Abstract
Multiple sclerosis (MS) is a T cell-dependent chronic inflammatory disease of the central nervous system. The role of chemokines in MS and its different stages is uncertain. Recent data suggest a bias in expression of chemokine receptors by Th1 vs. Th2 cells; human Th1 clones express CXCR3 and CCR5 and Th2 clones express CCR3 and CCR4. Chemokine receptors expressed by Th1 cells may be important in MS, as increased interferon-γ (IFN-γ) precedes clinical attacks, and IFN-γ injection induces disease exacerbations. We found CXCR3+ T cells increased in blood of relapsing-remitting MS, and both CCR5+ and CXCR3+ T cells increased in progressive MS compared with controls. Furthermore, peripheral blood CCR5+ T cells secreted high levels of IFN-γ. In the brain, the CCR5 ligand, MIP-1α, was strongly associated with microglia/macrophages, and the CXCR3 ligand, IP-10, was expressed by astrocytes in MS lesions but not unaffected white matter of control or MS subjects. Areas of plaque formation were infiltrated by CCR5-expressing and, to a lesser extent, CXCR3-expressing cells; Interleukin (IL)-18 and IFN-γ were expressed in demyelinating lesions. No leukocyte expression of CCR3, CCR4, or six other chemokines, or anti-inflammatory cytokines IL-5, IL-10, IL-13, and transforming growth factor-β was observed. Thus, chemokine receptor expression may be used for immunologic staging of MS and potentially for other chronic autoimmune/inflammatory processes such as rheumatoid arthritis, autoimmune diabetes, or chronic transplant rejection. Furthermore, these results provide a rationale for the use of agents that block CCR5 and/or CXCR3 as a therapeutic approach in the treatment of MS.
Chemokines, chemotactic cytokines that mediate the attraction of leukocytes to tissues, are essential for inflammatory responses. More than 40 chemokines and 10 chemokine receptors are known, as recently reviewed (1). Recent data suggest a bias in expression of selected chemokine receptors by Th1 cells compared with Th2 cells. In particular, human Th1 clones express CXCR3 (receptor for IP-10 and Mig) and CCR5 (receptor for MIP-1α, MIP-1β, and RANTES), whereas Th2 cells express CCR3 (receptor for eotaxin, RANTES, MCP-3, MCP-4), CCR4 (receptor for TARC and MDC) (2–4) and CCR8 (receptor for I-309) (5).
Multiple sclerosis (MS) is a T cell-dependent chronic inflammatory disease of the central nervous system with a likely autoimmune etiology (6). A central mechanism in the pathogenesis of MS is the organ-specific traffic of T cells into the brain. Though it is known that activated T cells can cross the blood–brain barrier, the mechanisms by which activated T cells are recruited and remain in the brain, and whether chemokines are involved in the pathogenesis of MS, are unknown. Chemokine receptors that are expressed by Th1 cells may be especially important, as increased production of interferon-γ (IFN-γ) precedes clinical attacks (7, 8) and injection of MS patients with recombinant IFN-γ induced exacerbations of the disease (9). In addition, MS involves different stages, usually beginning with a relapsing–remitting phase and later, a progressive form. Immune factors associated with different stages of the disease are not well understood. We studied chemokine receptor expression by mononuclear cells in MS. We found that the numbers of CXCR3+ T cells were increased in relapsing– remitting MS and that both CCR5+ and CXCR3+ T cells were increased in peripheral blood of progressive MS patients compared with healthy controls. In addition, their ligands MIP-1α and IP-10 were strongly associated with microglia/macrophages and astrocytes, respectively, in the MS lesions but not in the control white matter areas of postmortem central nervous system (CNS) samples. Additionally, CCR5+ T cells isolated from peripheral blood of progressive MS patients secreted IFN-γ at a high level. Hence, the selective chemotaxis of IFN-γ-producing CCR5+ T cells into the CNS may be an important mechanism in the pathogenesis of MS, and differential chemokine receptor expression may be associated with different stages of the disease.
MATERIALS AND METHODS
Subjects.
MS patients from the outpatient MS clinic of the Brigham and Women’s Hospital were studied. Relapsing–remitting patients (n = 14, average age = 40 ± 8 years) had an average expanded disability status (EDSS) of 1.1 ± 0.8, and chronic progressive MS patients (n = 20, average age = 47 ± 6 years) had an EDSS of 5.1 ± 1.9. A disability of 6 or greater involves use of a cane or other support. Patients had not received immunosuppressive therapy in the past, or steroid treatment in the 6 months prior to blood drawing. The control group consisted of healthy subjects (n = 20, average age = 48 ± 8 years). The number of patients used for each individual experiment is given in the corresponding table or figure legend.
Cell Separation.
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized venous blood by centrifugation over Ficoll–Hypaque (Pharmacia LKB Biotechnology). Cells were resuspended (106 cells per ml) in RPMI medium 1640 supplemented with 10% fetal bovine serum, 4 mM l-glutamine, 25 mM Hepes buffer, 50 units/ml penicillin, and 50 μg/ml streptomycin (all from BioWhittaker). T cells were separated from PBMC by negative depletion of non-T cells with a human T cell enrichment column (R & D Systems), according to the manufacturer’s instructions. T cells were separated into CCR5-depleted (CCR5−) and CCR5-enriched (CCR5+) T cells by using anti-CCR5 mAb (10 μg/ml), goat anti-mouse IgG-coated magnetic microbeads, and a type MS Separation column from Miltenyi Biotec (Auburn, CA), according to the manufacturer’s recommendations.
Flow Cytometry.
Unlabeled mouse mAbs directed against human CCR3 mAb (7B11, IgG2a), CCR5 (2D7, IgG1), CCR6 (11A9, IgG1), and CXCR3 mAb (1C6, IgG1) were obtained from LeukoSite (Cambridge, MA); anti-CXCR4 mAb (1G5, IgG2a) was purchased from PharMingen (San Diego); and anti-human CD28 mAb (EX5–3D10, IgG2a) was provided by Ed Greenfield (Dana–Farber Cancer Institute, Boston). Control unlabeled mouse IgG1, IgG2a, fluorescein isothiocyanate (FITC)-conjugated polyclonal anti-mouse Ig, phycoerythrin (PE)-conjugated anti-human CD3 mAb (IgG1), PE-anti-human CD4 mAb (IgG1), PE-anti-human CD8 mAb (IgG1), and isotype control PE-IgG1 were purchased from PharMingen. Two-color flow cytometry was performed by incubating 2 × 105 cells in RPMI medium 1640 plus 2% fetal bovine serum with unlabeled mAb (10 μg/ml), followed by FITC-goat anti-mouse Ig (5 μg/ml), and the appropriate directly conjugated mAb(s). Each incubation (20 min, 4°C) was followed with two washes. Unlabeled and labeled appropriate control mouse Ig of identical isotype were used in control samples. Flow cytometric analysis of 1 × 104 cells from each sample was performed on a FACSort flow cytometer (Becton Dickinson) according to standard procedures.
T Cell Activation and Cytokine Assay.
Isolated T cells (1 × 105 cells per well) were added to the wells of a 96-well flat-bottom plate (final volume of 0.2 ml) containing immobilized anti-CD3 (OKT3, IgG2a, 1 μg/ml) and anti-CD28 (EX5–3D10, IgG2a, 1 μg/ml) or immobilized control mouse IgG2a. Culture supernatants were collected after incubation for 20 hr (37°C, 5% CO2). Assays of IFN-γ, interleukin (IL)-4, and IL-5 in culture supernatants were performed by using a cytokine capture ELISA protocol from PharMingen. Components included unlabeled, and corresponding biotinylated, mouse mAbs to human IFN-γ, IL-4, and IL-5, plus recombinant human IFN-γ (GIBCO/BRL), IL-4 (Sigma), and IL-5 (PharMingen) standards. Assay sensitivities were 32 pg/ml (IFN-γ) and 8 pg/ml (IL-4, IL-5).
Human Brain Immunopathology.
Frozen brain specimens were obtained from the Rocky Mountain Multiple Sclerosis Center Tissue Bank, Englewood, CO. Samples were collected from cadavers within 6 hr post mortem and snap-frozen in liquid nitrogen. There were five specimens from patients (45 ± 8 years) with active MS in which the tissues were dissected to encompass an area of MS plaque and adjacent normal-appearing white matter, plus corresponding white matter; five patients (44 ± 9 years) with inactive MS who died from other causes; and five non-MS patients (68 ± 21 years) with normal-appearing brain tissues as controls. Cryostat sections were initially stained by hematoxylin and eosin, and luxol fast blue, and appropriate areas were chosen for evaluation by immunohistology using an avidin–biotin complex method (10). After overnight incubation with primary mAbs and developing reagents, sections were incubated with diaminobenzidine, counterstained with hematoxylin, and evaluated independently by two pathologists (J.E.B. and W.W.H.). Primary mAbs against human chemokines and chemokines receptors were prepared at LeukoSite, mAbs to cell markers were obtained from Dako, and anti-cytokine mAbs were from R & D. These included mAbs to the human chemokine receptors, CCR1 (2D4), CCR2 (1D9), CCR3 (7B11), CCR4 (1G1), CCR5 (5C7), CCR6 (11A9), and CXCR3 (1C6); the chemokines eotaxin (9G3), eotaxin-2 (10G12), IP-10 (6G10), Mig (4G10), ITAC (9E9), MIP-1α (11A3), MIP-3a (6A12), MCP-1 (10F7), MCP-2 (2D5), MCP-3 (9H11), and TARC (208); markers for all leukocytes (CD45, PD7/26), T cells (CD2, MT910), and mononuclear phagocytes (CD68, EBM11); and mAbs to human IFN-γ (MAB-285), IL-4 (MAB204), IL-5 (MAB205), IL-10 (MAB217), IL-13 (MAB213), IL-18 (MAB318), and transforming growth factor-β1 (MAB240), plus isotype-matched control mAbs.
Statistical Analysis.
Results are presented as mean ± SEM or mean ± SD for each group. Statistical significance was calculated by using Student’s t test.
RESULTS AND DISCUSSION
Production of IFN-γ, a cytokine that is a hallmark of Th1 type immune responses, is important to the pathogenesis of MS, as increased production of IFN-γ precedes clinical attacks (7, 8), and injection of MS patients with recombinant IFN-γ induces exacerbations of the disease (9). Activated blood mononuclear cells are known to produce significantly higher levels of IFN-γ in progressive MS (11, 12). Furthermore, active lesions in the CNS of MS patients are characterized by mononuclear cell infiltrates including both CD4+ and CD8+ T cells and macrophages (13) and are associated with increased IFN-γ expression (14). However, little is known about the immune basis for selective traffic of IFN-γ-secreting T cells into the CNS or the expression of chemokine of different chemokine receptors in different stages of the disease. Since expression of chemokine receptors by particular T cells subsets may play a role in this selective T cell migration and immune activation and differentiation, we studied chemokine receptor expression by peripheral blood cells from MS patients with relapsing–remitting or chronic progressive forms of the disease, as well as healthy individuals.
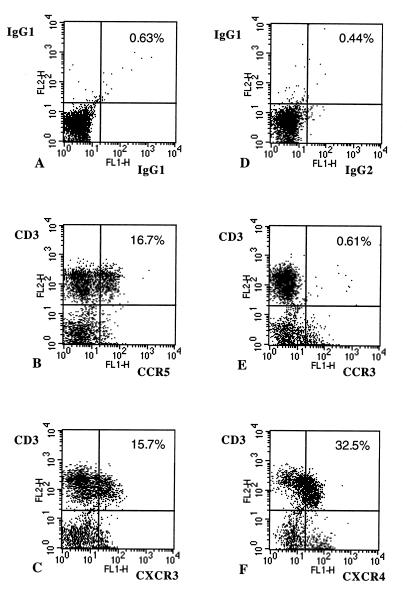
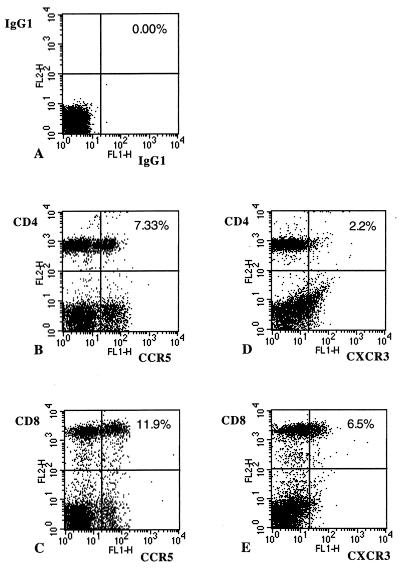
CCR5+ and CXCR3+ T cells were identified by flow cytometry. As shown in Fig. 1, CCR5, CXCR3, and CXCR4 were expressed primarily by CD3+ cells, whereas CCR3 expression was associated primarily with non-T cells. As shown in Fig. 2, CCR5 and CXCR3 were expressed by both CD4+ and CD8+ T cells. We then compared the levels of T cells expressing chemokine receptors in peripheral blood samples from a series of healthy controls (n = 20), and patients with relapsing–remitting (n = 14) or progressive MS (n = 20) (Table 1). We tested chemokine receptors associated with Th1 cells, CCR5 and CXCR3, as well as testing CCR3, a chemokine receptor associated with Th2 cells. CCR6 and CXCR4 served as control chemokine receptors because their expression is not selectively linked with either Th1 or Th2 subset (2–4). Both CD3+ CCR5+ and CD3+ CXCR3+ cells were elevated in progressive MS patients (P < 0.005), whereas the percentage of CXCR3+ T cells was increased in relapsing-remitting MS (P < 0.05) compared with controls. In contrast, the numbers of CXCR4+, CCR3+, and CCR6+ T cells were not different between MS patients and controls. We found no increase in CD3+ cells expressing CCR4 (associated with the Th2 cells) in chronic progressive MS patients (9.6% ± 2.7%), as compared with control subjects (9.0% ± 1.1%) or relapsing–remitting patients (7.1% ± 0.6%).
Figure 1.
Separated PBMC from a progressive MS patient were stained with mAb and isotype control, as described in the text. Two-color flow cytometry analysis of 1 × 104 cells from each sample was performed. The percentage of double-positive cells from the lymphocyte populations analyzed is shown. A shows staining with an isotype control (mouse IgG1-FITC and mouse IgG1-PE) that is the control for B (CD3+ CCR5+ cells) and C (CD3+ CXCR3+ cells). D represents staining with an isotype control (mouse IgG2a-FITC and mouse IgG1-PE) that is the control for E (CD3+ CCR3+ cells) and F (CD3+ CXCR4+ cells).
Figure 2.
PBMC from a progressive MS patient were stained with mAb and isotype-matched control Ig, as described in the text. Two-color flow cytometry analysis of 1 × 104 cells from each sample was performed. The percentage of double-positive cells from the lymphocyte populations analyzed is shown. A shows staining with an isotype control (mouse IgG1-FITC and mouse IgG1-PE) that is the control for B (CD4+ CCR5+ cells), C (CD8+ CCR5+ cells), D (CD4+ CXCR3+ cells), and E (CD8+ CXCR3+ cells).
Table 1.
Expression of chemokine receptors by peripheral blood T cells in MS patients
| Receptors | Double-positive cells, %
|
||
|---|---|---|---|
| Controls | RR MS | Progressive MS | |
| CD3+ CCR3+ | 1.4 ± 0.3 (20) | 2.0 ± 0.6 (14) | 2.1 ± 0.3 (20) |
| CD3+ CCR4+ | 9.0 ± 1.1 (6) | 7.1 ± 0.6 (14) | 9.6 ± 2.7 (7) |
| CD3+ CCR5+ | 7.9 ± 0.8 (20) | 9.9 ± 1.3 (14) | 13.1 ± 1.4** (20) |
| CD3+ CCR6+ | 8.6 ± 1.3 (9) | Not tested | 9.2 ± 0.7 (10) |
| CD3+ CXCR3+ | 9.1 ± 1.1 (20) | 14.4 ± 1.9* (14) | 14.8 ± 1.5** (20) |
| CD3+ CXCR4+ | 47.1 ± 8.1 (9) | Not tested | 30.9 ± 5.3 (10) |
PBMC from control healthy donors, relapsing–remitting (RR) MS, and progressive MS patients were separated and a three-step staining procedure, with mouse anti-chemokine receptor mAb followed by FITC-conjugated goat anti-mouse IgG and then PE-labeled anti-CD3 mAb, was performed as described in the text. Two-color flow cytometry was performed, and percentage of double-positive cells was analyzed and presented as mean ± SEM, with the number of patients in parentheses. ∗, P < 0.05 vs. controls; ∗∗, P < 0.005 vs. controls.
We (12) and others (11) have observed that activated peripheral blood T cells from patients with progressive MS produce high levels of IFN-γ, a cytokine characteristic of Th1 lymphocytes. Moreover, recent studies with human T cell clones suggest that CCR5 or CXCR3 chemokine receptors may be markers for Th1 cells, and that CCR3, CCR4, and CCR8 are corresponding markers for Th2 cells (2–5). We therefore investigated whether the expression of CCR5 and CXCR3 receptors by peripheral blood T cells of patients with progressive MS was associated with increased production of IFN-γ. T cells from patients with progressive MS were sorted into CCR5+, CCR5−, CXCR3+, and CXCR3− T cell subsets and were stimulated with immobilized anti-CD3 and anti-CD28 mAbs. The results from five individuals are presented in Table 2. In five of five experiments, CCR5+ T cells produced increased levels of IFN-γ compared with CCR5− T cells (4,669 ± 1,169 pg/ml vs. 1,038 ± 226 pg/ml, P < 0.02). There was also an increase of IFN-γ secretion in CXCR3+ T cells in two of four patients tested (Table 2). These studies indicate that progressive MS is associated with an increase in circulating activated T cells expressing CCR5 that produce IFN-γ. To assess the possible pathophysiological significance for active disease within the CNS, we undertook immunopathologic evaluation of MS and control brain samples.
Table 2.
IFN-γ production by T cell subsets in progressive MS patients
| Exp. | IFN-γ production by CCR5 cell subsets
|
IFN-γ production by CXCR3 cell subsets
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Whole T cells
|
CCR5− T cells
|
CCR5+ T cells
|
Whole T cells
|
CXCR3− T cells
|
CXCR3+ T cells
|
|||||
| pg/ml | pg/ml | % | pg/ml | % | pg/ml | pg/ml | % | pg/ml | % | |
| 1 | 3,375 | 1,407 | 42 | 7,658 | 224 | 3,375 | 3,006 | 89 | 2,928 | 87 |
| 2 | 2,479 | 1,526 | 62 | 2,258 | 91 | 2,479 | 1,474 | 59 | 1,474 | 59 |
| 3 | 1,203 | 896 | 74 | 5,144 | 428 | 1,203 | 282 | 23 | 1,625 | 135 |
| 4 | 1,001 | 250 | 25 | 1,713 | 171 | 1,001 | 425 | 42 | 2,935 | 293 |
| 5 | 3,735 | 1,112 | 30 | 6,572 | 176 | ND | ND | |||
| Mean | 2,359 ± 553 | 31,038 ± 226 | 47 ± 9* | 4,669 ± 1,169 | 218 ± 57** | 2,015 ± 559 | 1,297 ± 629 | 53 ± 14*** | 2,241 ± 400 | 144 ± 52 |
T cells from five progressive MS patients were isolated, separated into T cells subsets, and activated with immobilized anti-CD3 plus anti-CD28 mAb. Cell supernatants were collected at 20 h and IFN-γ, IL-4, IL-5 production were measured by ELISA. IL-4 production by purified T cells in all experiments was less than 40 pg/ml. IL-5 production by purified T cells was tested in experiments 2, 3, and 4 and was less that 16 pg/ml. Since donors had different background levels of IFN-γ production prior to T cell subset separation, IFN-γ production for separated subsets is also expressed as a percentage of IFN-γ production by the subset compared to the whole T cell population [(separated subset/whole T cells) × 100%]. ND, not done. ∗, P < 0.001 vs. whole T cells; ∗∗, P < 0.02 vs. CCR5− T cells; ∗∗∗, P < 0.02 vs. whole T cells.
Samples of brain from patients with active MS (n = 5), inactive MS (n = 5), and no MS (n = 5) were examined. All samples consisted primarily of white matter. In the active MS brain specimens, extensive areas of the white matter were infiltrated with large numbers of macrophages/microglia, occasional lymphocytes, and scattered neutrophils. The infiltrating cells often formed linear aggregates parallel to swollen axons, and in some areas, the white matter was completely effaced, with only massive numbers of foamy gitter cells and tangled astrocyte processes remaining. Gemistocytic astrocytes were prominent along the periphery of lesions. In some brain specimens, the leukocyte infiltrate extended into the gray matter, resulting in neuronal necrosis.
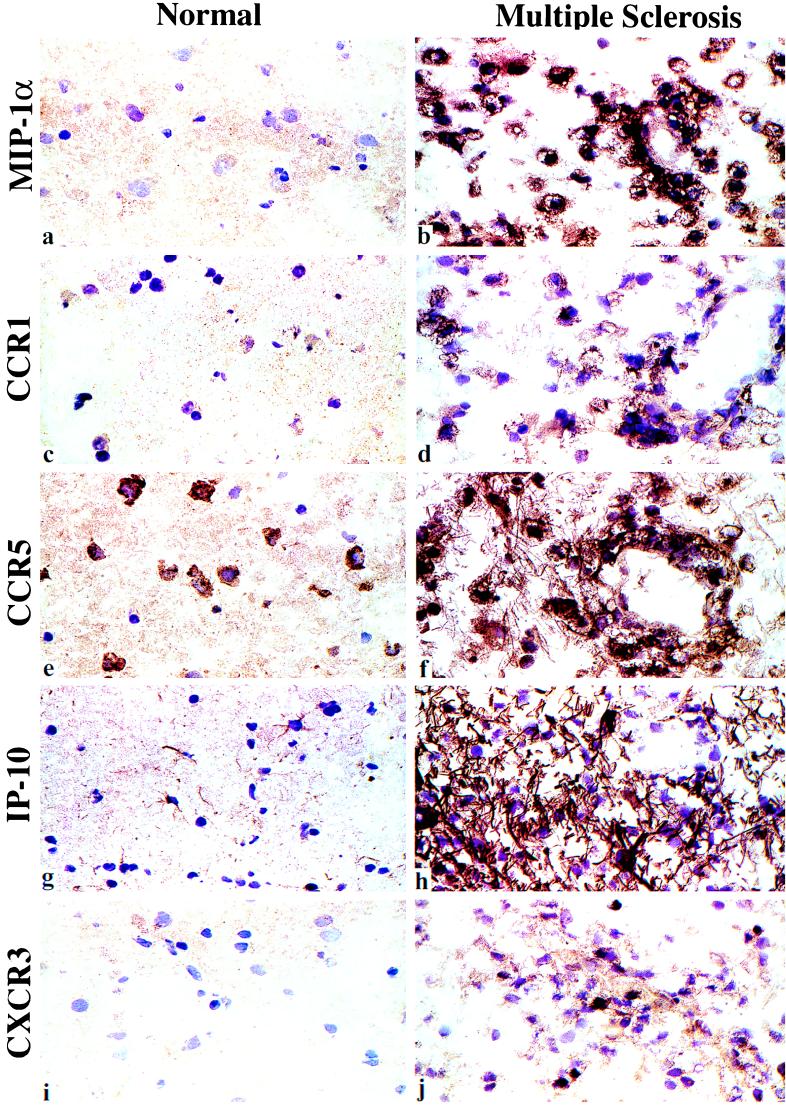
Immunohistologic studies of serial brain sections allowed analysis of expression of the chemokine receptor/chemokine pairs CCR1/MIP-1α, CCR5/MIP-1α, CXCR3/IP-10/Mig, and CCR2/MCP-1 (Table 3, Fig. 3). Macrophages/microglia in areas of severe inflammation and necrosis were intensely immunoreactive for CCR1, CCR5, and the ligand for both receptors, MIP-1α. Expression of CXCR3 was restricted to small numbers of lymphocytes, which were also CCR5+; these cells were typically detected adjacent to blood vessels of inflamed brain specimens. Perivascular and plaque-associated lymphocytes showed cytoplasmic staining for IFN-γ, and macrophages in these areas expressed the potent IFN-γ-inducing factor, IL-18. Hence, multiple feedback and amplification loops are present within MS lesions, including, in the example of leukocytes alone, CCR5+ IFN-γ-producing T cells, plus the chemokine, IP-10, which is induced by IFN-γ, and corresponding IP-10-responsive CXCR3+ mononuclear cells. These findings contrasted with the lack of leukocyte expression of other chemokine receptor/chemokine pairs, including CCR3/eotaxin/eotaxin-2, CCR4/TARC, and CCR6/MIP-3α.
Table 3.
CNS expression of chemokines and their receptors in MS
| Marker | Normal brain or inactive MS | Active MS |
|---|---|---|
| CCR1 | Negative | Macrophages/microglia |
| CCR2 | Negative | Occasional macrophages/microglia |
| CCR5 | Microglia, | Marked up-regulation |
| some neurons | (mononuclear cells) | |
| CXCR3 | Negative | Lymphocytes |
| MIP-1α | Negative | Macrophages/microglia |
| MCP-1 | Astrocytes | Up-regulated (astrocytes/blood vessels) |
| IP-10 | Astrocytes | Marked up-regulation (same cells) |
| Mig | Negative | Focal macrophages/microglia |
| IFN-γ | Negative | Lymphocytes |
| IL-18 | Negative | Focal macrophages/microglia |
Based upon assessment of serial sections of brain tissue from five patients with active disease vs. five patients with inactive disease and five normal brain samples; no significant inflammatory cell labeling was seen with mAbs to CCR3, CCR4, or CCR6; the chemokines eotaxin, eotaxin-2, ITAC, MCP-2, MCP-3, MIP-3α, or TARC; or the cytokines IL-4, IL-10, IL-13, or transforming growth factor-β.
Figure 3.
Immunopathology of progressive MS illustrating chemokine and chemokine receptor expression within the CNS in an area of demyelination (Right). Left micrographs show the very limited expression of these proteins in normal brain, with focal expression of CCR5 by neurons, and IP-10 expression by astrocytes; only minor expression, comparable to normal brain, was seen in the uninvolved tissues of MS patients with inactive disease (not shown). In contrast, areas of demyelination showed dense surface staining of macrophages for MIP-1α (a and b). Macrophages and small numbers of lymphocytes in these areas showed corresponding expression of CCR1 (c and d) and CCR5 (e and f). Lesions contained dense IP-10 expression, associated with astrocytes and their tangled processes (g and h), plus small numbers of CXCR3+ lymphocytes (i and j). (Cryostat sections, hematoxylin counterstain, ×100.)
In addition to leukocytes, astrocytes and vascular cells showed chemokine or chemokine receptor expression. Thus, as with macrophages, astrocytes in regions of inflammation showed dense expression of CCR5, and astrocytes also showed cytoplasmic labeling for the ligands MCP-1 and IP-10. Vascular smooth muscle and endothelium in inflamed brain specimens were immunoreactive for MCP-1, as compared with normal brain specimens, suggesting up-regulation. Negligible CNS expression of the MCP-1 receptor, CCR2, or additional ligands, MCP-2 and MCP-3, was observed, and the results of staining of the available areas of white matter from brains of patients with inactive MS or non-MS patients were similar; overall findings of active MS vs. control samples are summarized in Table 3.
In the present study, we observed increased expression of CXCR3+ T cells in relapsing–remitting patients and of CXCR3+ and CCR5+ T cells in progressive patients. Earlier studies from our group suggest that chronic activation of the immune system occurs when a patient changes from the relapsing–remitting to the progressive stage, as measured by IFN-γ and IL-12 production (12). In the present report we have found that this change in the stage of MS also appears to be linked to chemokine receptor expression. Interestingly, relapsing–remitting MS has been reported in patients with a mutation in the CCR5 gene (15), but the effect of this mutation on progressive MS is unknown. We have also noted an increased number of CD40 ligand (CD40L)-expressing T cells in the peripheral blood of progressive MS patients (12), and CD40L+ T cells have been identified in direct contact with CD40+ macrophages within MS lesions (16). This interaction may be particularly important in the context of MS, because CD40L+ T cells can induce production of MIP-1α, MIP-1β, RANTES, and MCP-1 by CD40+ macrophages (17) and thus may contribute to the chemokine induction we observed in active MS brain lesions.
Since IFN-γ has been reported to be elevated in MS, we tested whether the elevated expression of CXCR3 and CCR5 was related to IFN-γ. We carried out experiments in which we added IFN-γ (10 ng/ml) or IL-12 (1 ng/ml) to PBMC and measured chemokine receptor expression. We found no increase of expression in MS patients after culture with IFN-γ or IL-12, but rather a decrease in expression in the range of 5–40%, depending on the donor (n = 4). Similar reductions were observed in healthy controls. These results suggest that although cells expressing CCR5 produce more IFN-γ in MS, the IFN-γ itself does not appear to be directly responsible for the increased expression of this Th1 type chemokine receptor. The linkage of CCR5 expression and IFN-γ production thus appears related to the chemotaxis of IFN-γ-producing Th1 cells to sites of inflammation rather than the induction of CCR5 by IFN-γ.
On the basis of our results in the progressive cases in which there was increased chemokine receptor expression, one might predict more CNS inflammation in progressive cases, something we did not observe in our immunohistologic studies. However, we do not know whether activated Th1 type cells that migrate to the CNS undergo rapid apoptosis in the target organ, thus not being visible in the CNS despite their increased expression in the bloodstream. Furthermore, the CNS samples studied were postmortem samples and not from the patients from whom the blood samples were studied. It thus may be that this question can be answered only when T cells can be labeled in the periphery and imaged in vivo in the CNS. Further studies are also required to determine whether T cells expressing CXCR3 and/or CCR5 are specific for one of the several myelin autoantigens postulated to play a role in MS, or are specific for an as-yet-undefined antigen.
Chemokine receptor expression has not been studied before in MS, to our knowledge, although previous studies have examined some of the chemokines we analyzed, and the chemokine data assembled thus far are consistent. Studies by McManus et al. (18) and Simpson et al. (19) detected MCP-1 production by astrocytes, and MIP-1α and MIP-1β production by macrophages, within sections of brains from patients with chronic MS. In addition, MIP-1α has been detected within the cerebrospinal fluid of patients with active disease (20). Last, an anti-MIP-1α antiserum (21) or generation of an immune response to MIP-1α DNA injected as a vaccine (22) prevented CNS infiltration by PBMC in mice with experimental allergic encephalomyelitis, a rodent model of MS.
The studies presented here suggest important roles for MIP-1α, acting via CCR5, and potentially IP-10, acting via CXCR3, in the activation and recruitment of host mononuclear cells to the CNS in MS. In combination with what has been shown in rodent models (23), our data provide a rationale for the use of agents that block CCR5 and/or CXCR3 as a therapeutic approach in the treatment of MS, as well as the use of corresponding mAbs for the peripheral blood monitoring of the clinical course and response to therapy of MS patients. Furthermore, our results suggest that chemokine receptor expression may have relevance for immunologic staging of MS and potentially for other chronic autoimmune or inflammatory processes such as rheumatoid arthritis, autoimmune diabetes, or chronic transplant rejection.
Acknowledgments
We thank Dr. Catalin Butoni, of the Rocky Mountain Multiple Sclerosis Center Tissue Bank, Englewood, CO, for supply of tissue samples. This work was supported by National Institutes of Health Grant NS-23132-11A, the National Multiple Sclerosis Society, and the Nancy Davis Center Without Walls. K.E.B. is a Susan Fuhrbacher Conroy Fellow.
ABBREVIATIONS
- CNS
central nervous system
- MS
multiple sclerosis
- PBMC
peripheral blood mononuclear cells
- PE
phycoerythrin
Note Added in Proof
Sorensen et al. (24) have recently reported on the expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients.
References
- 1.Luster A D. New Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 2.Sallusto F, Mackay C R, Lanzavecchia A. Science. 1997;277:2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 3.Sallusto F, Lenig D, Mackay C R, Lanzavecchia A. J Exp Med. 1988;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonecchi R, Biachi G, Bordignon P P, D’Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray P A, Mantovani A, et al. J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zingoni A, Soto H, Hedrick J A, Stoppacciaro A, Storlazzi C T, Sinigaglia F, D’Ambrosio D, Ogarra A, Robinson D, Rocchi M, et al. J Immunol. 1998;161:547–551. [PubMed] [Google Scholar]
- 6.Martin R, McFarland H F, McFarlin D E. Annu Rev Immunol. 1992;10:153–187. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- 7.Beck J, Rondot P, Catinot L, Falcoff E, Kirchner H, Wietzerbin J. Acta Neurol Scand. 1988;78:318–323. doi: 10.1111/j.1600-0404.1988.tb03663.x. [DOI] [PubMed] [Google Scholar]
- 8.Lu C Z, Jensen M A, Arnason B G W. J Neuroimmunol. 1993;46:123–128. doi: 10.1016/0165-5728(93)90241-p. [DOI] [PubMed] [Google Scholar]
- 9.Panitch H S, Hirsch R L, Haley A S, Johnson K P. Lancet. 1987;i:893–895. doi: 10.1016/s0140-6736(87)92863-7. [DOI] [PubMed] [Google Scholar]
- 10.Khoury S J, Hancock W W, Weiner H L. J Exp Med. 1992;176:1355–1364. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noronha A, Toscas A, Jensen M A. J Neuroimmunol. 1993;46:145–154. doi: 10.1016/0165-5728(93)90244-s. [DOI] [PubMed] [Google Scholar]
- 12.Balashov K E, Smith D R, Khoury S J, Hafler D A, Weiner H L. Proc Natl Acad Sci USA. 1997;94:599–603. doi: 10.1073/pnas.94.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Traugott U, Reinherz E L, Raine C S. Science. 1983;219:308–310. doi: 10.1126/science.6217550. [DOI] [PubMed] [Google Scholar]
- 14.Woodroofe M N, Cuzner M L. Cytokine. 1993;5:583–588. doi: 10.1016/s1043-4666(05)80008-0. [DOI] [PubMed] [Google Scholar]
- 15.Bennetts B H, Teutsch S M, Buhler M M, Heard R N S, Stewart G J. Human Immunol. 1997;58:52–59. doi: 10.1016/s0198-8859(97)00207-3. [DOI] [PubMed] [Google Scholar]
- 16.Gerritse K, Laman J D, Noelle R J, Aruffo A, Ledbetter J A, Boersma W J A, Claassen E. Proc Natl Acad Sci USA. 1996;93:2499–2504. doi: 10.1073/pnas.93.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kornbluth R S, Kee K, Richman D D. Proc Natl Acad Sci USA. 1998;95:5205–5210. doi: 10.1073/pnas.95.9.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McManus C, Berman J W, Brett F M, Staunton H, Farrell M, Brosnan C F. J Neuroimmunol. 1998;86:20–29. doi: 10.1016/s0165-5728(98)00002-2. [DOI] [PubMed] [Google Scholar]
- 19.Simpson J E, Newcombe J, Cuzner M L, Woodroofe M N. J Neuroimmunol. 1998;84:238–249. doi: 10.1016/s0165-5728(97)00208-7. [DOI] [PubMed] [Google Scholar]
- 20.Miyagishi R, Kikuchi S, Fukazawa T, Tashiro K. J Neurol Sci. 1995;129:223–227. doi: 10.1016/0022-510x(95)00004-l. [DOI] [PubMed] [Google Scholar]
- 21.Karpus W J, Lukacs N W, McRae B L, Strieter R M, Kunkel S L, Miller S D. J Immunol. 1995;155:5003–5010. [PubMed] [Google Scholar]
- 22.Youssef S, Wildbaum G, Maor G, Lanir N, Gour-Lavie A, Grabie N, Karin N J. J Immunol. 1998;161:3870–3879. [PubMed] [Google Scholar]
- 23.Karpus W J, Ransohoff R M. J Immunol. 1998;161:2667–2671. [PubMed] [Google Scholar]
- 24.Sorensen T L, Tani M, Jensen J, Pierce V, Lucchinetti C, Folcik V A, Qin S, Rottman J, Sellebjerg F, Strieter R M, et al. J Clin Invest. 1999;103:807–815. doi: 10.1172/JCI5150. [DOI] [PMC free article] [PubMed] [Google Scholar]