 500 nM or above, but at 300 nM, where near maximal γ-globin mRNA induction was seen, there was no effect on cell proliferation. When differentiation kinetics were studied, as measured by the proportion of cells expressing glycophorin A, there was no consistent dose-related change (Figure 2F). Using propidium iodide staining and flow cytometry, we examined cell-cycle distribution during differentiation at 5-Aza dose levels of 0, 300, and 500 nM. The proportion of cells in G1, S, and G2 phases was determined. Our results show that there was a reciprocal relationship between the proportion of cells in G1 and S with the proportion of cells in S phase peaking on day 11. These results are similar to those previously reported by Wojda et al.22 There was no significant change in the proportion of cells in G1 (Figure 2G), S (Figure 2H), or G2 (data not shown) with 5-Aza treatment. This experiment demonstrated that 5-Aza, at doses that strongly induce fetal gene expression, does not alter cell-cycle distribution during differentiation.
Figure 2.
Dose-response effects of 5-azacytidine during in vitro erythroid differentiation. Effects of different doses of 5-azacytidine on (A) γ-globin gene expression during differentiation, (B) β-globin gene expression during differentiation, (C) total γ- and β-globin gene expression, expressed as the area under the curve (AUC) for each dose, (D) γ/γ plus βAUC for each dose, (E) cell expansion, (F) differentiation kinetics as assessed by CD235a (glycophorin A) cell surface expression, (G) the proportion of cells in the G1 phase of the cell cycle, and (H) the proportion of cells in the S phase of the cell cycle. CD34+ cells were from different normal donors were used for experiments in panels A-F and G,H. Each RT-PCR analysis was performed in triplicate. Error bars represent 1 SD.
5-Azacytidine induced a disproportionate increase in fetal hemoglobin production
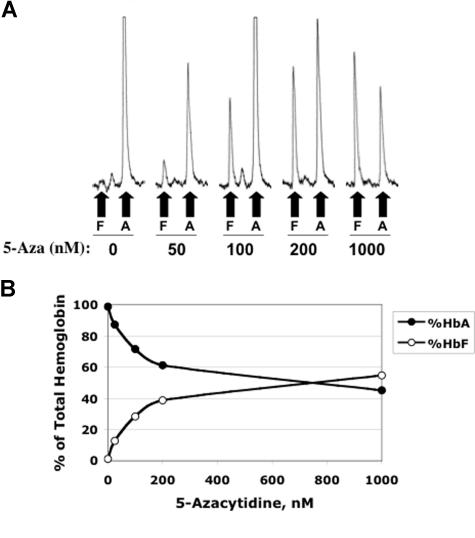
We next determined whether the observed increase in γ-globin mRNA resulted in an increase in HbF using HPLC. These determinations were made using hemolysates from cells at the end of the culture period. The primary data for this experiment are presented in Figure 3A and the summary dose-response curves are shown in Figure 3B. This experiment showed an increase in the proportion of HbF to more than 50% of total hemoglobin at the 1000 nM 5-Aza dose. Note also the reciprocal drop in HbA levels as the dose of 5-Aza is increased, and that a dose of 5-Aza as low as 50 nM induced an approximately 10-fold increase in HbF.
Figure 3.
Dose-response effects of 5-azacytidine on hemoglobin production. (A) Ion exchange HPLC tracings of hemolysates from cells at the completion of the in vitro differentiation procedure. Fetal (F) and adult (A) hemoglobin peaks are indicated. (B) Summary of Hb HPLC data showing a reciprocal effect of 5-azacytidine on fetal and adult hemoglobin levels.
5-Aza produced hypomethylation of the γ-globin promoter during differentiation of adult erythroid cells
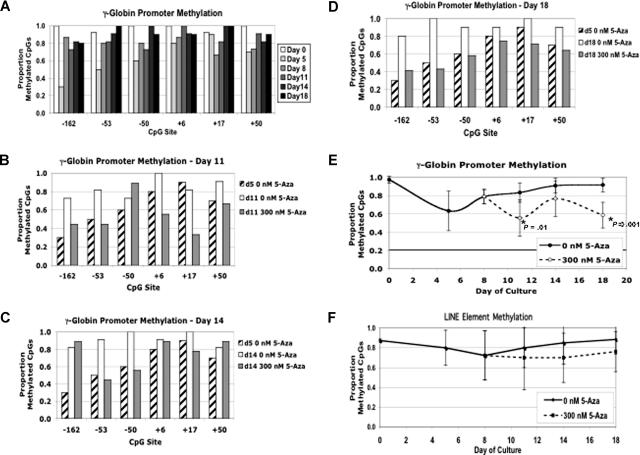
The demethylation model of γ-globin induction by 5-Aza is based on the fact that this agent is a general, nonspecific inhibitor of DNMTs, including the 3 human enzymes DNMT1, DNMT3a, and DNMT3b.7 This inhibition of DNMT activity is hypothesized to produce global demethylation of DNA in differentiating erythroid cells, including the γ-globin gene promoters, resulting in activation of these genes. Using the bisulfite conversion method, we examined the effect of 5-Aza on the 7 CpGs between −256 and +50 bp of the γ promoter. The methylation changes for each CpG in untreated cells during differentiation are shown in Figure 4A. In CD34+ progenitor cells (day 0 of differentiation), these CpGs were highly methylated. Early in differentiation the promoter CpGs became hypomethylated. This change was most notable for the CpGs upstream of the transcriptional start site. Later, following the addition of Epo, they returned to the highly methylated state seen in normal human adult marrow cells.13 The effect of daily 300 nM 5-Aza on methylation of individual promoter CpGs at days +11, +14, and +18 is shown in Figure 4B-D. This dose of 5-Aza was chosen because it produced near maximal stimulation of fetal globin gene expression and HbF induction without altering cell growth or differentiation. For comparison, the methylation status of each CpG on day +5 is also shown. In general, 5-Aza caused a decrease in methylation to levels near those seen at day +5 of normal differentiation. This period of hypomethylation was associated with the time when γ-globin mRNA levels were most rapidly increasing (days ∼7-9). Increased methylation (by days 9-10) was associated with the leveling off and falling of γ-globin mRNA levels (see Figures 1C and 2A).13 Note also that, just as during normal differentiation, the decreased methylation associated with 5-Aza appeared to be more pronounced at CpGs located upstream of the transcriptional start site. Our results for the CpG at −53 agreed with earlier findings that this CpG becomes hypomethylated with in vivo 5-Aza treatment.4,5 A summary of these data are presented in Figure 4E, where the averages for all of the promoter CpGs are plotted for each time point. Following demethylation early in differentiation, there was a gradual remethylation in the untreated cells. In the presence of 5-Aza, overall promoter methylation was decreased to levels equivalent to those seen at day +5. To determine whether the demethylating effects of 5-Aza were representative of genome-wide demethylation or were a localized effect at the globin promoters, we used a method developed by the Issa laboratory to assess global levels of DNA methylation.17 Following standard bisulfite modification of genomic DNA, this method employed PCR primers that amplify a conserved segment of long interspersed nuclear element (LINE) DNA. Several hundred thousand copies of these highly methylated retrotransposons are scattered throughout the genome.23 Each PCR product included approximately 7 to 10 CpGs, depending on the sequence of the individual element. This method has been used to monitor the effects of 5-Aza on genomic DNA methylation in the hematopoietic cells of leukemia patients on investigational trials.24 In contrast to what was seen for the γ-globin promoters, there was no significant decrease in global methylation early in differentiation (Figure 4F). Although there was a trend toward lower methylation during 5-Aza treatments, it was not significantly different compared with the untreated cells. A median of 8 LINE elements were sequenced for each time point in Figure 4F, producing an average of 56 individual CpGs for each data point. While our methylation data did show the expected demethylation of the γ globin promoters by 5-Aza, it suggested that at the dose used, this was not a generalized genome-wide effect, but may be a localized secondary effect of the drug.
Figure 4.
5-Azacytidine decreases methylation of the γ-globin promoter. (A) The sodium bisulfite modification method was used to determine the methylation status of the 6 CpGs between −162 and +50 of the γ-globin promoter during in vitro differentiation. Each bar represents the proportion of methylated CpGs at each site for different time points during differentiation. (B) Methylation of individual γ-globin CpGs on day +11 of differentiation with ( ) and without (□) 300 nM 5-azacytidine treatment. For comparison, the methylation of the CpGs at day +5 without 5-azacytidine is shown (▨). (C) Methylation of individual γ-globin CpGs on day +14. (D) Methylation of individual γ-globin CpGs on day +18. (D) Comparison of total γ-globin promoter methylation during differentiation with and without 300 nM 5-azacytidine. (E) Comparison of total γ-globin promoter methylation during differentiation with and without 300 nM 5-azacytidine. (F) As a measure of genome-wide DNA methylation, the effect of 300 nM 5-azacytidine on LINE methylation was determined. CD34+ cells from a single normal donor were used for this experiment. Error bars represent 1 SD. P values were determined by t test.
) and without (□) 300 nM 5-azacytidine treatment. For comparison, the methylation of the CpGs at day +5 without 5-azacytidine is shown (▨). (C) Methylation of individual γ-globin CpGs on day +14. (D) Methylation of individual γ-globin CpGs on day +18. (D) Comparison of total γ-globin promoter methylation during differentiation with and without 300 nM 5-azacytidine. (E) Comparison of total γ-globin promoter methylation during differentiation with and without 300 nM 5-azacytidine. (F) As a measure of genome-wide DNA methylation, the effect of 300 nM 5-azacytidine on LINE methylation was determined. CD34+ cells from a single normal donor were used for this experiment. Error bars represent 1 SD. P values were determined by t test.
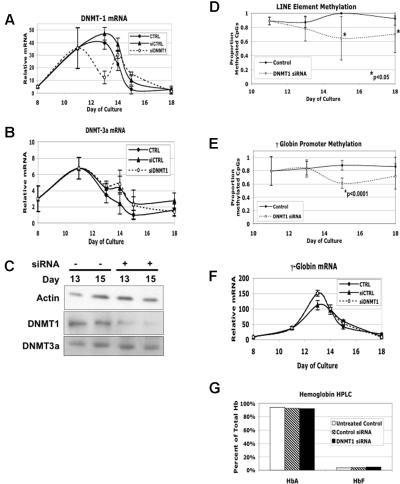
siRNA and shRNA suppression of DNMT1 expression caused promoter demethylation but not HbF induction
One of the difficulties in determining the mechanism of 5-Aza is that it has many activities beyond inhibition of DNA methylation. These include cytotoxicity, induction of apoptosis and DNA repair pathways, inhibition of DNA, RNA and protein synthesis, and alteration of tRNA and rRNA function.7 To critically test the hypothesis that DNA demethylation, in the absence of these other effects, is sufficient to induce γ-globin gene expression, we used siRNA to suppress the production of DNMT1 during erythroid differentiation. DNMT1 is the principal mammalian maintenance methyltransferase25 and is therefore the most likely to be involved in methylating globin gene promoter CpGs following DNA replication. It is also the most highly induced of the 3 human DNMTs during in vitro differentiation.13 If the major mechanism by which 5-Aza stimulates γ globin gene expression and HbF production is through global DNA hypomethylation, then this manipulation should produce similar results.
For these experiments, the DNMT1 siRNA and a scrambled, fluorescent control siRNA were transfected into differentiating erythroid cells on day +11. This time point was picked for siRNA treatment to suppress the previously identified peak in DNMT1 mRNA.13 An untreated control was mock transfected without any added siRNA. An average transfection efficiency of 77% was determined using the fluorescently tagged control siRNA and flow cytometry (data not shown). As shown in Figure 5A, transfection with the DNMT1 siRNA results in a significant decrease in DNMT1 mRNA compared with the mock transfected and control siRNA treated cells. Figure 5B shows that these treatments did not alter DNMT-3a mRNA levels. In Figure 5C, levels of DNMT1 protein were assessed by Western blotting. Here we see a reduction in protein levels only with the DNMT1 siRNA treatment, and levels of actin and DNMT3a were not changed. We next examined the effect of DNMT1 siRNA on global DNA methylation. As shown in Figure 5D, this treatment caused a significant decrease in LINE element methylation. We next evaluated the effect of DNMT1 siRNA on γ-globin promoter methylation (Figure 5E). Here we saw a significant decrease in promoter methylation (61% ± 7% of CpGs methylated vs 88% ± 8% in untreated controls, P < .001). This change is similar in magnitude to that seen with 5-Aza (59% ± 14% methylated CpGs vs 92% ± 8.1% in controls, P < .001; Figure 4E). We next determined whether the changes in γ promoter methylation were associated with γ-globin mRNA induction (Figure 5F). While the nonspecific control showed slightly less expression at one time point, there was no difference at all at the other time points for the 3 treatments. Finally, as shown in Figure 5G, we determined the effect of DNMT1 siRNA on hemoglobin production. In contrast to the high levels of induction seen with 5-Aza, we saw no increase in HbF despite demethylation of LINE and γ-globin promoter CpGs.
Figure 5.
siRNA knock-down of DNMT1 produces decreased γ-globin promoter methylation but no increase in γ-globin mRNA or fetal hemoglobin. siRNA for the DNMT1 gene was transiently transfected into cells on day +11 of in vitro erythroid differentiation. Controls for this experiment were cells from the same initial culture that were treated with a nonspecific siRNA (siCTRL) or underwent mock transfection without siRNA (CTRL). (A) The effect of siRNA treatment on DNMT1 mRNA levels during differentiation as determined by quantitative RT-PCR. (B) The effect of siRNA treatment on DNMT3a mRNA levels. (C) The effect of siRNA treatment on DNMT1 protein levels as determined by Western blotting. β-actin and DNMT3a serve as nonspecific controls. (D) The effect of siRNA treatment on global DNA methylation as determined by bisulfite conversion analysis of LINE elements. Note that for the day 15 control sample no error bars are shown. This is because all 5 sequences, which included a total of 42 individual CpGs, were 100% methylated so the standard deviation for this data point was 0. (E) The effect of siRNA treatment on γ-globin promoter DNA methylation. (F) The effect of siRNA treatment on γ-globin mRNA during differentiation. (G) The effect of siRNA treatment on hemoglobin production as assessed by HPLC analysis of cell lysates at the end of in vitro differentiation. CD34+ cells from a single normal donor were used for this experiment. Error bars represent 1 SD. P values were determined by t test.
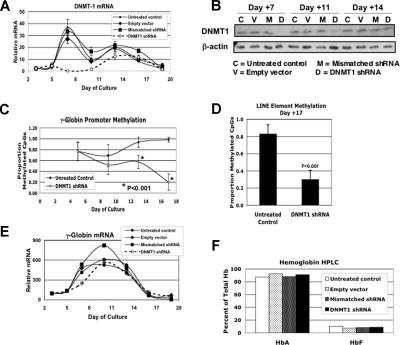
The preceding results argue against the hypothesis that 5-Aza induces HbF production through generalized DNA demethylation. Because this was an unexpected finding and could, if correct, lead to new ideas about fetal globin gene regulation and induction, we wanted to confirm our results using a method that could achieve even greater levels of global DNA demethylation. To accomplish this, we constructed a lentiviral vector expressing anti-DNMT1 shRNA and green fluorescent protein (GFP). Negative controls included a vector with a mismatched version of the DNMT1 shRNA, an “empty” vector with no shRNA, and an untransfected population of cells. On day +5 of differentiation, cells were transfected with the vectors, and on day +7 cells expressing GFP were selected by FACS and then continued on the standard differentiation protocol. As shown in Figure 6A, this strategy completely eliminated the early peak in DNMT1 expression. Near the end of the differentiation process, levels of DNMT1 mRNA rose to control levels. This may have been due to silencing of the integrated vector as the DNA became compacted and genes were down-regulated near the end of erythroid differentiation. DNMT1 protein levels are shown in Figure 6B. On days +7 and +11, protein levels decreased only with anti-DNMT1 shRNA but, consistent with the mRNA data, they rose later in differentiation. Figure 6C shows that suppression of DNMT1 resulted in a potent demethylation of γ promoter DNA as, by the end of differentiation, only 20% (± 11%) of promoter CpGs were methylated compared with 98% (± 4%) in the control cells (P < .001). This level of promoter hypomethylation was equivalent to that seen in primary fetal erythroid cells (13% ± 7%).13 To verify that this was a global effect on DNA methylation, we determined LINE element methylation on cells from day +17. As shown in Figure 6D, these sequences were also highly demethylated when DNMT1 was suppressed. A potential explanation for the continued fall in DNA methylation despite the rise in DNMT1 protein levels later in differentiation is that, due to the lack of the enzyme early in differentiation, hemimethylated and then fully demethylated sequences were produced. Because DNMT1 methylates the unmethylated strand of newly synthesized hemimethylated DNA, any unmethylated DNA will not be remethylated despite the return of normal levels of DNMT1. Finally, Figure 6E,F shows that, despite demethylation of the γ-globin promoters to levels seen in fetal liver cells, there was no increase in γ-globin steady-state mRNA or HbF.
Figure 6.
shRNA knock-down of DNMT1 produces decreased γ-globin promoter methylation but no increase in γ-globin mRNA or fetal hemoglobin. Lentiviral vector LL3.7 containing DNMT1 shRNA and GFP sequences was used to transduce differentiating cells on day +5. On day +7 cells expressing GFP were sorted and allowed to differentiate into erythroid cells. Controls for this experiment were cells from the same initial culture transduced with vectors carrying a mismatched version of the DNMT1 shRNA, an empty vector, or cells that were untreated. Effects of DNMT1 shRNA on (A) DNMT1 steady-state mRNA levels during in vitro differentiation, (B) DNMT1 protein levels as determined by Western blot, (C) γ-globin promoter DNA methylation, (D) LINE element DNA methylation, (E) γ-globin steady-state mRNA, and (F) Hb levels as determined by HPLC at the end of differentiation. CD34+ cells from a single healthy donor were used for this experiment. Error bars represent 1 SD. P values were determined by t test.
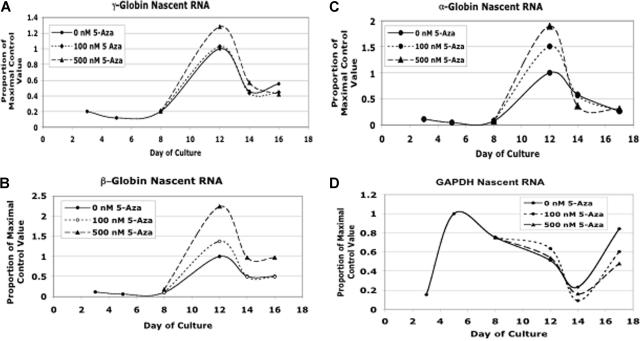
5-Azacytidine induced nascent RNA production from multiple globin genes
As a final investigation into the mechanism of 5-Aza, we sought to determine the basis for the increased γ-globin mRNA levels. As shown in Figures 1 and 2, γ-globin message not only peaked at a higher level with 5-Aza, but levels remained elevated later into differentiation. This result led us to hypothesize that the major action of 5-Aza was to extend the period of γ-globin gene expression, resulting in accumulation of higher levels of mRNA. We further hypothesized that β-globin gene expression would be suppressed. Such a model would be consistent with the LCR being partially redirected from the β to the γ-globin promoter by 5-Aza treatment. To address this model, we performed RT PCR for nascent globin gene transcripts using PCR primers that either crossed intron-exon boundaries (α, β, and γ-globin) or were within an intron (GAPDH). Because introns are excised even as RNA is being synthesized, this assay provided information on current mRNA synthesis as opposed to steady-state globin mRNA levels, which have long half-lives. Controls without reverse transcriptase were run for each experiment and showed no detectable PCR product, indicating that we were not amplifying residual DNA (data not shown). Data for nascent γ-globin gene expression are shown in Figure 7A. While there was an increase in peak γ-globin nascent RNA on day 12 with 500 nM 5-Aza, the period of expression is not prolonged. When we looked at nascent β-globin transcripts (Figure 7B), we were surprised to see a dose-dependent increase in gene expression. This is in contrast to the decrease in β-globin steady-state mRNA levels seen with 5-Aza treatment. We next evaluated α-globin nascent RNA production (Figure 7C) and found that it too was increased in a dose-dependent fashion. Finally, as a nonglobin control, we examined nascent expression of the GAPDH gene. As seen in Figure 7D, this gene had an expression pattern that was very different from the globin genes. Furthermore, the 5-Aza dependent increase in globin gene expression seen at day +12 of differentiation, were not part of a generalized phenomenon, as 5-Aza, at the doses tested in this experiment, did not alter GAPDH gene expression. These results suggest that while 5-Aza affected γ-globin gene expression, posttranscriptional effects on globin gene mRNA levels also played a role globin gene induction.
Figure 7.
5-Azacytidine increases nascent mRNA production from the γ, β, and α-globin genes. Quantitative RT-PCR was performed using primers that crossed intron-exon boundaries or were within an intron to determine levels of nascent or pre-mRNA as an indicator of gene activity. (A) Effect of 5-azacytidine on nascent γ-globin gene expression. (B) Effect of 5-azacytidine on nascent β-globin gene expression. (C) Effect of 5-azacytidine on nascent α-globin gene expression. (D) Effect of 5-azacytidine on nascent GAPDH gene expression. CD34+ cells from a single normal donor were used for this experiment. Each RT-PCR analysis was performed in triplicate.
Discussion
Comparing models of 5-azacytidine action
Our initial focus was to determine which of 2 proposed models of 5-Aza action was most likely to be responsible for fetal Hb induction. The predictions of the kinetics model include a slowing of cell expansion as cells die or are arrested following drug treatment, changes in cell-cycle distribution, and changes in the kinetics of differentiation. While each of these effects is well known to occur at higher doses of 5-Aza, we found that a 5-Aza dose of 300 nM, which produced near maximal induction of steady-state γ-globin mRNA and HbF production, did not significantly affect these parameters.
The demethylation model of 5-Aza and decitabine action is based on the drugs' ability to decrease DNA methylation. While our experiments confirming the previously described demethylating effect of 5-Aza on upstream γ-globin promoter CpGs were consistent with this model, other data were not. First, the 5-Aza–induced demethylation was more pronounced at upstream CpG sites, not at all promoter CpGs. Second, a 5-Aza dose of 300 nM resulted in an approximately 50% decrease in γ promoter methylation, but did not significantly change LINE1 element methylation. Our experiments are not the first to demonstrate such a result, as Charache et al, in their report of 5-Aza stimulation of HbF production in an SCD patient, noted demethylation at one CpG of the upstream γ-globin promoter but not at several other CpGs in the globin locus.5 Third, while siRNA targeting of DNMT1 caused demethylation of the γ-globin promoters that was similar in magnitude to that seen with 5-Aza, there was no change in γ-globin mRNA levels or HbF production. Finally, anti-DNMT1 shRNA suppression of γ promoter methylation to levels as low as those seen in fetal liver erythroid cells also did not increase fetal globin mRNA or HbF production. These results suggest that the decrease in γ-globin promoter methylation seen with 5-Aza is not the primary cause of γ gene induction and HbF production, but is a secondary effect related to gene activation by some other mechanism.
Besides inhibition of DNA methylation and changes in RNA structure and function and protein synthesis, 5-Aza has other effects on cellular function. One possibility is that low-level activation of DNA damage, cell stress or other signaling pathways leads to the binding of positive-acting transcription complexes to the globin promoters that exclude DNMT1 and other repressive factors. 5-Aza or decitabine have been shown to activate p53 related pathways for DNA damage,26 apoptosis27 and the mitogen-activated protein kinases (MAPK).28 Several cell-signaling pathways have been implicated in fetal globin gene regulation in a variety of experimental systems, including those involving nitric oxide,29 p38 MAPK,30 STAT3β,31 cGMP,29,32 cAMP,33 and cytokine signaling.34,35 It is possible that 5-Aza and decitabine, as well as other inducers of fetal Hb, act through these or other pathways.
The role of posttranscriptional effects in globin gene induction
To date, most research on HbF induction has focused on changes in steady-state mRNA levels. While our findings do confirm the results of previous studies showing that 5-Aza treatment causes increased γ-globin and decreased β-globin mRNA levels, we have also observed that nascent RNA production from the α-, β-, and γ-globin genes is increased in our system. We also found that while 5-Aza induces a relatively modest, approximately 2-fold increase in total γ-globin mRNA, there is a much larger increase in HbF. This increase, to levels exceeding 50% of total Hb, is greater than has been seen in clinical trials. One potential explanation for this result is that patients have typically been treated with drug for courses of only 5 to 7 days. With these dosing schedules, only a fraction of the subjects' circulating erythrocytes actually underwent differentiation in the presence of drug. In contrast, all of the cells in our culture system were exposed to 5-Aza for the full duration of their differentiation.
Our results suggest posttranscriptional regulation of globin mRNA and protein levels. There is an extensive body of work on the mechanisms that mediate α-globin message stability and processing.36,37 Recently, similar mechanisms have been shown to mediate human ϵ and β-globin mRNA stability.38,39 In the case of 5-Aza induction, globin RNA processing could be affected by at least 2 broad mechanisms. First, 5-Aza is incorporated into RNA transcripts as they are synthesized. The incorporated nucleoside analogues could disrupt (or enhance) the interactions of proteins required for nuclear processing, nuclear export, cytoplasmic stability, cytoplasmic localization, or efficient translation of mRNAs. Butyrate has recently been shown to increase HbF production through enhanced translation efficiency of γ-globin message,40 and induction of HbF in K562 cells by GTP has been linked to a prolongation of γ-globin mRNA half-life.41
A second possibility is that the posttranscriptional effects we have observed following 5-Aza treatment are a reflection of an underlying system which coordinately regulates globin mRNA and/or protein levels. Evidence of coordinated Hb production comes from the work of Dover and Boyer, who showed that in a variety of clinical conditions associated with elevated HbF levels, the mean corpuscular Hb was equivalent in F and non-F cells from the same subject.42 To explain their findings, they proposed that once a critical total cellular Hb concentration is reached in the differentiating erythroblast, further Hb production is shut down. Noting that HbF is produced earlier in differentiation than HbA, they proposed that increased HbF synthesis would lead to the early termination of HbA formation and a higher proportion of HbF. Chakalova et al recently studied Hb production in people with the Corfu deletional δβ thalassemia mutation.15 In heterozygotes, HbF levels are normal (1% to 2%) despite elevated expression of nascent γ-globin transcripts in cis to the mutation. In homozygotes, who exhibit only a moderate anemia, most of the hemoglobin is HbF. The authors suggested that γ mRNA is able to efficiently accumulate only if β-globin mRNA falls below a specific level. Our results with 5-Aza are consistent with these clinical observations as we found that a dose-dependent increase in HbF is associated with a reciprocal decrease in HbA so that total cellular Hb was unchanged.
Implications for the pharmacologic manipulation of HbF production
Our results suggest that the pharmacologic induction of HbF by 5-Aza may not be the result of effects on general mechanisms such as differentiation kinetics or genome-wide DNA methylation, but may represent a more focused effect. If our results are verified, then strategies targeting specific pathways may be able to produce clinically significant induction of HbF without the side effects and other problems which limit the wide spread use of current agents. Our results also suggest that future studies on the posttranscriptional regulation of globin mRNA and Hb levels will be important for understanding normal and induced fetal Hb production.
Acknowledgments
We thank Dr Shelly Heimfeld and his staff at the National Heart, Lung, and Blood Institute's Programs of Excellence for Gene Therapy Hematopoietic Cell Processing Core of the University of Washington for providing peripheral blood CD34+ cells, Dr Lionel Lewis and Bernie Beaulieu for assistance with Hb HPLC, Dr Jean-Pierre Issa for assistance with the LINE element methylation assay, and Drs Steven Fiering, Jeffrey Miller, Susan Perrine, Shirley Taylor, Eric Russell, William Rigby, and George Stamatoyannopoulos for helpful discussions.
This work was supported by National Institutes of Health grant HL73442 to C.H.L. and from funding provided by the Knights of the York Cross of Honour.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.M., M.R.G., C.A.R., S.C., and C.K. helped design, perform, and analyze experiments. C.H.L. designed the overall project, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christopher H. Lowrey, MD, Section of Hematology/Oncology, Dartmouth-Hitchcock Medical Center, One Medical Center Drive, Lebanon, NH 03756; e-mail: c.lowrey@dartmouth.edu.
References
- 1.Ali SA. Milder variant of sickle-cell disease in Arabs in Kuwait associated with unusually high level of foetal haemoglobin. Br J Haematol. 1970;19:613–619. doi: 10.1111/j.1365-2141.1970.tb01645.x. [DOI] [PubMed] [Google Scholar]
- 2.Prchal J, Stamatoyannopoulos G. Two siblings with unusually mild homozygous beta-thalassemia: a didactic example of the effect of a nonallelic modifier gene of the expressivity of a monogenic disorder. Am J Med Genet. 1981;10:291–300. doi: 10.1002/ajmg.1320100312. [DOI] [PubMed] [Google Scholar]
- 3.Pace BS, Zein S. Understanding mechanisms of gamma-globin gene regulation to develop strategies for pharmacological fetal hemoglobin induction. Dev Dyn. 2006;235:1727–1737. doi: 10.1002/dvdy.20802. [DOI] [PubMed] [Google Scholar]
- 4.Ley TJ, DeSimone J, Anagnou NP, et al. 5-azacytidine selectively increases gamma-globin synthesis in a patient with beta+ thalassemia. N Engl J Med. 1982;307:1469–1475. doi: 10.1056/NEJM198212093072401. [DOI] [PubMed] [Google Scholar]
- 5.Dover GJ, Charache SH, Boyer SH, Talbot CC, Jr., Smith KD. 5-Azacytidine increases fetal hemoglobin production in a patient with sickle cell disease. Prog Clin Biol Res. 1983;134:475–488. [PubMed] [Google Scholar]
- 6.Lowrey CH, Nienhuis AW. Brief report: treatment with azacitidine of patients with end-stage beta-thalassemia [see comments]. N Engl J Med. 1993;329:845–848. doi: 10.1056/NEJM199309163291205. [DOI] [PubMed] [Google Scholar]
- 7.Christman JK. 5-Azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene. 2002;21:5483–5495. doi: 10.1038/sj.onc.1205699. [DOI] [PubMed] [Google Scholar]
- 8.DeSimone J, Heller P, Hall L, Zwiers D. 5-Azacytidine stimulates fetal hemoglobin synthesis in anemic baboons. Proc Natl Acad Sci U S A. 1982;79:4428–4431. doi: 10.1073/pnas.79.14.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saunthararajah Y, Lavelle D, DeSimone J. DNA hypo-methylating agents and sickle cell disease. Br J Haematol. 2004;126:629–636. doi: 10.1111/j.1365-2141.2004.05064.x. [DOI] [PubMed] [Google Scholar]
- 10.Stamatoyannopoulos G, Veith R, Galanello R, Papayannopoulou T. Hb F production in stressed erythropoiesis: observations and kinetic models. Ann N Y Acad Sci. 1985;445:188–197. doi: 10.1111/j.1749-6632.1985.tb17188.x. [DOI] [PubMed] [Google Scholar]
- 11.Fathallah H, Atweh GF. DNA hypomethylation therapy for hemoglobin disorders: molecular mechanisms and clinical applications. Blood Rev. 2006;20:227–234. doi: 10.1016/j.blre.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Stamatoyannopoulos G. Control of globin gene expression during development and erythroid differentiation. Exp Hematol. 2005;33:259–271. doi: 10.1016/j.exphem.2004.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mabaera R, Richardson CA, Johnson K, Hsu M, Fiering S, Lowrey CH. Developmental- and differentiation-specific patterns of human {gamma}- and {beta}-globin promoter DNA methylation. Blood. 2007;110:1343–1352. doi: 10.1182/blood-2007-01-068635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larionov A, Krause A, Miller W. A standard curve based method for relative real time PCR data processing. BMC Bioinformatics. 2005;6:62. doi: 10.1186/1471-2105-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakalova L, Osborne CS, Dai YF, et al. The Corfu deltabeta thalassemia deletion disrupts gamma-globin gene silencing and reveals post-transcriptional regulation of HbF expression. Blood. 2005;105:2154–2160. doi: 10.1182/blood-2003-11-4069. [DOI] [PubMed] [Google Scholar]
- 16.Ou CN, Rognerud CL. Rapid analysis of hemoglobin variants by cation-exchange HPLC. Clin Chem. 1993;39:820–824. [PubMed] [Google Scholar]
- 17.Yang AS, Estecio MR, Doshi K, Kondo Y, Tajara EH, Issa JP. A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res. 2004;32:e38. doi: 10.1093/nar/gnh032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ting AH, Jair KW, Suzuki H, Yen RW, Baylin SB, Schuebel KE. CpG island hypermethylation is maintained in human colorectal cancer cells after RNAi-mediated depletion of DNMT1. Nat Genet. 2004;36:582–584. doi: 10.1038/ng1365. [DOI] [PubMed] [Google Scholar]
- 19.Rubinson DA, Dillon CP, Kwiatkowski AV, et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet. 2003;33:401–406. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- 20.Farquhar MN, Turner PA, Papayannopoulou T, Brice M, Nienhuis AW, Stamatoyannopoulos G. The asynchrony of gamma- and beta-chain synthesis during human erythroid cell maturation. III. gamma- and beta-mRNA in immature and mature erythroid clones. Dev Biol. 1981;85:403–408. doi: 10.1016/0012-1606(81)90271-2. [DOI] [PubMed] [Google Scholar]
- 21.Israili ZH, Vogler WR, Mingioli ES, Pirkle JL, Smithwick RW, Goldstein JH. The disposition and pharmacokinetics in humans of 5-azacytidine administered intravenously as a bolus or by continuous infusion. Cancer Res. 1976;36:1453–1461. [PubMed] [Google Scholar]
- 22.Wojda U, Noel P, Miller JL. Fetal and adult hemoglobin production during adult erythropoiesis: coordinate expression correlates with cell proliferation. Blood. 2002;99:3005–3013. [PubMed] [Google Scholar]
- 23.Smit AF. The origin of interspersed repeats in the human genome. Curr Opin Genet Dev. 1996;6:743–748. doi: 10.1016/s0959-437x(96)80030-x. [DOI] [PubMed] [Google Scholar]
- 24.Yang AS, Doshi KD, Choi SW, et al. DNA methylation changes after 5-aza-2′-deoxycytidine therapy in patients with leukemia. Cancer Res. 2006;66:5495–5503. doi: 10.1158/0008-5472.CAN-05-2385. [DOI] [PubMed] [Google Scholar]
- 25.Goll MG, Bestor TH. Eukaryotic cytosine methyltransferases. Annu Rev Biochem. 2005;74:481–514. doi: 10.1146/annurev.biochem.74.010904.153721. [DOI] [PubMed] [Google Scholar]
- 26.Karpf AR, Moore BC, Ririe TO, Jones DA. Activation of the p53 DNA damage response pathway after inhibition of DNA methyltransferase by 5-aza-2′-deoxycytidine. Mol Pharmacol. 2001;59:751–757. [PubMed] [Google Scholar]
- 27.Ueno M, Katayama K, Yamauchi H, Nakayama H, Doi K. Cell cycle and cell death regulation of neural progenitor cells in the 5-azacytidine (5AzC)-treated developing fetal brain. Exp Neurol. 2006;198:154–166. doi: 10.1016/j.expneurol.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 28.Pulukuri SM, Rao JS. Activation of p53/p21Waf1/Cip1 pathway by 5-aza-2′-deoxycytidine inhibits cell proliferation, induces pro-apoptotic genes and mitogen-activated protein kinases in human prostate cancer cells. Int J Oncol. 2005;26:863–871. [PubMed] [Google Scholar]
- 29.Cokic VP, Smith RD, Beleslin-Cokic BB, et al. Hydroxyurea induces fetal hemoglobin by the nitric oxide-dependent activation of soluble guanylyl cyclase. J Clin Invest. 2003;111:231–239. doi: 10.1172/JCI16672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pace BS, Qian XH, Sangerman J, et al. p38 MAP kinase activation mediates gamma-globin gene induction in erythroid progenitors. Exp Hematol. 2003;31:1089–1096. doi: 10.1016/s0301-472x(03)00235-2. [DOI] [PubMed] [Google Scholar]
- 31.Foley HA, Ofori-Acquah SF, Yoshimura A, Critz S, Baliga BS, Pace BS. Stat3 beta inhibits gamma-globin gene expression in erythroid cells. J Biol Chem. 2002;277:16211–16219. doi: 10.1074/jbc.M106556200. [DOI] [PubMed] [Google Scholar]
- 32.Ikuta T, Ausenda S, Cappellini MD. Mechanism for fetal globin gene expression: role of the soluble guanylate cyclase-cGMP-dependent protein kinase pathway. Proc Natl Acad Sci U S A. 2001;98:1847–1852. doi: 10.1073/pnas.041599798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keefer JR, Schneidereith TA, Mays A, Purvis SH, Dover GJ, Smith KD. Role of cyclic nucleotides in fetal hemoglobin induction in cultured CD34+ cells. Exp Hematol. 2006;34:1151–1161. doi: 10.1016/j.exphem.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 34.Bhanu NV, Trice TA, Lee YT, et al. A sustained and pancellular reversal of gamma-globin gene silencing in adult human erythroid precursor cells. Blood. 2005;105:387–393. doi: 10.1182/blood-2004-04-1599. [DOI] [PubMed] [Google Scholar]
- 35.Perrine SP, Castaneda SA, Boosalis MS, White GL, Jones BM, Bohacek R. Induction of fetal globin in beta-thalassemia: Cellular obstacles and molecular progress. Ann N Y Acad Sci. 2005;1054:257–265. doi: 10.1196/annals.1345.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waggoner SA, Liebhaber SA. Regulation of alpha-globin mRNA stability. Exp Biol Med (Maywood) 2003;228:387–395. doi: 10.1177/153537020322800409. [DOI] [PubMed] [Google Scholar]
- 37.Ji X, Kong J, Carstens RP, Liebhaber SA. The 3′ untranslated region complex involved in stabilization of human alpha-globin mRNA assembles in the nucleus and serves an independent role as a splice enhancer. Mol Cell Biol. 2007;27:3290–3302. doi: 10.1128/MCB.02289-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He Z, Russell JE. Dynamic posttranscriptional regulation of epsilon-globin gene expression in vivo. Blood. 2007;109:795–801. doi: 10.1182/blood-2006-06-027946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang Y, Xu XS, Russell JE. A nucleolin-binding 3′ untranslated region element stabilizes beta-globin mRNA in vivo. Mol Cell Biol. 2006;26:2419–2429. doi: 10.1128/MCB.26.6.2419-2429.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinberg RS, Ji X, Sutton M, et al. Butyrate increases the efficiency of translation of gamma-globin mRNA. Blood. 2005;105:1807–1809. doi: 10.1182/blood-2004-02-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morceau F, Dupont C, Palissot V, et al. GTP-mediated differentiation of the human K562 cell line: transient overexpression of GATA-1 and stabilization of the gamma-globin mRNA. Leukemia. 2000;14:1589–1597. doi: 10.1038/sj.leu.2401890. [DOI] [PubMed] [Google Scholar]
- 42.Dover GJ, Boyer SH. Fetal hemoglobin-containing cells have the same mean corpuscular hemoglobin as cells without fetal hemoglobin: a reciprocal relationship between gamma- and beta-globin gene expression in normal subjects and in those with high fetal hemoglobin production. Blood. 1987;69:1109–1113. [PubMed] [Google Scholar]