Abstract
The response of hepcidin transcription to iron has been repeatedly documented in living mice, but it is difficult to demonstrate the response in ex vivo systems. We have hydrodynamically transfected mice with plasmid constructs composed of a murine hepcidin 1 promoter and fragments of the promoter fused to a firefly luciferase reporter. This method enabled us to quantitate the response of the hepcidin promoter to short-term feeding of a high-iron diet to mice that have been maintained on an iron-deficient diet. We show that the region of the promoter between 1.6 Kb and 1.8 Kb upstream from the start of translation is essential for the response to iron. The promoter region between −260 bp and −1.6 Kb is not essential for the iron responsiveness of hepcidin promoter. The iron-responsive region that we have mapped is the same region required for the in vitro response of HepG2 cells to stimulation with bone morphogenetic proteins and differs from the LPS/IL-6 responsive area.
Introduction
The regulation of hepcidin expression is of major interest because the mature 25–amino acid hepcidin peptide is a central regulator of iron homeostasis. Dysregulation of hepcidin transcription is the cause of most forms of hemochromatosis, and may play an important role in the anemia of chronic inflammation.1 Hepcidin transcription is up-regulated by iron; by the cytokines IL-6, IL-1α, and IL-1β; and by the bone morphogenetic proteins (BMPs). It is down-regulated by anemia and by hypoxia. Until now, the basis of hepcidin gene expression in response to iron has been elusive because a robust response is only observed in intact animals. Recent studies by Lin et al show that there is a limited response in primary hepatocytes in vitro,2 provided that the iron-transferrin stimulus is applied within 2 hours of harvesting the cells. This response is much less than that observed in vivo and, in any case, is not suitable for promoter mapping.
Study design
We have now devised a means of studying the in vivo response of the hepcidin promoter to iron. Details of the methodology have been published previously.3 In brief, we hydrodynamically transfected mice with constructs containing fragments of the hepcidin promoter fused to the firefly luciferase (luc) reporter. To elicit a response to iron, mice were maintained for at least 2 weeks on an iron-poor diet containing only 2 to 5 ppm of iron, as suggested by Rivera et al,4 and then fed a diet containing 2 × 104 ppm iron for 24 hours. Luciferase expression driven by the different hepcidin promoter regions was measured in the intact animals using an IVIS Live image instrument (Xenogen, Hopkinton, MA).
Results and discussion
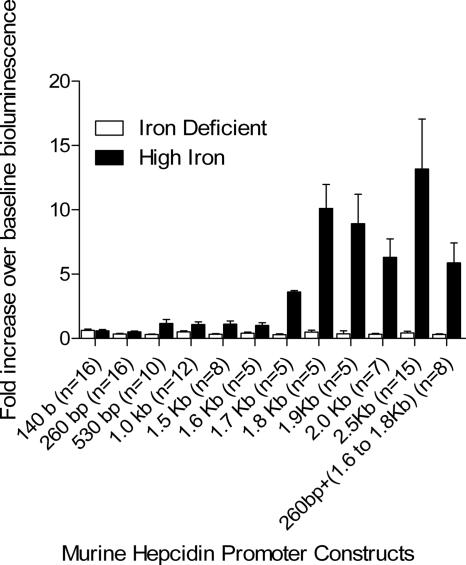
The results of our studies are shown in Figure 1. It is apparent that there is a region of the promoter between 1.6 and 1.8 Kb upstream from the start of translation that is required for the response to iron. This region, together with the first 260 bp of the promoter, is sufficient to provide a near-maximal response to iron stimulation. Interestingly, this is the same region required for the in vitro response of HepG2 cells to stimulation with BMP-4 or BMP-9.5
Figure 1.
Location of the iron-responsive element in vivo. Mice receiving an iron-deficient diet (2-5 ppm) were hydrodynamically transfected with a pGL3 reporter plasmid containing the firefly luciferase gene (luc) under the control of various lengths of the murine hepcidin 1 (Hamp1) promoter. After 3 days, the basal level of bioluminescence was determined and mice were divided into 2 groups; 1 received a high-iron diet (2 × 104 ppm), and the second group remained on the iron-deficient diet (2–5 ppm). After 24 hours, the mice were anesthetized and reinjected with luciferin, and the bioluminescence was remeasured (day 4). The day-4 bioluminescence is expressed as fold change over baseline day 3 bioluminescence. Because the reporter is delivered by hydrodynamic transfection that results in transient expression, the actual levels of expressed reporter decreased with time. As a result, without stimulation, the day-4 bioluminescence is about one-third of the day-3 bioluminescence. Thus, the fold change of day-4 bioluminescence over day-3 baseline bioluminescence in mice on an iron-deficient diet is less than 1. The number of base pairs upstream of the start of translation is given for each promoter construct. The construct designated 260 bp + (1.6 to 1.8 Kb) contains the first 260 bp and the portion of the promoter between 1.6 and 1.8 Kb after the start of translation. The number of animals in each group on iron-deficient and high-iron diets was equivalent and is shown in the brackets; error bars represent 1 SEM.
Previous investigations of the cytokine responsiveness of hepcidin promoter constructs have focused on regions less than 1.0 Kb upstream from the start of translation. These studies have demonstrated that IL-6 stimulation is mediated by STAT3 activation and subsequent hepcidin promoter binding by STAT3 in the proximal 150-bp region.6,7 There are many potential transcription factor–binding sites in the iron-regulatory region, and the technology that we have developed to map the in vivo iron response area is much more cumbersome than that using cells transfected with reporter constructs. However, the present studies show clearly that the in vivo response to short-term iron loading resides in an area far distant from inflammatory cytokine response elements, and helps to focus our attention on a part of the hepcidin promoter (above−1.6 Kb) which has, up to now, not been explored. The region between −260 and −1.6 Kb from the start of translation is not needed for the iron response.
Acknowledgments
This is manuscript no. 18912-MEM.
This work was supported by the National Institutes of Health grant DK53505-09, and the Stein Endowment Fund.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.T. aided in design of the experiments and performed the studies with the assistance of H.P. P.L. aided J.T. in the design of studies. J.F. carried out some of the experiments. E.B. conceived the experimental approach and provided overall supervision.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ernest Beutler, Department of Molecular and Experimental Medicine, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037; e-mail: beutler@scripps.edu.
References
- 1.Nemeth E, Ganz T. Regulation of iron metabolism by hepcidin. Annu Rev Nutr. 2006;26:323–342. doi: 10.1146/annurev.nutr.26.061505.111303. [DOI] [PubMed] [Google Scholar]
- 2.Lin L, Valore EV, Nemeth E, Goodnough JB, Gabayan V, Ganz T. Iron-transferrin regulates hepcidin synthesis in primary hepatocyte culture through hemojuvelin and BMP2/4. Blood. doi: 10.1182/blood-2007-04-087593. Prepublished May 31, 2007, as DOI 10.1182/blood-2007-04-087593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flanagan JM, Truksa J, Peng H, Lee P, Beutler E. In vivo imaging of hepcidin promoter stimulation by iron and inflammation. Blood Cells Mol Dis. 2007;38:253–257. doi: 10.1016/j.bcmd.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivera S, Nemeth E, Gabayan V, Lopez MA, Farshidi D, Ganz T. Synthetic hepcidin causes rapid dose-dependent hypoferremia and is concentrated in ferroportin-containing organs. Blood. 2005;106:2196–2199. doi: 10.1182/blood-2005-04-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Truksa J, Peng H, Lee P, Beutler E. Different regulatory elements are required for response of hepcidin to IL-6 and bone morphogenetic proteins BMP 4 and 9. Br J Haematol. 2007;139:138–147. doi: 10.1111/j.1365-2141.2007.06728.x. [DOI] [PubMed] [Google Scholar]
- 6.Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108:3204–3209. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verga Falzacappa MV, Vujic SM, Kessler R, Stolte J, Hentze MW, Muckenthaler MU. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007;109:353–358. doi: 10.1182/blood-2006-07-033969. [DOI] [PubMed] [Google Scholar]