Abstract
As risk for secondary breast cancer is elevated among cancer survivors treated with conventional therapy, we sought to determine the risk among 3337 female 5-year survivors who underwent an allogeneic hematopoietic cell transplantation (HCT) at the Fred Hutchinson Cancer Research Center or at one of 82 centers reporting to the European Bone Marrow Transplant Registry. Risk was calculated using standardized incidence ratios (SIRs), and risk factors were evaluated with a multivariable Cox proportional hazards model. Fifty-two survivors developed breast cancer at a median of 12.5 (range: 5.7-24.8) years following HCT (SIR = 2.2). Twenty-five–year cumulative incidence was 11.0%, higher among survivors who received total body irradiation (TBI) (17%) than those who did not receive TBI (3%). In multivariable analysis, increased risk was associated with longer time since transplantation (hazard ratio [HR] for 20+ years after transplantation = 10.8), use of TBI (HR = 4.0), and younger age at transplantation (HR = 9.5 for HCT < 18 years). Hazard for death associated with breast cancer was 2.5 (95% CI: 1.1-5.8). We conclude that female survivors of allogeneic HCT are at increased risk of breast cancer and should be educated about the need for regular screening.
Introduction
Hematopoietic cell transplantation (HCT) is now successfully used in the treatment of malignant and nonmalignant diseases, resulting in a growing cohort of long-term survivors. Accompanying this survivorship has been the development of adverse long-term outcomes, including subsequent malignant neoplasms (SMNs).1 New cancers of solid organs have been reported among HCT survivors, with an excess risk for cancers of the liver, thyroid, brain, bone, connective tissue, cervix, oral cavity, and skin.2–11
Survivors of cancer treated with conventional chemoradiotherapy are at an increased risk for the development of subsequent breast cancer.12–21 We therefore hypothesized that survivors of HCT would also have an excess risk for subsequent breast cancer, with the risk modified by the use of total body irradiation (TBI) and the occurrence of chronic graft-versus-host disease (GVHD), as has been reported for other solid organ cancers following HCT.4–6,8,9
Methods
Patients
Data were available for 3337 female patients who underwent transplantation at the Fred Hutchinson Cancer Research Center (FHCRC, n = 968) or affiliated hospitals in Seattle or at one of 82 centers reporting to the European Group of Bone and Marrow Transplantation (EBMT, n = 2369) between November 1969 and December 2000 and who survived at least 5 years after HCT. Additional participants in the EBMT Study Group may be found in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article.)
Patient follow-up and data collection
Clinical surveillance of patients who underwent HCT was approved by the FHCRC Institutional Review Board and by the review boards at each of the participating institutions reporting to the EBMT. Informed consent was provided according to the Declaration of Helsinki. Patient characteristics, HCT treatment regimens, and clinical outcome data were collected prospectively and stored in the FHCRC and the EBMT databases. At the FHCRC, a long-term follow-up department has been in place for more than 30 years to capture late events and better characterize and determine the incidence of delayed complications, such as SMNs. Patients and their physicians are contacted annually for information on the patient's overall condition and targeted long-term complications, including the development of SMNs. In addition, there are alert forms for specific targeted outcomes, such as SMNs or mortality. In the EBMT, patients are also followed long term at their centers to capture long-term outcomes. Patients are also seen annually at their transplant centers for evaluation. If they are unable to return to the transplant center, the information is collected from their primary health care provider. Participating centers then report to the EBMT central office annually on follow-up, which includes reporting of SMNs. Eighty-seven percent of the combined FHCRC-EBMT cohort have had contact with their transplant center within the past 6 years.
Breast cancers were initially ascertained by report from either survivors or their physicians. Subsequent verification and validation was undertaken for all reports, with requests for pathology reports or more detailed clinical notes. Detailed physician records were available for all cases of breast cancer and pathology was available for 30 cases (58%).
Statistical analyses
Cumulative incidence estimates of secondary breast cancer were calculated,22 using time from 5 years after HCT to first occurrence of breast cancer, treating death or second transplantation as a competing risk event and censoring at the date of last contact. Standard errors of cumulative incidence estimates were calculated and used to evaluate 95% confidence intervals (CIs).23 Cumulative incidence curves were compared using Gray test.24 Age- and calendar year–adjusted standardized incidence ratios (SIRs) of observed to expected malignancies were evaluated using United States Surveillance Epidemiology End Results (SEER) for expected rates.25,26 Because EBMT subjects were from 22 different countries, it was difficult to ascertain exact expected breast cancer rates for this population. Since the US rates would be expected to be slightly higher than European rates, and there is some evidence that the differences would not be large between developed countries such as these,27 SEER rates were selected to calculate SIR for all subjects. The possible bias induced by this assumption would be to overestimate expected breast cancer numbers and therefore underestimate the SIR; thus these estimates should be viewed as conservative. Cox proportional hazards models were used to evaluate potential risk factors using age as the time scale to more directly control for the increased risk of breast cancer with increasing age.28 Under this framework, subjects enter the risk set for analysis at the age at which they enter the cohort (5 years after HCT). They contribute to the Cox model until the age at which they develop breast cancer, or at which follow up is censored. This method ensures that HRs are calculated based on comparisons between subjects at the same age. Candidate risk factors evaluated included age at HCT, primary disease, status of disease at HCT, donor/patient HLA compatibility, use of total body irradiation (TBI), acute and chronic graft-versus-host disease (GVHD), cohort (EBMT vs FHCRC), and years since HCT. All models were adjusted for length of follow up since HCT. Final multivariable models included those factors that markedly influenced hazard ratios (HRs) for other factors (confounders) or that were statistically significant themselves in step-down modeling procedures. All statistical analyses were carried out using Stata 9.2 software (StataCorp, College Station, TX).
Results
Of the 3337 female allogeneic transplant patients who survived at least 5 years after transplantation, 52 developed breast cancer at a median of 12.5 (range: 5.7-24.8) years after HCT, at a median age of 47.5 (range: 25.5-65.8) years. Of those, 43 (83%) remain alive at a median of 17.4 (range: 7.4-33.1) years after HCT. Of the 3285 patients who have not developed breast cancer, 3036 (92%) remain alive at a median of 9.9 (range: 7.0-32.2) years after transplantation, while 249 died at a median of 8.7 (range: 6.5-26.9) years after transplantation. Eighty-five percent of breast cancer cases were older than 17 years at HCT, while 69% of those without breast cancer were in the same age group. Twenty-nine of the breast cancers (55.8%) occurred in women treated at EBMT centers, which also contributed 71% of those without breast cancer. Twenty-three (44.2%) of the breast cancer cases were treated at the FHCRC, which contributed 29% of the non–breast cancer cases.
Source and dose of TBI as well as pretransplantation radiotherapy were not collected systematically on the entire cohort. However, 90% of breast cancers occurred among patients receiving TBI, while 64% of those without breast cancer received TBI. During this time period, the source of TBI changed from cobalt to linear accelerator in the cohort in 1998 to 2000, with changes from single-dose to fractionated-dose TBI taking place between 1977 and 1990, all dependent on protocol. Fractionated TBI doses ranged from 8 to 15.75 Gy. Single-dose TBI doses were 9.2 to 10 Gy. Among the 22 patients who developed breast cancer after HCT for acute leukemia, 5 had acute lymphoblastic leukemia (ALL) and only 2 were likely to have received craniospinal radiotherapy in addition to TBI. Splenic pedicle radiotherapy was rarely used in either cohort prior to transplantation for patients with chronic myelogenous leukemia (CML). Five patients developed breast cancer without receiving TBI. Three of these patients were treated for aplastic anemia, one for myelodysplastic syndrome, and the other for CML. None received pretransplantation radiotherapy.
Only 9.6% and 27.3% of the women who developed breast cancer had grades III to IV acute GVHD or chronic GVHD, respectively. In comparison, among those who did not develop breast cancer, 9.4% had grades III to IV acute GVHD and 28.9% had chronic GVHD (Table 1).
Table 1.
Characteristics of female 5-year survivors of allogeneic transplantation
| Characteristic | All patients | Patients with breast cancer | Patients without breast cancer |
|---|---|---|---|
| Patients, no. | 3337 | 52 | 3285 |
| Median age at transplantation, y (range) | 28.1 (0.2-70.3) | 32.9 (3.6-59.2) | 28.0 (0.2-70.3) |
| Median age at breast cancer, y (range) | N/A | 47.5 (25.5-65.8) | NA |
| Location of transplant center, no. (%) | |||
| EBMT | 2369 (71.0) | 29 (55.8) | 2340 (71.2) |
| FHCRC | 968 (29.1) | 23 (44.2) | 945 (28.8) |
| Age at HCT, no. (%) | |||
| Younger than 10 y | 549 (16.5) | 1 (1.9) | 548 (16.7) |
| 10 to 17 y | 477 (14.3) | 7 (13.5) | 470 (14.3) |
| 18 to 39 y | 1577 (47.2) | 25 (48.1) | 1552 (47.3) |
| 40 y or older | 734 (22.0) | 19 (36.5) | 715 (21.7) |
| Primary disease, no. (%) | |||
| Acute leukemia | 1364 (40.9) | 22 (42.3) | 1342 (40.9) |
| Chronic leukemia | 1060 (34.8) | 22 (42.3) | 1038 (31.6) |
| Myelodysplastic syndrome | 200 (6.0) | 1 (1.9) | 199 (6.1) |
| Multiple myeloma & plasma cell disorders | 52 (1.6) | 0 (0) | 52 (1.6) |
| Lymphoma | 94 (2.8) | 4 (7.7) | 90 (2.7) |
| Solid tumors | 7 (0.2) | 0 (0) | 7 (1.2) |
| Total body radiation (TBI), no. (%) | |||
| No | 1070 (32.1) | 5 (9.6) | 1065 (32.4) |
| Yes | 2162 (64.8) | 47 (90.4) | 2115 (64.4) |
| Unknown | 105 (3.1) | 0 | 105 (3.2) |
| Donor type, no. (%) | |||
| HLA-identical related | 2645 (79.3) | 43 (82.7) | 2602 (79.2) |
| HLA-mismatched related | 169 (5.0) | 3 (5.8) | 166 (5.0) |
| Unrelated | 494 (14.8) | 6 (11.5) | 488 (14.9) |
| Unknown | 29 (0.9) | 0 | 29 (0.9) |
| Acute graft-versus-host disease, no. (%) | |||
| None | 1142 (34.2) | 20 (38.5) | 1122 (34.2) |
| Grades 1 to 2 | 1723 (51.6) | 27 (51.9) | 1696 (51.6) |
| Grades 3 to 4 | 315 (9.4) | 5 (9.6) | 310 (9.4) |
| Unknown | 157 (4.8) | 0 | 157 (4.8) |
| Chronic graft-versus-host disease, no. (%) | |||
| No | 2366 (70.9) | 33 (63.5) | 2333 (71.0) |
| Yes | 913 (27.4) | 15 (28.9) | 898 (27.3) |
| Unknown | 58 (1.7) | 4 (7.6) | 54 (1.7) |
Histology was available on 30 of the 52 breast cancers. Twenty-six were infiltrating ductal, 2 were infiltrating lobular, and one each was fibrocarcinoma and cystosarcoma phyllodes. Stage was available on 31 patients and 16 were stage I, 7 were stage II, 5 were stage III, and 3 were stage IV. Three patients had bilateral disease.
Compared with the general population, female survivors of HCT had a modestly increased risk for breast cancer (SIR = 2.2; 95% CI: 1.7-2.9). The SIR was highest for patients aged 25 to 29 years (SIR = 27.1; 95% CI: 11.3-65.2), but remained elevated for subsequent age groups: 30 to 39 years (SIR = 4.1; 95% CI: 2.2-7.7), 40 to 59 years (SIR = 1.6; 95% CI: 1.1-2.3), and 60 years or older (SIR = 2.6; 95% CI: 1.3-5.5). The SIR also increased with time after HCT: At 5 to 9 years the SIR was 1.4 (95% CI: 0.9-2.3). It rose to 2.0 (95% CI: 1.2-3.3), 3.8 (95% CI: 2.1-6.8), and 10.3 (95% CI: 5.3-19.7) at 10 to 14, 15 to 19, and 20+ years after HCT, respectively. The SIR did not differ between the FHCRC (SIR = 2.1; 95% CI: 1.4-3.1) and EBMT (SIR = 2.3; 95% CI: 1.6-3.3) cohorts.
The SIR was highest for those younger than 18 years at time of HCT (SIR = 25.0; 95% CI: 12.5-50.1), but remained elevated for those ages 18 to 39 years (SIR = 2.1; 95% CI: 1.4-3.0) and those who received a transplant at 40 years or older (SIR = 1.7; 95% CI: 1.1-2.6).
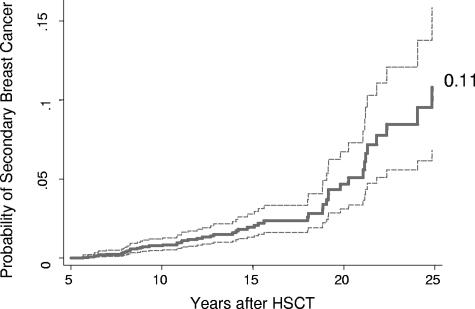
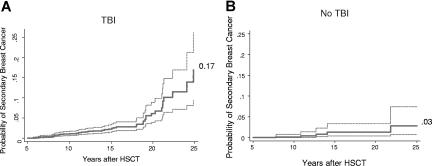
Cumulative incidence estimates for breast cancer markedly increased from 10 years (0.8%; 95% CI: 0.5%-1.2%) to 20 years (4.6%; 95% CI: 3.1%-6.7%) after HCT (Table 2). At 25 years the cumulative incidence was 11.0% (95% CI: 7%-16%; Figure 1), higher among survivors who had been treated with TBI (17%; 95% CI: 9%-26%) than those who did not receive TBI (3%; 95% CI: 1%-7%, P < .001; Figure 2).
Table 2.
Cumulative incidence estimates for breast cancer at 10, 20, and 25 years among allogeneic female HCT patients surviving at least 5 years after transplantation (N = 3337)
| Cumulative incidence, % (95% CI) |
|||
|---|---|---|---|
| 10 y | 20 y | 25 y | |
| Location | |||
| FHCRC | 1 (0.5-2.0) | 3.4 (1.9-5.5) | 13 (6.9-22.7) |
| EBMT | 0.6 (0.3-1.1) | 7.6 (4.0-12.8) | 9.1 (4.9-14.8) |
| TBI | |||
| No | 0.1 (0.02-0.8) | 1.3 (0.4-3.3) | 2.8 (0.7-7.4) |
| Yes | 1.1 (0.6-1.7) | 6.1 (3.9-8.9) | 16.9 (9.4-26.2) |
| Age at transplantation | |||
| Younger than 18 y | 0 (−) | 2.8 (1.0-6.1) | 6.5 (2.3-13.8) |
| 18 to 39 y | 0.6 (0.3-1.2) | 5.0 (2.7-8.3) | 13.2 (6.9-21.6) |
| 40 y or older | 2.6 (1.3-4.5) | 7.3 (3.8-12.4) | 19.7 (3.9-44.3) |
| Total | 0.8 (0.5-1.2) | 4.6 (3.1-6.7) | 10.8 (6.8-15.8) |
CI indicates confidence interval; and −, no CI.
Figure 1.
Overall cumulative incidence of breast cancer among 5-year female allogeneic HCT survivors.
Figure 2.
Cumulative incidence of breast cancer divided into subgroups defined by TBI conditioning. (A) TBI. (B) No TBI. P < .001 based on Gray test.
We also determined the impact of breast cancer on mortality. Adjusted for age at transplantation, TBI, disease status at transplantation, chronic GVHD, and cohort (EBMT vs FHCRC), the hazard for death for those who developed breast cancer was 2.5 (95% CI: 1.1-5.8; P = .03) compared with those without breast cancer.
Initially, we examined the potential impact of each host, disease, and treatment-related risk factor on the development of breast cancer in separate Cox proportional hazards regression analyses adjusted only for time since transplantation and treating current age as the time scale. Compared with subjects 40 years or older at HCT, younger age at HCT (< 18 years) significantly increased the risk of breast cancer (HR = 6.8; 95% CI: 1.3-35.8). Use of a TBI-containing conditioning regimen was a significant risk factor (HR = 3.8; 95% CI: 1.5-9.6). There was no significant interaction between age at HCT and TBI (P = .67). Transplantation for lymphoma conveyed a significant risk (HR = 9.2; 95% CI: 1.9-43.7) compared with bone marrow failure or other nonmalignant disorders. Donor type, development of acute or chronic GVHD, and cohort did not impact the risk of breast cancer.
In multivariable Cox regression, time since transplantation, TBI-containing conditioning, and age at HCT were significant independent risk factors (Table 3). Because of the strong correlation between TBI and primary cancer diagnosis, we did not include the latter in this model. In an effort to separate the effects of TBI and lymphoma, the same multivariate model was fit among the subpopulation of nonlymphoma subjects, and the risks due to TBI remained significantly elevated (HR = 3.8; 95% CI: 1.5-9.8). It was not possible to fit such a model among lymphoma patients since all breast cancer cases in this group had received TBI. In a separate, secondary, multivariable model that included the effect of lymphoma as a primary cancer diagnosis, the risk conferred by lymphoma compared with other diagnoses was elevated with only marginal significance (HR = 2.7; 95% CI: 0.95-7.6; P = .06) while TBI remained significant (P < .001).
Table 3.
Multivariable Cox proportional hazards model for breast cancer among 5-year female allogeneic HCT survivors (age as time scale)
| Factor | Adjusted hazard ratio | P | 95% confidence interval |
|---|---|---|---|
| Follow-up time | |||
| 5 to 9.9 y* | 1.0 | — | — |
| 10 to 14.99 y | 2.7 | .022 | (1.2-6.4) |
| 15 to 19.99 y | 5.1 | .001 | (1.9-13.6) |
| 20 y or older | 10.8 | <.001 | (3.2-36.1) |
| TBI | |||
| No* | 1.0 | — | — |
| Yes | 4.0 | .004 | (1.6-10.3) |
| Age at transplantation | |||
| Younger than 18 y | 9.5 | .009 | (1.8-51.1) |
| 18 to 39 y | 1.6 | .30 | (0.7-3.8) |
| 40 y or older* | 1.0 | — | — |
| Location | |||
| EBMT* | 1.0 | — | — |
| FHCRC | 0.65 | .15 | (0.35-1.2) |
— indicates not applicable.
Reference category.
Discussion
With considerable improvement in the cure rate of patients undergoing HCT over the last 4 decades, there has been increased interest in long-term outcomes and complications, including subsequent malignancies. The combined FHCRC-EBMT patient population presents an ideal cohort to study risk factors for such adverse long-term outcomes, as HCT has been carried out successfully by these groups since 1969, and excellent follow up has been secured, with mechanisms in place for survivors or health care providers to report late events. This research should contribute to improved understanding of the pathogenesis of secondary carcinogenesis and generate information on prevention and screening strategies for those at increased risk.
In this analysis, we identified an elevated risk of breast cancer among female patients treated with allogeneic HCT at the FHCRC or at one of 82 centers reporting to the EBMT. Younger age at time of HCT and the use of TBI in the conditioning regimen were the predominant risk factors. Risk increased with ongoing time since HCT. Compared with those in the cohort who did not develop breast cancer, mortality risk was increased in patients with secondary breast cancer. Cumulative incidence increased with follow up, without current evidence of a plateau.
Use of TBI was a strong independent risk factor for secondary breast cancer. The risk conferred by radiotherapy in our cohort is consistent with reports of secondary breast cancer following conventional therapy for Hodgkin lymphoma, where one of the most significant risk factors is thoracic radiotherapy, with higher doses, particularly more than 40 Gy, conferring the highest risk.13–21,29 A limitation of the study is that we did not have pretransplantation radiotherapy exposure available for all patients. However, 48 of the 52 breast cancers occurred in patients treated for malignancies in which radiotherapy to the thorax would have been unlikely, and therefore it is unlikely that pretransplantation radiotherapy was a significant risk factor in the majority of cases. Two patients likely received craniospinal radiotherapy for ALL during childhood. However, large cohort studies of secondary cancers following childhood ALL do not show an increased risk of breast cancer as a second malignancy.30–32 TBI doses in our cohort ranged from 8 to 15 Gy, considerably lower than those used for the treatment of Hodgkin lymphoma, but homogenous across breast tissue. These doses may, therefore, well be comparable with the doses received by the breast as a result of mantle radiotherapy.33–35 In addition, they were delivered as a single fraction or over 2 to 5 days, as opposed to several weeks, potentially increasing the dosimetry to breast tissue, particularly for those with single fraction dose. These data are consistent with the finding that TBI increases the risk for other solid organ malignancies after HCT.3–11 During this period, TBI doses decreased overall, with increased use of fractionation. However, with long latency between exposure and onset of breast cancer, it will be only through continued follow up of this cohort that the impact of these changes on breast cancer incidence may be recognized.
In our analysis, the diagnosis of lymphoma carried an increased risk of secondary breast cancer, 9-fold in analyses adjusted only for length of follow up; however, when adjusted for the other risk factors such as age and use of TBI, the effect was only of borderline significance. This contrasts with some studies in the nontransplantation setting, where survivors of Hodgkin lymphoma appear to be at uniquely increased risk, compared with other diagnostic groups, with Hodgkin lymphoma an independent risk factor in some studies.12–21 Our findings may be a reflection of the fact that more patients with lymphoma received TBI (88%) than patients with other diagnoses (68%). It is difficult, therefore, to completely separate the risk conferred by primary disease from that of TBI. In addition, there were only 94 lymphoma survivors in the cohort, limiting our ability to discern an independent effect. It should be noted, however, that among these 94 lymphoma survivors, 4 developed breast cancer (4.2%) compared with 48 breast cancers (1.5%) among the remaining 3243 patients.
The risk of breast cancer remained elevated throughout age groups, but decreased with increasing age at time of HCT; patients younger than 18 years had the highest risk. This is again consistent with what has been reported among Hodgkin lymphoma patients treated with conventional therapy12–14,16,17,19 as well as for other solid organ and skin cancers following HCT4–8,10,11 and pediatric cancer survivors.9,17,36 However, although those treated in the pediatric age group were at highest risk, it should be noted that elevated risk remained through patients treated at 60 years and older compared with population incidence.
With longer follow up after HCT, both the cumulative incidence of breast cancer and the SIR increased in our cohort. In evaluating risk factors for second cancer, the question of whether to use time since transplantation or age as the scale in the analysis is an important one to consider. Our analysis addressed the issue of changing risk of breast cancer with attained age by using age as the time scale for Cox regression models, while adjusting for years of follow-up.28
We found no association between secondary breast cancer and GVHD, although both acute and chronic GVHD have been reported to be risk factors for several types of solid tumors, often in sites where GVHD is most prominent—liver, skin, and mucosal surfaces.2,4–6,9 The lack of association with GVHD may be because breast tissue is unaffected by GVHD, or it is possible that the risk conferred by TBI obscured such an effect in many patients who received TBI and developed GVHD.
Despite the associations between second breast cancers and TBI, younger age at transplantation, and time since HCT, there were likely other factors that contributed to the breast cancer risk, for example hormonal influences. It is known that females who have undergone HCT may develop either primary or secondary ovarian failure,37–41 which may require hormonal replacement therapy and may affect childbearing, which in turn may modify the risk of breast cancer. A limitation of our analysis is indeed that we did not have available complete data on hormonal exposures in our population, or other population-based risk factors for breast cancer, such as age at menarche, pregnancy history, family history of cancer, or pertinent environmental exposures. As the histology and stage distribution in the cohort was similar to de novo breast cancer, these would be valuable data to collect. With the excellent follow up in our combined cohort, it is possible to obtain this information, an initiative for future research.
In summary, female survivors of HCT are at increased risk for developing breast cancer, with younger age at diagnosis, increased time since transplantation, and use of TBI being the major risk factors. Given the lack of plateau evident in the cumulative incidence, it is likely that the risk of breast cancer will continue to increase over the ensuing decades after HCT. Screening for breast cancer is now recommended for women treated for Hodgkin lymphoma with radiotherapy, and similar lifelong screening should be considered for women who underwent allogeneic HCT, particularly in those younger at time of HCT with TBI as part of the conditioning regimen. Such screening may include self- and provider examination, mammography, or breast magnetic resonance imaging (MRI), as has been recommended for survivors of childhood cancer and Hodgkin lymphoma.3,42–44
The successes in treating many cancers by HCT are not negated by the increased risk of breast cancer. However, survivors and their physicians should be aware of the increased risk, in order that screening for early detection is implemented. Further research is required to better define the subset of female transplantation survivors at highest risk, so that modifiable risk factors such as diet, weight, exercise, alcohol and tobacco exposure, and hormonal therapy45–49 can be appropriately addressed.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Cancer Institute (CA18029, CA15704, CA103728, and CA102542) and the National Heart, Lung and Blood Institute (HL36444).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: D.L.F. and A.R. designed and performed the research, analyzed data, and wrote the paper; W.L. analyzed data and contributed to writing the paper; A.L., M.E.D.F., A.T., J.E.S., H.J.D., and G.S. contributed to data collection, analysis, and writing of the paper.
A list of participants in the EBMT Study Group can be found in Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Debra L. Friedman, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D5-280, PO Box 19024, Seattle, WA 98109; e-mail: dfriedma@fhcrc.org.
References
- 1.Kolb HJ, Socie G, Duell T, et al. Malignant neoplasms in long-term survivors of bone marrow transplantation: Late Effects Working Party of the European Cooperative Group for Blood and Marrow Transplantation and the European Late Effect Project Group. Ann Intern Med. 1999;131:738–744. doi: 10.7326/0003-4819-131-10-199911160-00004. [DOI] [PubMed] [Google Scholar]
- 2.Curtis RE, Metayer C, Rizzo JD, et al. Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: an international case-control study. Blood. 2005;105:3802–3811. doi: 10.1182/blood-2004-09-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ades L, Guardiola P, Socie G. Second malignancies after allogeneic hematopoietic stem cell transplantation: new insight and current problems. Blood Rev. 2002;16:135–146. doi: 10.1054/blre.2002.0010. [DOI] [PubMed] [Google Scholar]
- 4.Baker KS, DeFor TE, Burns LJ, Ramsay NK, Neglia JP, Robison LL. New malignancies after blood or marrow stem-cell transplantation in children and adults: incidence and risk factors. J Clin Oncol. 2003;21:1352–1358. doi: 10.1200/JCO.2003.05.108. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia S, Louie AD, Bhatia R, et al. Solid cancers after bone marrow transplantation. J Clin Oncol. 2001;19:464–471. doi: 10.1200/JCO.2001.19.2.464. [DOI] [PubMed] [Google Scholar]
- 6.Curtis RE, Rowlings PA, Deeg HJ, et al. Solid cancers after bone marrow transplantation. N Engl J Med. 1997;336:897–904. doi: 10.1056/NEJM199703273361301. [DOI] [PubMed] [Google Scholar]
- 7.Deeg HJ, Socie G. Malignancies after hematopoietic stem cell transplantation: many questions, some answers. Blood. 1998;91:1833–1844. [PubMed] [Google Scholar]
- 8.Friedman DL, Leisenring W, Schwartz JL, Deeg HJ. Second malignant neoplasms following hematopoietic stem cell transplantation. Int J Hematol. 2004;79:229–234. doi: 10.1532/ijh97.03178. [DOI] [PubMed] [Google Scholar]
- 9.Leisenring W, Friedman DL, Flowers ME, Schwartz JL, Deeg HJ. Nonmelanoma skin and mucosal cancers after hematopoietic cell transplantation. J Clin Oncol. 2006;24:1119–1126. doi: 10.1200/JCO.2005.02.7052. [DOI] [PubMed] [Google Scholar]
- 10.Socie G. Secondary malignancies. Curr Opin Hematol. 1996;3:466–470. doi: 10.1097/00062752-199603060-00011. [DOI] [PubMed] [Google Scholar]
- 11.Socie G, Curtis RE, Deeg HJ, et al. New malignant diseases after allogeneic marrow transplantation for childhood acute leukemia. J Clin Oncol. 2000;18:348–357. doi: 10.1200/JCO.2000.18.2.348. [DOI] [PubMed] [Google Scholar]
- 12.Metayer C, Lynch CF, Clarke EA, et al. Second cancers among long-term survivors of Hodgkin's disease diagnosed in childhood and adolescence. J Clin Oncol. 2000;18:2435–2443. doi: 10.1200/JCO.2000.18.12.2435. [DOI] [PubMed] [Google Scholar]
- 13.Hill DA, Gilbert E, Dores GM, et al. Breast cancer risk following radiotherapy for Hodgkin lymphoma: modification by other risk factors. Blood. 2005;106:3358–3365. doi: 10.1182/blood-2005-04-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhatia S, Yasui Y, Robison LL, et al. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin's disease: report from the Late Effects Study Group. J Clin Oncol. 2003;21:4386–4394. doi: 10.1200/JCO.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 15.Guibout C, Adjadj E, Rubino C, et al. Malignant breast tumors after radiotherapy for a first cancer during childhood. J Clin Oncol. 2005;23:197–204. doi: 10.1200/JCO.2005.06.225. [DOI] [PubMed] [Google Scholar]
- 16.Kenney LB, Yasui Y, Inskip PD, et al. Breast cancer after childhood cancer: a report from the Childhood Cancer Survivor Study. Ann Intern Med. 2004;141:590–597. doi: 10.7326/0003-4819-141-8-200410190-00006. [DOI] [PubMed] [Google Scholar]
- 17.Neglia JP, Friedman DL, Yasui Y, et al. Second malignant neoplasms in five-year survivors of childhood cancer: childhood cancer survivor study. J Natl Cancer Inst. 2001;93:618–629. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 18.Swerdlow AJ, Barber JA, Hudson GV, et al. Risk of second malignancy after Hodgkin's disease in a collaborative British cohort: the relation to age at treatment. J Clin Oncol. 2000;18:498–509. doi: 10.1200/JCO.2000.18.3.498. [DOI] [PubMed] [Google Scholar]
- 19.Travis LB, Hill DA, Dores GM, et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA. 2003;290:465–475. doi: 10.1001/jama.290.4.465. [DOI] [PubMed] [Google Scholar]
- 20.van Leeuwen FE, Klokman WJ, Stovall M, et al. Roles of radiation dose, chemotherapy, and hormonal factors in breast cancer following Hodgkin's disease. J Natl Cancer Inst. 2003;95:971–980. doi: 10.1093/jnci/95.13.971. [DOI] [PubMed] [Google Scholar]
- 21.Wolden SL, Lamborn KR, Cleary SF, Tate DJ, Donaldson SS. Second cancers following pediatric Hodgkin's disease. J Clin Oncol. 1998;16:536–544. doi: 10.1200/JCO.1998.16.2.536. [DOI] [PubMed] [Google Scholar]
- 22.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 23.Marubini E, Valsecchi MG. Analysing Survival Data from Clinical Trials and Observational Studies. Chichester, England: Wiley; 1995. [Google Scholar]
- 24.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 25.Breslow N, Day N. Statistical Methods in Cancer Research: Volume II: The Design and Analysis of Cohort Studies. Lyon, France: IARC Scientific Publications; 1987. [PubMed] [Google Scholar]
- 26.National Cancer Institute, Cancer Surveillance Research Program. Surveillance, Epidemiology, and End Results (SEER) Program Public-Use CD-ROM. Released April 2000, based on August 1999 submission.
- 27.Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000: the global picture. Eur J Cancer. 2001;37(suppl 8):S4–S66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 28.Yasui Y, Liu Y, Neglia JP, et al. A methodological issue in the analysis of second-primary cancer incidence in long-term survivors of childhood cancers. Am J Epidemiol. 2003;158:1108–1113. doi: 10.1093/aje/kwg278. [DOI] [PubMed] [Google Scholar]
- 29.Raj KA, Marks LB, Prosnitz RG. Late effects of breast radiotherapy in young women. Breast Dis. 2005;23:53–65. doi: 10.3233/bd-2006-23108. [DOI] [PubMed] [Google Scholar]
- 30.Bhatia S, Sather HN, Pabustan OB, Trigg ME, Gaynon PS, Robison LL. Low incidence of second neoplasms among children diagnosed with acute lymphoblastic leukemia after 1983. Blood. 2002;99:4257–4264. doi: 10.1182/blood.v99.12.4257. [DOI] [PubMed] [Google Scholar]
- 31.Hijiya N, Hudson MM, Lensing S, et al. Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia. JAMA. 2007;297:1207–1215. doi: 10.1001/jama.297.11.1207. [DOI] [PubMed] [Google Scholar]
- 32.Loning L, Zimmermann M, Reiter A, et al. Secondary neoplasms subsequent to Berlin-Frankfurt-Munster therapy of acute lymphoblastic leukemia in childhood: significantly lower risk without cranial radiotherapy. Blood. 2000;95:2770–2775. [PubMed] [Google Scholar]
- 33.Kowalski A, Smith S. Measurement of radiation dose delivered to breast tissue during mantle field irradiation for Hodgkin's disease. Med Dosim. 1998;23:31–36. doi: 10.1016/s0958-3947(97)00108-8. [DOI] [PubMed] [Google Scholar]
- 34.Rajaratnam S, Nisce LZ, Avanzato J. Dose to breast in breast undergoing mantle treatments. Med Dosim. 1994;19:145–146. doi: 10.1016/0958-3947(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 35.Zellmer DL, Wilson JF, Janjan NA. Dosimetry of the breast for determining carcinogenic risk in mantle irradiation. Int J Radiat Oncol Biol Phys. 1991;21:1343–1351. doi: 10.1016/0360-3016(91)90296-g. [DOI] [PubMed] [Google Scholar]
- 36.Tucker MA, Jones PH, Boice JD, Jr, et al. Therapeutic radiation at a young age is linked to secondary thyroid cancer: The Late Effects Study Group. Cancer Res. 1991;51:2885–2888. [PubMed] [Google Scholar]
- 37.Tauchmanova L, Selleri C, De Rosa G, et al. Gonadal status in reproductive age women after haematopoietic stem cell transplantation for haematological malignancies. Hum Reprod. 2003;18:1410–1416. doi: 10.1093/humrep/deg295. [DOI] [PubMed] [Google Scholar]
- 38.Sklar C. Maintenance of ovarian function and risk of premature menopause related to cancer treatment. J Natl Cancer Inst Monogr. 2005:25–27. doi: 10.1093/jncimonographs/lgi018. [DOI] [PubMed] [Google Scholar]
- 39.Cumber PM, Whittaker JA. Ovarian failure after total body irradiation. BMJ. 1990;300:464. doi: 10.1136/bmj.300.6722.464-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keilholz U, Korbling M, Fehrentz D, Bauer H, Hunstein W. Long-term endocrine toxicity of myeloablative treatment followed by autologous bone marrow/blood derived stem cell transplantation in patients with malignant lymphohematopoietic disorders. Cancer. 1989;64:641–645. doi: 10.1002/1097-0142(19890801)64:3<641::aid-cncr2820640313>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 41.Sanders JE, Buckner CD, Amos D, et al. Ovarian function following marrow transplantation for aplastic anemia or leukemia. J Clin Oncol. 1988;6:813–818. doi: 10.1200/JCO.1988.6.5.813. [DOI] [PubMed] [Google Scholar]
- 42.Kaste SC, Hudson MM, Jones DJ, et al. Breast masses in women treated for childhood cancer: incidence and screening guidelines. Cancer. 1998;82:784–792. doi: 10.1002/(sici)1097-0142(19980215)82:4<784::aid-cncr23>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 43.Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: the Children's Oncology Group Long-Term Follow-Up Guidelines from the Children's Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22:4979–4990. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 44.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 45.Li CI, Daling JR, Malone KE, et al. Relationship between established breast cancer risk factors and risk of seven different histologic types of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:946–954. doi: 10.1158/1055-9965.EPI-05-0881. [DOI] [PubMed] [Google Scholar]
- 46.Li CI, Malone KE, Daling JR. Interactions between body mass index and hormone therapy and postmenopausal breast cancer risk (United States). Cancer Causes Control. 2006;17:695–703. doi: 10.1007/s10552-005-0001-7. [DOI] [PubMed] [Google Scholar]
- 47.Hamajima N, Hirose K, Tajima K, et al. Alcohol, tobacco and breast cancer: collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer. 2002;87:1234–1245. doi: 10.1038/sj.bjc.6600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McDonald JA, Mandel MG, Marchbanks PA, et al. Alcohol exposure and breast cancer: results of the women's contraceptive and reproductive experiences study. Cancer Epidemiol Biomarkers Prev. 2004;13:2106–2116. [PubMed] [Google Scholar]
- 49.McTiernan A, Kooperberg C, White E, et al. Recreational physical activity and the risk of breast cancer in postmenopausal women: the Women's Health Initiative Cohort Study. JAMA. 2003;290:1331–1336. doi: 10.1001/jama.290.10.1331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.