Abstract
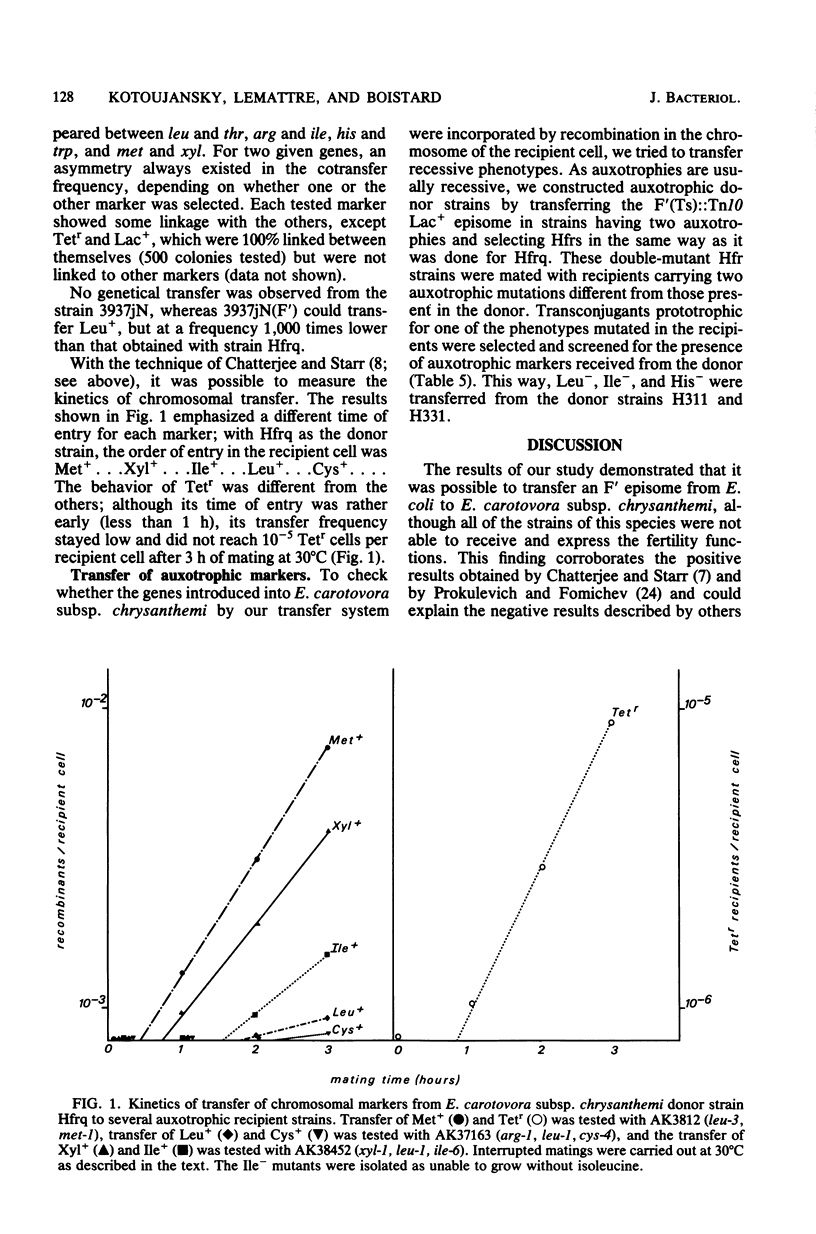
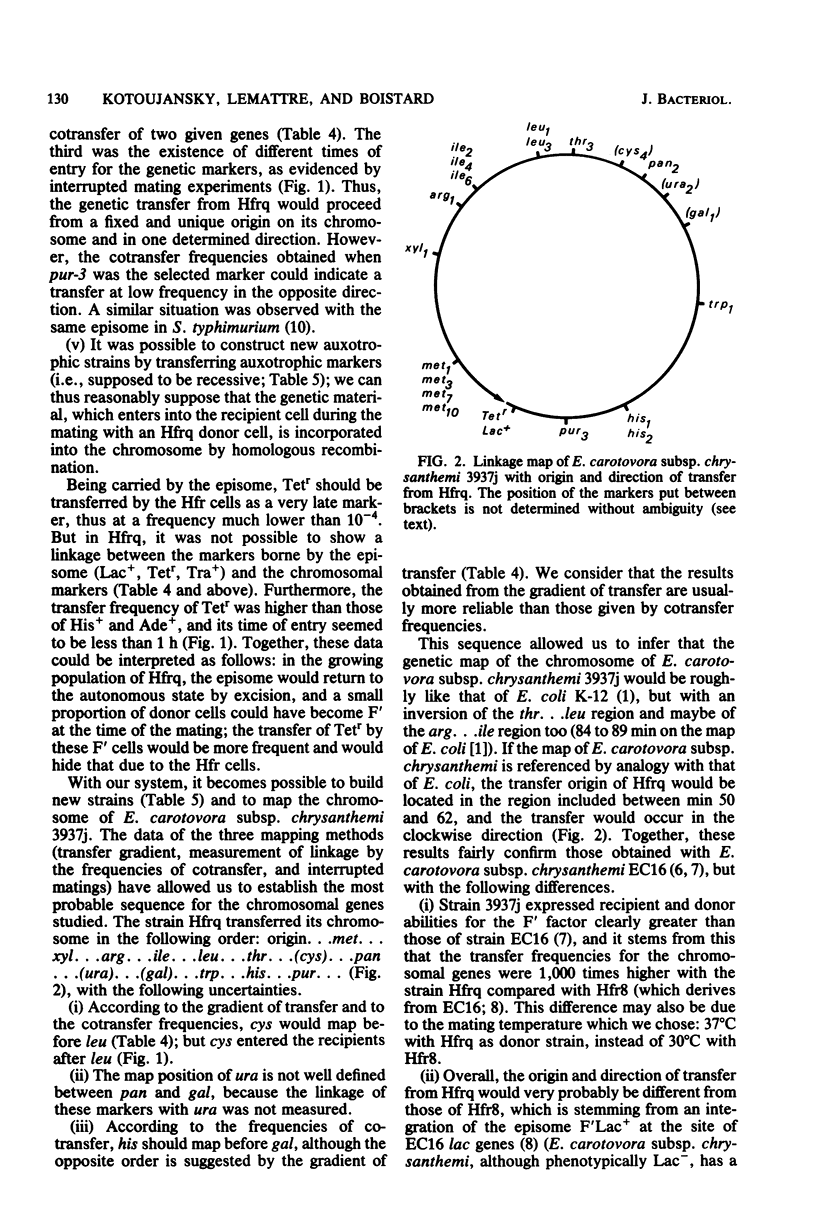
A thermosensitive episome bearing the transposon Tn10, F(Ts)::Tn10 Lac+, has been successfully transferred from Escherichia coli to several wild strains of the enterobacteria Erwinia carotovora subsp. chrysanthemi, which are pathogenic on Saintpaulia ionantha. In one of these strains, all of the characters controlled by this episome (Lac+, Tetr, Tra+) were expressed, and its replication was stopped at 40 degrees C and above. At 30 degrees C, the episome was easily transferred between strains derived from E. carotovora subsp. chrysanthemi 3937j and to E coli. Hfr donor strains were obtained from a F' strain of 3937j by selecting clones which grew at 40 degrees C on plates containing tetracycline. One of these strains, Hfrq, was examined in more detail: the characters Lac+ and Tetr were stabilized and did not segregate higher than its parental F' strain. The mating was most efficient at 37 degrees C on a membrane. Hfrq transferred its chromosome to recipient strains at high frequency and in a polarized fashion, as evidenced by the gradient of transfer frequencies, the kinetics of marker entry (in interrupted mating experiments), and the analysis of linkage between different markers. The chromosome of Hfrq was most probably transferred in the following sequence: origin...met...xyl...arg...ile...leu...thr...cys...pan...ura...gal...trp...his. ..pur... Moreover, this genetic transfer system proved to be efficient in strain construction.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith J. R., Signer E. R., Epstein W. Transposition of the Lac region of E. coli. Cold Spring Harb Symp Quant Biol. 1966;31:393–401. doi: 10.1101/sqb.1966.031.01.051. [DOI] [PubMed] [Google Scholar]
- Beutin L., Achtman M. Two Escherichia coli chromosomal cistrons, sfrA and sfrB, which are needed for expression of F factor tra functions. J Bacteriol. 1979 Sep;139(3):730–737. doi: 10.1128/jb.139.3.730-737.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. K., Starr M. P. Donor strains of the soft-rot bacterium Erwinia chrysanthemi and conjugational transfer of the pectolytic capacity. J Bacteriol. 1977 Dec;132(3):862–869. doi: 10.1128/jb.132.3.862-869.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. K., Starr M. P. Genetic transfer of episomic elements among Erwinia species and other enterobacteria: F'Lac+. J Bacteriol. 1972 Jul;111(1):169–176. doi: 10.1128/jb.111.1.169-176.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. K., Starr M. P. Genetics of Erwinia species. Annu Rev Microbiol. 1980;34:645–676. doi: 10.1146/annurev.mi.34.100180.003241. [DOI] [PubMed] [Google Scholar]
- Chumley F. G., Menzel R., Roth J. R. Hfr formation directed by tn10. Genetics. 1979 Apr;91(4):639–655. doi: 10.1093/genetics/91.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen R. A., Reznikoff W. S. Organization of structural and regulatory genes that mediate tetracycline resistance in transposon Tn10. J Bacteriol. 1979 Jun;138(3):705–714. doi: 10.1128/jb.138.3.705-714.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Roth J., Botstein D. Genetic engineering in vivo using translocatable drug-resistance elements. New methods in bacterial genetics. J Mol Biol. 1977 Oct 15;116(1):125–159. doi: 10.1016/0022-2836(77)90123-1. [DOI] [PubMed] [Google Scholar]
- McEwen J., Silverman P. Chromosomal mutations of Escherichia coli that alter expression of conjugative plasmid functions. Proc Natl Acad Sci U S A. 1980 Jan;77(1):513–517. doi: 10.1073/pnas.77.1.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y., Caro L., Berg C. M., Hirota Y. Chromosome replication in Escherichia coli. IV. Control of chromosome replication and cell division by an integrated episome. J Mol Biol. 1971 Feb 14;55(3):441–456. doi: 10.1016/0022-2836(71)90328-7. [DOI] [PubMed] [Google Scholar]
- Prokulevich V. A., Fomichev Iu K. Peredacha F'lac-plazmidy ot Escherichia coli K-12 bakteriiam roda Erwinia. Genetika. 1978 Nov;14(11):1892–1899. [PubMed] [Google Scholar]
- Shapiro J. A. Molecular model for the transposition and replication of bacteriophage Mu and other transposable elements. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1933–1937. doi: 10.1073/pnas.76.4.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman P., Nat K., McEwen J., Birchman R. Selection of Escherichia coli K-12 chromosomal mutants that prevent expression of F-plasmid functions. J Bacteriol. 1980 Sep;143(3):1519–1523. doi: 10.1128/jb.143.3.1519-1523.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLLMAN E. L., JACOB F., HAYES W. Conjugation and genetic recombination in Escherichia coli K-12. Cold Spring Harb Symp Quant Biol. 1956;21:141–162. doi: 10.1101/sqb.1956.021.01.012. [DOI] [PubMed] [Google Scholar]



