Abstract
Thrombin activatable fibrinolysis inhibitor (TAFI), when activated, forms a basic carboxypeptidase that can inhibit fibrinolysis. Potential physiologic activators include both thrombin and plasmin. In vitro, thrombomodulin and glycosaminoglycans increase the catalytic efficiency of TAFI activation by thrombin and plasmin, respectively. The most relevant (patho-) physiologic activator of TAFI has not been disclosed. Our purpose was to identify the physiologic activator of TAFI in vivo. Activation of protein C (a thrombin-thrombomodulin–dependent reaction), prothrombin, and plasminogen occurs during sepsis. Thus, a baboon model of Escherichia coli–induced sepsis, where multiple potential activators of TAFI are elaborated, was used to study TAFI activation. A monoclonal antibody (mAbTAFI/TM#16) specifically inhibiting thrombin-thrombomodulin–dependent activation of TAFI was used to assess the contribution of thrombin-thrombomodulin in TAFI activation in vivo. Coinfusion of mAbTAFI/TM#16 with a lethal dose of E coli prevented the complete consumption of TAFI observed without mAbTAFI/TM#16. The rate of fibrin degradation products formation is enhanced in septic baboons treated with the mAbTAFI/TM#16; therefore, TAFI activation appears to play a key role in the extent of fibrin(ogen) consumption during E coli challenge, and thrombin-thrombomodulin, in a baboon model of E coli–induced sepsis, appears to be the predominant activator of TAFI.
Introduction
Fibrinolysis is achieved primarily through the proteolytic action of plasmin on fibrin polymers that reinforce and maintain the integrity of a thrombus. In the hemostatic response, fibrin is a critical component preventing blood loss due to injury. However, in certain circumstances inappropriate fibrin-rich thrombus formation occludes vessels required to maintain the viability of a tissue area. Degradation of fibrin by plasmin exposes basic C-terminal residues (lysines and arginines) as the fibrin strands continually truncate.1 Generation of C-terminal basic amino acids provides positive feedback enhancing plasminogen activation.1–4
Fibrin is a key component of the fibrinolytic cascade, not only as a substrate but as a cofactor and modulator, and is also the end product of the coagulation cascade. Therefore, fibrinolysis is regulated by all of the regulatory elements attributed to control of coagulation. Recently, another connection between coagulation and fibrinolysis has been identified and is mediated through thrombin activatable fibrinolysis inhibitor (TAFI, also known as procarboxypeptidase U and procarboxypeptidase R).5 Activation of TAFI to TAFIa generates a carboxypeptidase capable of catalyzing the removal of C-terminal basic amino acids.5–9 Therefore, by modulating the steady-state concentration of these C-terminal basic amino acids, TAFIa modulates the steady-state concentrations of various forms of bound and free plasminogen and plasmin: activated, inhibited, and inhibitable.5,6,10–13
TAFI, secreted by the liver, circulates in plasma at approximately 100 nM.5,6,14 The potential carboxypeptidase activity generated from TAFI activation is currently difficult to assess, and so the inferred role of TAFI and its correlation with pathologies are unclear.15 New tools to measure TAFIa (reviewed in Willemse and Hendriks15) will undoubtedly contribute to a better understanding of the physiologic role of TAFI. Activation of TAFI has been studied in vitro, and although TAFI is activated by thrombin it is also activated by other proteases such as trypsin, plasmin, and elastase.5,6,9,16–19 These proteases activate TAFI poorly alone; however, TAFI activation by thrombin and plasmin can be cofactor dependent. Thrombomodulin, a cellular receptor involved in activation of protein C,20 also potentiates thrombin-dependent TAFI activation 1250-fold.21,22 Interestingly, TAFIa and activated protein C inactivate cofactors involved in regulating fibrinolysis and coagulation, respectively.11,23 In addition to thrombin-thrombomodulin–dependent activation, carbohydrate moieties, including heparin, potentiate the rate of activation of TAFI by plasmin.17 Both thrombomodulin and carbohydrate moieties are physiologic cofactors able to stimulate thrombin- and plasmin-dependent activation of TAFI in vivo. The key to understanding the (patho)physiologic role of TAFI is to understand how TAFI is activated.
To study TAFI activation in vivo, a baboon model of Escherichia coli–induced sepsis was used. Protein C concentrations are decreased in patients with sepsis,24 which is recapitulated in a baboon sepsis model.25 During sepsis, thrombin formation, thrombin-thrombomodulin-dependent protein C activation, and plasmin production are observed.26–28 Therefore, because the likely (patho-) physiologic activators of TAFI are all elaborated in sepsis (thrombin, thrombin-thrombomodulin, plasmin), a baboon model of sepsis is well suited to disclose the (patho)physiologic activator of TAFI. In addition, we demonstrate the utility of an antibody that specifically inhibits TAFI activation induced by thrombin-thrombomodulin complex but not by thrombin, plasmin, or plasmin-heparin complex. Infusing this antibody into septic baboons we show that, during sepsis, TAFI activation occurs primarily through activation induced by thrombin in complex with thrombomodulin. This study, therefore, comprises the first demonstration that in certain circumstances, specifically during sepsis, thrombin-thrombomodulin is the predominant (patho)physiologic activator of TAFI. Furthermore, in pathologic conditions, TAFIa plays a role in modulation of the fibrino(geno)lytic cascade in vivo as indicated by increased loss of fibrinogen and production of fibrin degradation products (FDPs) in challenged animals treated with the antibody.
Materials and methods
Approval was obtained from the Health Research Ethics Board (Biomedical Panel) at the University of Alberta for the use of human plasma in these studies.
Proteins and reagents
Pooled normal human plasma and polyclonal anti-TAFI antibody (lot AP286) were purchased from Affinity Biologicals (ABI, Ancaster, ON). Numerous generous gifts were gratefully received: human thrombin from Dr M. E. Nesheim (Kingston, ON); activase, the recombinant tissue-type plasminogen activator (tPA), from Dr G. Vehar at Genentech (South San Francisco, CA); soluble thrombomodulin (Solulin) from Paion Deutschland (Aachen, Germany); and tick anticoagulant peptide (TAP) from Dr S. Krishnaswamy (The Children's Hospital of Philadelphia, Philadelphia, PA). Unfractionated heparin (UH), 10 000 U/mL in HBS, was prepared from heparin sodium salt (Sigma-Aldrich, Oakville, ON). Plasmin was prepared from pooled normal human plasma as described.29 TAFI and baboon TAFI were prepared from normal human plasma and normal baboon plasma, respectively, as previously described for the purification of human TAFI.5 The potato tuber carboxypeptidase inhibitor (PTCI) was purchased from Sigma-Aldrich. 3-(2-furyl)acryloyl-Ala-Arg-OH (FAAR) was purchased from Bachem Bioscience (King of Prussia, PA). The mAbTAFI/TM#16 was produced at the University of Vermont in the hybridoma core facility as previously described.30
In vivo experimentation
Papio cyanocephalus cynocephalus or P cyanocephalus anubis baboons were purchased from a breeding colony maintained at the University of Oklahoma Health Science Center (UOHSC) animal facility. The Institutional Animal Care and Use Committees of the UOHSC and the Oklahoma Medical Research Foundation approved the study protocol. Animals weighed 5 to 8.5 kg, had hematocrit exceeding 0.36 (36%), and were tuberculosis free. Animals were held for 30 days prior to infusion studies conducted at the UOHSC. All animals were observed for a minimum of 10 hours following infusion of test materials. Surviving animals were returned to the colony.
Infusion procedures
Baboons were fasted overnight before each experiment with water available ad libitum. Following sedation using ketamine hydrochloride (14 mg/kg, intramuscularly) on the morning of the study, each baboon was anesthetized with sodium pentobarbital administered in the cephalic vein through a percutaneous catheter to maintain light level surgical anesthesia (2 mg/kg approximately every 45 minutes). Oral intubation allowed animals to breathe spontaneously. The femoral artery and vein were cannulated aseptically and used for measuring arterial pressure and obtaining blood samples. E coli and other agents were infused through a percutaneous catheter. The anesthetized animal was positioned on its side on a heating pad. E coli infusions, when performed, began at T = 0 and ran over a 2-hour period after which colony counts were performed on blood taken at T = 120 minutes. Sublethal E coli (SLEC) doses administered were 1 × 108 CFU/kg and lethal doses (LDEC) were 1 × 1010 CFU/kg. When present, mAbTAFI/TM#16 doses were 5 mg/kg given as a bolus injection at T = 0.
Sampling procedures
Mean systemic arterial pressure (MSAP) and heart rate were monitored with a Stathem pressure transducer and Hewlett Packard recorder (Avondale, PA). Temperature was monitored with a Telethermometer (Yellow Springs Instrument, Yellow Springs, OH). These measurements were made and blood samples were collected at T = −60, 0, +60, +120, +180, +240, +360, +480, and +600 minutes and +24 hours unless otherwise indicated. No more than 10% of the animals' calculated blood volume (70 mL/kg) was drawn over the 10-hour monitoring period.
Preparation of plasma for lysis assays
Pooled normal human plasma, from a minimum of 20 donors, was dialyzed against 4 L of 0.02 M Hepes, 0.15 M NaCl at pH 7.4 (HBS) at 4°C with 4 buffer changes. The dialysate, normal human plasma (NHP), was stored at −70°C in 2-mL aliquots. TAFI-depleted plasma (TdP) was produced by passing an aliquot, less than 50 mL, of NHP over a 2-mL anti-TAFI affinity column (mAbTAFI/TM#16-Sepharose CL-4B) equilibrated in HBS. Depletion of TAFI was verified by Western blot analysis of a sample from each individual fraction (data not shown) using a polyclonal anti-TAFI antibody.
Clot lysis experiments
Baboon plasma was diluted with HBS to an A280 of 16, typically 1 part plasma to 2 parts buffer containing PCPS vesicles at a final concentration of 10 μM. Clots (100 μL) were formed in the wells of a microtiter plate by the addition of 91 μL diluted plasma to 2 μL tPA (1.8 nM) and 2 μL thrombin (6 nM) in CaCl2 (5 mM). The remaining 5 μL volume comprised buffer or various combinations of thrombomodulin (0-50 nM), tick anticoagulant peptide (TAP, 1.5 μM), mAbTAFI/TM#16 (0-125 nM), and PTCI (6 μM). All concentrations are stated as the final concentrations. Turbidity of each clot was monitored at 405 nm and 37°C using a SpectraMax Plus Platereader (Molecular Devices, Sunnyvale, CA). The lysis time was defined as the time at which the turbidity of the clot equals one half of the maximal turbidity value attained.
For function determination of mAbTAFI/TM#16 in baboon plasma samples, a modified clot lysis assay was used. The minimal volume of normal baboon plasma required to observe the effect of bTAFI activation was determined by titrating normal baboon plasma (NBP) into TAFI-depleted human plasma (TdP). All parameters were as described in the previous paragraph except for the use of 441 pM tPA, 20 nM thrombomodulin, and 1 μM TAP. NBP (10 μL) in a 100-μL clot produced maximal prolongation of lysis time (data not shown). This procedure was necessary to use a minimal volume of baboon plasma.
TAFIa activity assay
Purified baboon TAFIa (bTAFIa) activity was determined using the chromolytic substrate FAAR. The bTAFIa samples (100 μL) were mixed with 100 μL FAAR (final concentration 1 mM, diluted in HBS 0.01% Tween 80 [HBST]) in the wells of a microtiter plate, and the absorbance (340 nm) of duplicate reactions was monitored continuously over 3 hours at ambient temperature. bTAFIa samples consisted of 100 nM affinity-purified bTAFI, 5 mM Ca2+, mAbTAFI/TM#16 (1 μM when present) and an activator. TAFI was activated by either thrombin alone (600 nM); thrombin (20 nM) and thrombomodulin (20 nM); thrombin (20 nM), thrombomodulin (20 nM), and unfractionated heparin (UH) (200 U/mL); plasmin alone (20 nM); or plasmin (20 nM) and UH (200 U/mL).
Assays
mAbTAFI/TM#16 concentrations were determined in plasma samples from infused baboons by enzyme-linked immunosorbent assay (ELISA) specific for mouse IgG. Concentrations of antibody were also evaluated using clot lysis assays (as described above) to verify the relationship between activity and antigen presence of the mAbTAFI/TM#16. Relative inhibition of thrombomodulin/TAFI-dependent prolongation of clot lysis was compared with a standard curve comprising known concentrations of mAbTAFI/TM#16 for quantification. Fibrinogen concentration was determined using the thrombin clotting time.31 Fibrin degradation products (FDPs) were measured by latex agglutination assay.32
Western blotting
Samples were diluted in sample preparation buffer (1% sodium dodecyl sulfate [SDS], 1% 2-mercaptoethanol [BME], and 10% glycerol with bromophenol blue) and subjected to SDS–polyacrylamide gel electrophoresis (SDS-PAGE) using a 5% to 15% gradient gel in a minislab gel electrophoresis apparatus (Hoefer; Technical Marketing, Ottawa, ON) according to the procedure of Neville.33 Protein was then transferred to nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA) at 500 mA for 1 hour as described by Towbin et al.34 The blot was blocked with 5% nonfat milk in 20 mM Tris, 0.15 M NaCl, 0.05% Tween 20 at pH 7.4 (TTBS), and TAFI antigen was probed for 1 hour with an affinity-purified polyclonal sheep anti-TAFI antibody (1 μg/mL in TTBS). The anti-TAFI antibody was detected using a secondary horseradish peroxidase–conjugated donkey antisheep antibody (ABI) and enhanced chemiluminescence (Chemiluminescence Detection Kit; DuPont, Boston, MA) with X-Omat film (Kodak Scientific Imaging) and an M35A X-Omat processor (Eastman Kodak, New Haven, CT).
Results
mAbTAFI/TM#16 specifically inhibits thrombomodulin-mediated activation of purified baboon TAFI
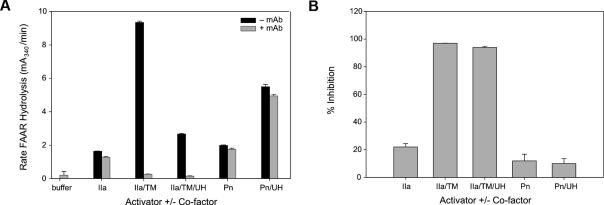
Activated TAFI (TAFIa) catalyzes the removal of C-terminal lysines and arginines from various substrates. The chromolytic substrate FAAR may be used to quantitate TAFIa activity. Maximal rates of FAAR hydrolysis presented in Figure 1 are proportional to TAFIa activity. Various activators of TAFI, alone and with cofactors, were incubated with purified baboon TAFI (bTAFI), in the absence and presence of mAbTAFI/TM#16. Activity associated with bTAFIa was inferred from rates of FAAR hydrolysis (Figure 1A). No reduction in the rate of FAAR hydrolysis with the mAbTAFI/TM#16 was observed when thrombin was the activator. When both thrombomodulin and thrombin were present, an increased rate of FAAR hydrolysis was observed (9.35 mA340nm/min), consistent with the cofactor dependence of this reaction; however, in the presence of the mAbTAFI/TM#16, bTAFI activation was reduced by 93% (0.27 mA340nm/min). Addition of 200 U/mL unfractionated heparin (UH) to 20 nM thrombin-thrombomodulin complex reduced bTAFI activation by only 65.6% in the absence of mAbTAFI/TM#16, however, remaining bTAFI activation was also attenuated by the inclusion of mAbTAFI/TM#16. Although present at 1/30th of the concentration of thrombin alone, plasmin was able to activate a comparable concentration of bTAFI that was increased in the presence of UH. The mAbTAFI/TM#16 had no effect on plasmin-dependent TAFI activation. A replot of the data expressed as the percentage inhibition of bTAFI activity is shown in Figure 1B, where the specificity of the mAbTAFI/TM#16 toward inhibition of thrombin-thrombomodulin–dependent activation of bTAFI is clearly illustrated.
Figure 1.
mAbTAFI/TM#16 specifically inhibits thrombin-thrombomodulin activation of purified baboon TAFI (bTAFI). (A) In the absence of the mAbTAFI/TM#16, bTAFI is activated by thrombin (IIa) alone, IIa/thrombomodulin (TM)/unfractionated heparin (UH), plasmin (Pn) alone, and Pn/UH, but most effectively by IIa/TM as determined by activity of bTAFIa (inferred from rate of 3-(2-furyl)acryloyl-Ala-Arg-OH [FAAR] hydrolysis). Addition of the mAbTAFI/TM#16 dramatically reduces bTAFIa activity involving TM-associated activation. (B) The same data displayed as percentage inhibition of TAFI activity, illustrating only activation by IIa/TM (± UH) is blocked by the mAbTAFI/TM#16. Data are expressed as mean (± range); n = 2.
mAbTAFI/TM#16 specifically inhibits thrombin-thrombomodulin–mediated activation of baboon TAFI in plasma
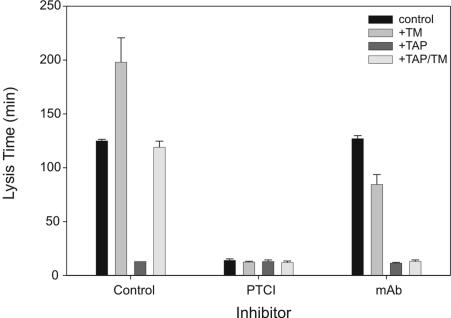
Activation of bTAFI during lysis prolongs clot lysis time, and at specific concentrations of tPA the amount of bTAFIa generated is proportional to prolongation. Inhibition of bTAFI activation in plasma, inferred from change in lysis times, by mAbTAFI/TM#16 was compared with that achieved with PTCI, an inhibitor of TAFIa. Each inhibitor was assessed for its ability to shorten lysis time of clots formed from baboon plasma in the presence and absence of thrombomodulin (to promote TAFI activation) and TAP (to prevent endogenous thrombin formation) shown in Figure 2. Addition of thrombomodulin prolonged clot lysis by 73 minutes over control values. Inclusion of TAP reduced control lysis time by 112 minutes to 13 minutes, and inclusion of thrombomodulin with TAP extended lysis time comparable with control. Therefore, 6 nM thrombin with thrombomodulin and without endogenous prothrombin activation is sufficient to activate significant concentrations of TAFI in baboon clots. That prolongation is TAFIa dependent is supported by addition of PTCI, which caused all clots to lyse in 11 to 15 minutes. In contrast to the effects of PTCI, mAbTAFI/TM#16 inhibited prolongation of clot lysis only in assays where thrombomodulin was present, confirming the inhibitory specificity of mAbTAFI/TM#16 for thrombomodulin-dependent bTAFI activation. This observation is consistent with those observed with purified components and indicates that the inhibitory properties of mAbTAFI/TM#16 remain intact in a more complex milieu such as in plasma-derived clots. In the presence of the mAbTAFI/TM#16, addition of thrombomodulin shortened lysis time compared with the control. This is likely attributable to the activation of protein C to activated protein C, which is known to be profibrinolytic.35 This suggests that mAbTAFI/TM#16 is able to uncouple thrombomodulin-mediated anticoagulation (protein C–dependent) and antifibrinolytic (TAFI-dependent) reactions, possibly allowing for bleed through of TAFI activation by any generated thrombin.
Figure 2.
mAbTAFI/TM#16 specifically inhibits thrombomodulin-mediated activation of baboon TAFI (bTAFI) in plasma. Clot lysis assays, containing one-third diluted baboon plasma, were performed in the absence and presence of potato tuber carboxypeptidase inhibitor (PTCI) and mAbTAFI/TM#16. Assay conditions were as follows: control, thrombomodulin (TM), tick anticoagulant peptide (TAP), and TAP/TM. In the control (no inhibitor), an increase in clot lysis prolongation is observed when TM is present. PTCI inhibits bTAFIa activity, whereas mAbTAFI/TM#16 specifically inhibits the effect of TM on bTAFI activation, inhibiting lysis prolongation. Data are expressed as mean (±) range; n = 2.
Effect of thrombomodulin concentration on bTAFI activation in plasma
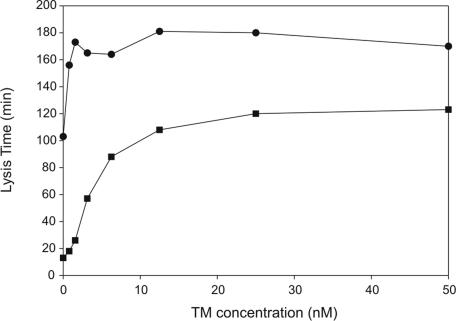
Thrombin-mediated activation of human TAFI is significantly enhanced by the addition of thrombomodulin.21 To determine the concentration dependence of thrombomodulin on bTAFI activation in plasma, thrombomodulin was titrated into clots formed with one-third diluted baboon plasma and lysis times were determined. Lysis assays were performed in the absence and the presence of TAP to prevent quantitative activation of prothrombin by factor Xa, which could otherwise circumvent the thrombomodulin-dependent reaction. Addition of thrombomodulin enhances prolongation of lysis time in clots formed from baboon plasma (Figure 3) as was observed with clots formed from human plasma. In the presence of TAP, 12.5 nM thrombomodulin with 6 nM thrombin was sufficient to prolong lysis time to that observed without TAP or thrombomodulin, 108 versus 103 minutes, respectively. Thrombin-thrombomodulin–mediated prolongation is concentration dependent and displays saturation higher than 1.56 nM thrombomodulin (Figure 3). Although prolongation was thrombomodulin dependent in the presence of TAP, saturation was achieved by 25 nM thrombomodulin at only 120 minutes, rather than by 3.13 nM thrombomodulin at 180 minutes without TAP. The 60-minute difference may indicate other antifibrinolytic roles for thrombin or it may indicate that thrombin alone plays a significant role in bTAFI activation. If so, the mAbTAFI/TM#16 may not be effective against TAFI activation in baboons in vivo due to its thrombin-thrombomodulin specificity.
Figure 3.
Thrombomodulin-dependent activation of endogenous TAFI in normal baboon plasma (NBP) results in prolongation of clot lysis. Lysis times were determined for clots formed with one third NBP, in the presence (■) and absence (●) of 2 μM tick anticoagulant peptide (TAP) with increasing concentrations of thrombomodulin (0-60 nM). Inhibition of thrombin activation by TAP resulted in reduced activation of baboon TAFI (bTAFI) both in the absence and presence of thrombomodulin. Thrombin alone activates bTAFI, and this effect is enhanced with increasing concentrations of thrombomodulin, achieving saturation by 12.5 nM thrombomodulin.
Determination of the effective inhibitory concentrations of mAbTAFI/TM#16 for infusion into normal baboons
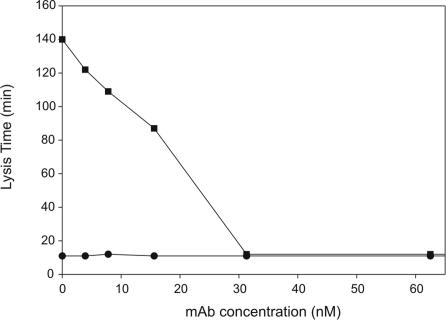
Prior to conducting in vivo studies to assess bTAFI activation, the concentration of mAbTAFI/TM#16 that could effectively inhibit thrombomodulin-mediated bTAFI activation in clots formed from plasma was determined. Clots containing one-third diluted baboon plasma, 6 nM thrombin, 5 mM CaCl2, 1.8 nM tPA, and 1.5 μM TAP were formed in the presence of various concentrations of mAbTAFI/TM#16. In the absence of thrombomodulin, all clots lysed by 11 minutes regardless of mAbTAFI/TM#16 concentration (Figure 4). In the presence of 20 nM thrombomodulin, mAbTAFI/TM#16 inhibited prolongation of clot lysis in a dose-dependent manner (Figure 4). Interestingly, full inhibition occurred at an antibody concentration of 31.3 nM, which, when corrected for the plasma dilution, is 93.9 nM and approximately equivalent to concentrations of TAFI determined for human plasma. Similar dose dependence was obtained in a system comprising purified components and thrombin-thrombomodulin with 100 nM hTAFI, suggesting a stoichiometry of 1:1 rather than 2:1 as would be expected for a bivalent antibody. The mAbTAFI/TM#16 had no effect on lysis times in clots formed in the absence of both TAP and thrombomodulin (data not shown). From these data, we calculated that 500 nM mAbTAFI/TM#16 (a 5-fold excess) would ensure complete inhibition of thrombin-thrombomodulin inhibition of bTAFI activation during infusion studies.
Figure 4.
mAbTAFI/TM#16 can effectively block baboon TAFI (bTAFI) activation in plasma, shutting off the prolongation of clot lysis. In the presence of 1.5 μM tick anticoagulant peptide (TAP), mAbTAFI/TM#16 was titrated into clots formed with one-third diluted normal baboon plasma (NBP) in the presence (■) and absence (●) of 20 nM thrombomodulin. At mAbTAFI/TM#16 concentrations of 31.3 nM and greater, no bTAFIa-induced prolongation of lysis times was observed.
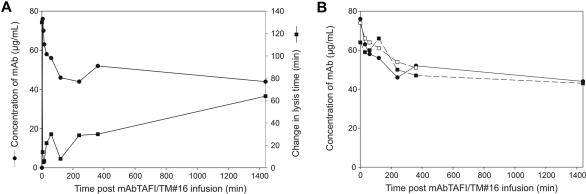
Determination of the half-life of mAbTAFI/TM#16
The clearance of both antigen and function of mAbTAFI/TM#16 were monitored following its infusion into baboons. In baboons infused with 5 mg/kg mAbTAFI/TM#16, the mAb concentration decreased rapidly within the first hour after infusion stabilizing at 58% after 24 hours as measured by ELISA (Figure 5A). The inhibitory activity of the mAbTAFI/TM#16 was determined using a clot lysis assay modified to minimize the volume of baboon plasma. Inhibitory activity of mAbTAFI/TM#16 was reduced over time after infusion, as measured by the ability of the antibody to prevent thrombomodulin-TAFI–dependent prolongation of lysis time. Clot lysis of the 0-minute sample, immediately prior to the infusion of mAbTAFI/TM#16, occurred in 130 minutes but was reduced to 14 minutes 5 minutes after mAbTAFI/TM#16 infusion. Lysis times gradually increased coincidently with the decrease in antibody concentration. Twenty-four hours after mAb infusion, clot lysis was prolonged to 64 minutes (Figure 5A). The concentration of mAbTAFI/TM#16 was also determined by ELISA to confirm that the half-life of the mAbTAFI/TM#16 in both a sublethal and lethal dose of E coli (Figure 5B, SLEC and LDEC, respectively) was similar to control baboons. The mAbTAFI/TM#16 concentration dropped similarly in all animals; however, mAbTAFI/TM#16 in the animal receiving the LDEC could be monitored for only 6 hours. Nonetheless, the half-life of the mAbTAFI/TM#16 was calculated to be greater than 24 hours.
Figure 5.
The half-life of mAbTAFI/TM#16 in vivo, measured by antigen and functional activity, is longer than 24 hours. (A) The concentration of mAbTAFI/TM#16, detected by ELISA, dropped rapidly in a control animal within the first hour after infusion, stabilizing during the 24-hour follow-up period (●, left-hand y-axis). Prolongation of clot lysis increased slowly over the 24-hour period, with a maximum of 64 minutes prolongation (49% of T = 0 lysis time) (■, right-hand y-axis). (B) The half-life of the antibody in a baboon model of sepsis, both a sublethal dose of E coli (SLEC) (■) and a lethal dose of E coli (LDEC) (□), is very similar to that seen in the control (●).
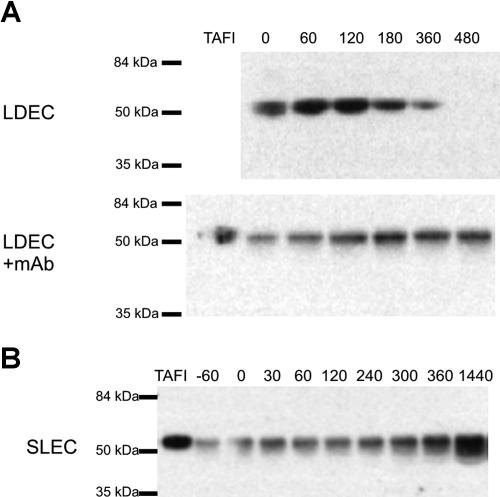
Effect of mAbTAFI/TM#16 on TAFI protein in septic baboons
Using Western blotting, TAFI was monitored in plasma samples taken from experimental animals immediately prior to and at various times following E coli infusion. Baboons were challenged with either a LDEC or SLEC, which influenced the duration of the experiment by 6 and 24 hours, respectively. Samples were probed with either monoclonal or polyclonal anti-TAFI antibodies and the results were identical (data not shown). Figure 6A illustrates that full-length TAFI is reduced but detectable in the plasma of animals receiving a LDEC until approximately 360 minutes after E coli challenge. However, TAFI is completely consumed by 480 minutes. In contrast, coadministration of the mAbTAFI/TM#16 with the LDEC completely abrogated consumption of TAFI as TAFI was detected at all time points. This indicates that the mAbTAFI/TM#16 decreases the activation and, indirectly, the resulting clearance of TAFIa observed with the LDEC. TAFIa can be observed in Western blots of baboon plasma samples under certain conditions (data not shown), however, in the current experiments either TAFIa was cleared too rapidly to be observed or TAFIa did not reach adequate concentrations for detection by Western blot. No difference was observed in TAFI antigen between animals receiving an SLEC alone or with the mAbTAFI/TM#16 infusion, and an increase in TAFI concentration was observed over the study period (Figure 6B). This observation is consistent with the observation that TAFI is an acute-phase protein in mice.36
Figure 6.
Rapid consumption of TAFI protein occurs with a lethal dose of E coli (LDEC), however treatment with the mAbTAFI/TM#16 slows consumption; TAFI consumption is not observed in the 24-hour period following a sublethal dose of E coli (SLEC). (A) Plasma samples from a representative animal that received a LDEC reveal consumption of TAFI protein at 480 minutes after E coli infusion by Western blotting. When the LDEC treatment was administered concurrently with the mAbTAFI/TM#16, rapid consumption of TAFI was not observed. (B) A representative animal that received a SLEC was monitored for 24 hours after E coli treatment. TAFI protein was present in all plasma samples and no difference was observed in the absence or presence (data not shown) of mAbTAFI/TM#16 infusion.
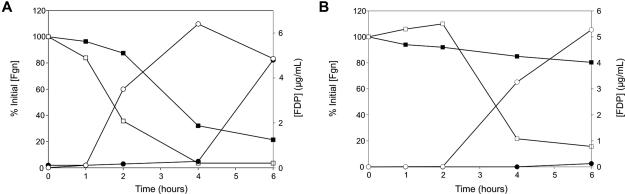
Rates of fibrinogen consumption and FDP production are increased in both LDEC and SLEC by the mAbTAFI/TM#16
We expected that because mAbTAFI/TM#16 prevents activation of TAFI and TAFIa inhibits fibrinolysis increased concentrations of markers of fibrinolysis would be observed when the mAbTAFI/TM#16 was administered. FDP concentrations were measured in plasma samples up to 6 hours after E coli challenge (Figure 7). Similar extents of fibrinogen losses and FDP increases were observed in 3 animals receiving a LDEC with or without concurrent mAbTAFI/TM#16 administration. Consumption of fibrinogen to 50% of initial values occurs earlier with the mAbTAFI/TM#16 (1.5 hours after E coli challenge vs 3.5 hours, Figure 7A). When the challenge was a SLEC without mAbTAFI/TM#16 treatment, a minimal loss in fibrinogen without change in FDP concentrations was observed. In contrast, with concurrent mAbTAFI/TM#16 treatment, SLEC-treated animals displayed a loss in fibrinogen and increase in FDP similar to those observed in the LDEC-treated animals occurring at 3.5 hours after E coli challenge (Figure 7B). These data indicate that activation of TAFI plays a key role in determining the extent of fibrinogen consumption as well as FDP formation induced during E coli challenge.
Figure 7.
Rates of fibrinogen (Fgn) loss and fibrin degradation product (FDP) production are enhanced in both lethal (LDEC) and sublethal (SLEC) doses of E coli by the mAbTAFI/TM#16. (A) In a representative animal that received a LDEC, Fgn loss (■, □) and FDP production (●, ○) in the absence of mAbTAFI/TM#16 (■, ●) occur slowly compared with in the presence of the mAbTAFI/TM#16 (□, ○). (B) After receiving a SLEC, Fgn loss (■) occurs slowly and FDP production (●) does not increase in the absence of mAbTAFI/TM#16; however in the presence of mAbTAFI/TM#16, both Fgn loss (□) and FDP production (○) were observed.
Discussion
Several investigations to establish a (patho)physiologic role for TAFI have been published.37–45 The antifibrinolytic effect of TAFIa in thrombolysis has been demonstrated in rabbits both by blocking the activity of TAFIa with a carboxypeptidase inhibitor derived from the potato tuber and by using a monoclonal antibody against factor XI to inhibit thrombin formation and subsequent TAFI activation.37,38 Others show that TAFI mRNA and protein levels are up-regulated in an abdominal sepsis model using wild-type mice. However, the role of TAFI, if any, is not immediately apparent because comparison of the effects of abdominal sepsis in wild-type and TAFI−/− mice disclosed neither an effect on fibrin deposition nor a differential dysregulation of hemostasis.39 In rats treated with high concentrations of endotoxin, TAFI was depleted rapidly in comparison with those treated with low-dose endotoxin where TAFI dropped slightly and then became significantly elevated. Depletion of TAFI was often associated with death of the animals, and this was attributed to lack of inactivation of anaphylatoxin by TAFIa.40,41 Concentrations of TAFI in humans, determined both by antigen and function, where function was determined by the conversion of the zymogen TAFI to active TAFIa, are reduced in cases of sepsis, disseminated intravascular coagulation (DIC), dengue hemorrhagic fever, and exposure of healthy individuals to endotoxin.42–45 In the latter investigations, elevated concentrations of soluble thrombomodulin and thrombin-antithrombin III (TAT) complexes appear to accompany the decrease in TAFI. It was concluded that in these conditions TAFI is activated and cleared. Contradictory results observed in small-animal models of sepsis and the human population might be related to the magnitude of the infection. Studies conducted in large-animal models, particularly nonhuman primates, are likely required to adequately understand the activation of TAFI in sepsis and to potentially expose a (patho)physiologic role for TAFI.
Elastase, trypsin, plasmin, and thrombin all activate TAFI in vitro.5,6,9,16–19 A compelling argument that the (patho)physiologic activator of TAFI is plasmin in conjunction with proteoglycans has been presented.17 The reasoning is based upon the Km for the activation of TAFI by both plasmin (55 nM) and plasmin-heparin (20 nM),17 which approximates the physiologic concentration of TAFI (100 nM) more closely than do the Km values of the other likely activators, thrombin (2140 nM) and thrombin-thrombomodulin (1100 nM).21 However, relatively high Km values for activation of TAFI by thrombin, with and without thrombomodulin, do not preclude the possibility that thrombin is a (patho)physiologic activator of TAFI. The catalytic efficiency (kcat/Km) for TAFI activation is greater for plasmin with and without heparin (0.13 and 0.008 μM−1s−1, respectively)17 than it is for thrombin (0.00098 μM−1s−1). However, the catalytic efficiency for thrombin-thrombomodulin activation (1.2 μM−1s−1)21 is 10-fold greater than for plasmin. This supports the concept that thrombin-thrombomodulin is a major physiologic activator of TAFI. Kinetics describing the various activation reactions in vitro cannot, by themselves, disclose the identity of the (patho)physiologic activator.
In addition to the simple chromolytic assay (Figure 1), the specificity of mAbTAFI/TM#16 to inhibit thrombomodulin and TAFIa-dependent inhibition of fibrinolysis was confirmed using an in vitro clot lysis assay using baboon plasma (Figure 2). This suggested the potential to use the mAbTAFI/TM#16 to identify the activator of TAFI in a baboon during sepsis. The mechanism by which the mAbTAFI/TM#16 is specific to thrombin-thrombomodulin activation of TAFI was not unambiguously determined, however, the antibody probably does not bind TAFI straddling the scissile bond because it does not prevent cleavage by all activators. It is possible that steric hindrance, whereby the antibody binds TAFI proximally to the scissile bond, prevents access or proper orientation of the relatively large thrombin-thrombomodulin complex. Species differences in regulating TAFI activation need to be considered.
The dependence of thrombomodulin as a cofactor for thrombin activation of bTAFI was confirmed (Figure 3), but unexpectedly it appears that bTAFIa has a larger effect on clot lysis than does hTAFIa. The effect of TAFIa maximally prolongs clot lysis by approximately 10-fold in clots formed from baboon plasma compared with only a 3-fold prolongation observed in clots formed with human plasma.46 The mechanism underlying this difference in effect of TAFIa is unclear; however, preliminary work appears to exclude the potential of bTAFIa being more stable than hTAFIa (data not shown). The role of thrombin in the activation of bTAFI to elicit an antifibrinolytic effect is also demonstrated in Figures 2,3. Inclusion of TAP blocks endogenous thrombin function, limiting the thrombin concentration in the clot to the input concentration of 6 nM human thrombin used to initiate clot formation. This difference in lysis time with or without TAP represents the contribution of thrombin-dependent TAFI activation on lysis time. Clots treated with TAP lysed in approximately 14 minutes, which is comparable with lysis times observed for clots formed in the presence of excess PTCI where all TAFIa activity is inhibited. In the absence of TAP, thrombin concentrations approximating quantitative activation of one third plasma concentrations of baboon prothrombin are likely achieved and result in a prolongation of lysis time from approximately 14 to approximately 125 minutes. Whether this reflects increased bTAFIa activity or increased thrombin-dependent activation of bTAFI relative to the human form is unknown. The lack of TAFIa-dependent prolongation at low thrombin concentrations suggests a reasonably high Km for activation of bTAFI as observed for hTAFI activation.
Analysis of Western blots of TAFI protein indicate complete consumption of TAFI without a concomitant increase in TAFIa in baboons receiving a LDEC. However, infusion of mAbTAFI/TM#16 prevents consumption of TAFI (Figure 6A). Thus, due to the specificity of the antibody, in a baboon model of E coli–induced sepsis, TAFI activation appears to occur primarily through a thrombin-thrombomodulin–dependent reaction. Despite the potential for high concentrations of both thrombin and plasmin, significant consumption of TAFI is not detectable by Western blotting. Further, in this model, activation of TAFI plays a role in determining the extent of fibrinolysis, as monitored by circulating concentrations of FDP, induced during both a SLEC and a LDEC challenge (Figure 7). Consumption of fibrinogen following an infusion of either SLEC or LDEC appears to increase by inhibition of TAFI activation, possibly indicating fibrinogenolysis. Therefore, TAFIa may play a critical role in reducing both/either fibrinolysis and fibrinogenolysis by limiting plasminogen activation, perhaps to local environments. A mechanism describing this effect is not unambiguously disclosed by the current study; however, some candidate mechanisms are more plausible than others. Circulating FDP could promote plasminogen activation and contribute to systemic fibrinogenolysis. The cofactor activity of FDP could be modified by TAFIa to down-regulate both plasminogen activation and subsequent fibrin(ogen)olysis. However, significant concentrations of circulating TAFIa would be required to act systemically to decrease the cofactor activity of FDP in comparison with low nanomolar concentrations of TAFIa required to act locally. In only one case was TAFIa detected by Western blots of plasma samples from challenged animals (data not shown). Therefore, the effects of TAFIa on fibrin(ogen)olysis may be due to limited plasminogen activation independent of fibrin and FDP or independent of plasminogen entirely. Although demonstrating the presence of TAFIa with the LDEC or activation of TAFI with SLEC has proved difficult, we are able to demonstrate its effect on fibrinolysis in both instances.
Previously it was shown in vitro that the coagulation and fibrinolytic cascades are coupled through the thrombomodulin-dependent activation of TAFI.5,21,35 However, this concept has not been extended to a (patho)physiologic condition. Here, we demonstrate that in severe sepsis, TAFI is rapidly activated and cleared. Further, we demonstrate the thrombomodulin-dependent consumption/activation of TAFI in vivo in a nonhuman primate model of sepsis. Inhibition of TAFI activation increases FDP generation. Together these data are consistent with the idea that thrombin-thrombomodulin is the primary (patho)physiologic activator of TAFI and that TAFIa down-regulates fibrinolytic activity. The possible contribution of TAFIa in regulation of fibrinogen consumption was an unexpected complication but may be explained by decreased plasmin-mediated fibrinogenolysis; however other mechanisms are possible. We conclude that thrombomodulin and TAFI link coagulation and fibrinolysis in vivo in certain pathologic challenges such as E coli–induced sepsis. Further, we demonstrate the potential clinical utility of a monoclonal antibody specifically inhibiting TAFI activation.
Acknowledgments
This work was supported by funding from the Heart and Stroke Foundation of Canada and the Canadian Institutes of Health Research (HL-04–005) and the National Institutes of Health/National Heart, Lung and Blood Institute (1R01 HL078658–01) to L.B. and from the National Institutes of Health/National Heart, Lung and Blood Institute R01 GM37704–16 to F.B.T.
This work would not have been possible without the generous contributions of TAP from Dr S. Krishnaswamy, mAbTAFI/TM#16 from Drs. M.E. Nesheim and K. Mann, as well as Solulin from Paion.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: F.B.T. and L.B. designed research; L.B. and T.M.B. drafted the paper. All authors analyzed data, interpreted data, and reviewed the paper.
Conflict-of-interest disclosure: Two of the authors (F.B.T. and L.B.) hold a patent related to the work that is described in the present study (US patent no. 6,838,432), and L.B. is a coholder on a patent for the use of inhibitory antibodies against TAFI (US patent no. 5,993,815). The other authors declare no competing financial interests.
Correspondence: Laszlo Bajzar, Department of Pediatrics, Faculty of Medicine, University of Alberta, 1–130 Dentistry Pharmacy Centre, 11304 89 Avenue NW, Edmonton, AB, T6G 2N8 Canada; e-mail: lbajzar@ualberta.ca.
References
- 1.Suenson E, Lutzen O, Thorsen S. Initial plasmin-degradation of fibrin as the basis of a positive feed-back mechanism in fibrinolysis. Eur J Biochem. 1984;140:513–522. doi: 10.1111/j.1432-1033.1984.tb08132.x. [DOI] [PubMed] [Google Scholar]
- 2.Norrman B, Wallen P, Ranby M. Fibrinolysis mediated by tissue plasminogen activator. Disclosure of a kinetic transition. Eur J Biochem. 1985;149:193–200. doi: 10.1111/j.1432-1033.1985.tb08911.x. [DOI] [PubMed] [Google Scholar]
- 3.Suenson E, Bjerrum P, Holm A, et al. The role of fragment X polymers in the fibrin enhancement of tissue plasminogen activator-catalyzed plasmin formation. J Biol Chem. 1990;265:22228–22237. [PubMed] [Google Scholar]
- 4.Fredenburgh JC, Nesheim ME. Lys-plasminogen is a significant intermediate in the activation of Glu-plasminogen during fibrinolysis in vitro. J Biol Chem. 1992;267:26150–26156. [PubMed] [Google Scholar]
- 5.Bajzar L, Manuel R, Nesheim ME. Purification and characterization of TAFI, a thrombin-activable fibrinolysis inhibitor. J Biol Chem. 1995;270:14477–14484. doi: 10.1074/jbc.270.24.14477. [DOI] [PubMed] [Google Scholar]
- 6.Eaton DL, Malloy BE, Tsai SP, Henzel W, Drayna D. Isolation, molecular cloning, and partial characterization of a novel carboxypeptidase B from human plasma. J Biol Chem. 1991;266:21833–21838. [PubMed] [Google Scholar]
- 7.Campbell W, Okada H. An arginine specific carboxypeptidase generated in blood during coagulation or inflammation which is unrelated to carboxypeptidase N or its subunits. Biochem Biophys Res Commun. 1989;162:933–939. doi: 10.1016/0006-291x(89)90762-6. [DOI] [PubMed] [Google Scholar]
- 8.Hendriks D, Scharpe S, van SM, Lommaert MP. Characterisation of a carboxypeptidase in human serum distinct from carboxypeptidase N. J Clin Chem Clin Biochem. 1989;27:277–285. doi: 10.1515/cclm.1989.27.5.277. [DOI] [PubMed] [Google Scholar]
- 9.Wang W, Hendriks DF, Scharpe SS. Carboxypeptidase U, a plasma carboxypeptidase with high affinity for plasminogen. J Biol Chem. 1994;269:15937–15944. [PubMed] [Google Scholar]
- 10.Redlitz A, Tan AK, Eaton DL, Plow EF. Plasma carboxypeptidases as regulators of the plasminogen system. J Clin Invest. 1995;96:2534–2538. doi: 10.1172/JCI118315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Boffa MB, Bajzar L, Walker JB, Nesheim ME. A study of the mechanism of inhibition of fibrinolysis by activated thrombin-activable fibrinolysis inhibitor. J Biol Chem. 1998;273:27176–27181. doi: 10.1074/jbc.273.42.27176. [DOI] [PubMed] [Google Scholar]
- 12.Walker JB, Bajzar L. The intrinsic threshold of the fibrinolytic system is modulated by basic carboxypeptidases, but the magnitude of the antifibrinolytic effect of activated thrombin-activable fibrinolysis inhibitor is masked by its instability. J Biol Chem. 2004;279:27896–27904. doi: 10.1074/jbc.M401027200. [DOI] [PubMed] [Google Scholar]
- 13.Walker JB, Bajzar L. Complete inhibition of fibrinolysis by sustained carboxypeptidase B activity: the role and requirement of plasmin inhibitors. J Thromb Haemost. 2007;5:1257–1264. doi: 10.1111/j.1538-7836.2007.02541.x. [DOI] [PubMed] [Google Scholar]
- 14.Van Tilburg NH, Rosendaal FR, Bertina RM. Thrombin activatable fibrinolysis inhibitor and the risk for deep vein thrombosis. Blood. 2000;95:2855–2859. [PubMed] [Google Scholar]
- 15.Willemse JL, Hendriks DF. Measurement of procarboxypeptidase U (TAFI) in human plasma: a laboratory challenge. Clin Chem. 2006;52:30–36. doi: 10.1373/clinchem.2005.055814. [DOI] [PubMed] [Google Scholar]
- 16.Tan AK, Eaton DL. Activation and characterization of procarboxypeptidase B from human plasma. Biochemistry. 1995;34:5811–5816. doi: 10.1021/bi00017a012. [DOI] [PubMed] [Google Scholar]
- 17.Mao SS, Cooper CM, Wood T, Shafer JA, Gardell SJ. Characterization of plasmin-mediated activation of plasma procarboxypeptidase B: modulation by glycosaminoglycans. J Biol Chem. 1999;274:35046–35052. doi: 10.1074/jbc.274.49.35046. [DOI] [PubMed] [Google Scholar]
- 18.Marx PF, Dawson PE, Bouma BN, Meijers JC. Plasmin-mediated activation and inactivation of thrombin-activatable fibrinolysis inhibitor. Biochemistry. 2002;41:6688–6696. doi: 10.1021/bi015982e. [DOI] [PubMed] [Google Scholar]
- 19.Kawamura T, Okada N, Okada H. Elastase from activated human neutrophils activates procarboxypeptidase R. Microbiol Immunol. 2002;46:225–230. doi: 10.1111/j.1348-0421.2002.tb02690.x. [DOI] [PubMed] [Google Scholar]
- 20.Esmon NL, Owen WG, Esmon CT. Isolation of a membrane-bound cofactor for thrombin-catalyzed activation of protein C. J Biol Chem. 1982;257:859–864. [PubMed] [Google Scholar]
- 21.Bajzar L, Morser J, Nesheim M. TAFI, or plasma procarboxypeptidase B, couples the coagulation and fibrinolytic cascades through the thrombin-thrombomodulin complex. J Biol Chem. 1996;271:16603–16608. doi: 10.1074/jbc.271.28.16603. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Nagashima M, Schneider M, Morser J, Nesheim M. Elements of the primary structure of thrombomodulin required for efficient thrombin-activable fibrinolysis inhibitor activation. J Biol Chem. 2000;275:22942–22947. doi: 10.1074/jbc.M001760200. [DOI] [PubMed] [Google Scholar]
- 23.Marlar RA, Kleiss AJ, Griffin JH. Mechanism of action of human activated protein C, a thrombin-dependent anticoagulant enzyme. Blood. 1982;59:1067–1072. [PubMed] [Google Scholar]
- 24.Griffin JH, Mosher DF, Zimmerman TS, Kleiss AJ. Protein C, an antithrombotic protein, is reduced in hospitalized patients with intravascular coagulation. Blood. 1982;60:261–264. [PubMed] [Google Scholar]
- 25.Taylor FB, Jr, Chang A, Esmon CT, et al. Protein C prevents the coagulopathic and lethal effects of Escherichia coli infusion in the baboon. J Clin Invest. 1987;79:918–925. doi: 10.1172/JCI112902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor FB, Jr, Emerson TE, Jr, Jordan R, Chang AK, Blick KE. Antithrombin-III prevents the lethal effects of Escherichia coli infusion in baboons. Circ Shock. 1988;26:227–235. [PubMed] [Google Scholar]
- 27.Wada H, Yamamuro M, Inoue A, et al. Comparison of the responses of global tests of coagulation with molecular markers of neutrophil, endothelial, and hemostatic system perturbation in the baboon model of E. colisepsis: toward a distinction between uncompensated overt DIC and compensated non-overt DIC. Thromb Haemost. 2001;86:1489–1494. [PubMed] [Google Scholar]
- 28.Taylor FB., Jr Staging of the pathophysiologic responses of the primate microvasculature to Escherichia coli and endotoxin: examination of the elements of the compensated response and their links to the corresponding uncompensated lethal variants. Crit Care Med. 2001;29:S78–S89. doi: 10.1097/00003246-200107001-00026. [DOI] [PubMed] [Google Scholar]
- 29.Walker JB, Nesheim ME. The molecular weights, mass distribution, chain composition, and structure of soluble fibrin degradation products released from a fibrin clot perfused with plasmin. J Biol Chem. 1999;274:5201–5212. doi: 10.1074/jbc.274.8.5201. [DOI] [PubMed] [Google Scholar]
- 30.Bajzar L, Nesheim ME, Tracy PB. The profibrinolytic effect of activated protein C in clots formed from plasma is TAFI-dependent. Blood. 1996;88:2093–2100. [PubMed] [Google Scholar]
- 31.Hougie C. Methods of estimating fibrinogen concentration-thrombin time test. In: Williams WJ, Beutler E, Erslev AJ, Lichtman MA, editors. Hematology. New York, NY: McGraw-Hill; 1983. pp. 1667–1668. [Google Scholar]
- 32.Wellcome Research Laboratories. Thombo-Wellcotest (Rapid Latex Kit) Purley, Surrey, United Kingdom: Southem Press; 1984. Fibrinogen Degredation Products. Ref Type: Pamphlet. [Google Scholar]
- 33.Neville DM., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971;246:6328–6334. [PubMed] [Google Scholar]
- 34.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bajzar L, Nesheim M. The effect of activated protein C on fibrinolysis in cell-free plasma can be attributed specifically to attenuation of prothrombin activation. J Biol Chem. 1993;268:8608–8616. [PubMed] [Google Scholar]
- 36.Sato T, Miwa T, Akatsu H, et al. Pro-carboxypeptidase R is an acute phase protein in the mouse, whereas carboxypeptidase N is not. J Immunol. 2000;165:1053–1058. doi: 10.4049/jimmunol.165.2.1053. [DOI] [PubMed] [Google Scholar]
- 37.Nagashima M, Werner M, Wang M, et al. An inhibitor of activated thrombin-activatable fibrinolysis inhibitor potentiates tissue-type plasminogen activator-induced thrombolysis in a rabbit jugular vein thrombolysis model. Thromb Res. 2000;98:333–342. doi: 10.1016/s0049-3848(00)00184-5. [DOI] [PubMed] [Google Scholar]
- 38.Minnema MC, Friederich PW, Levi M, et al. Enhancement of rabbit jugular vein thrombolysis by neutralization of factor XI: in vivo evidence for a role of factor XI as an anti-fibrinolytic factor. J Clin Invest. 1998;101:10–14. doi: 10.1172/JCI781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renckens R, Roelofs JJ, ter Horst SA, et al. Absence of thrombin-activatable fibrinolysis inhibitor protects against sepsis-induced liver injury in mice. J Immunol. 2005;175:6764–6771. doi: 10.4049/jimmunol.175.10.6764. [DOI] [PubMed] [Google Scholar]
- 40.Kato K, Shinagawa N, Hayakawa T, et al. Changes in arginine carboxypeptidase (CPR) activity in stressed rats. Pathophysiology. 1994;1:131–136. [Google Scholar]
- 41.Campbell WD, Lazoura E, Okada N, Okada H. Inactivation of C3a and C5a octapeptides by carboxypeptidase R and carboxypeptidase N. Microbiol Immunol. 2002;46:131–134. doi: 10.1111/j.1348-0421.2002.tb02669.x. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe R, Wada H, Watanabe Y, et al. Activity and antigen levels of thrombin-activatable fibrinolysis inhibitor in plasma of patients with disseminated intravascular coagulation. Thromb Res. 2001;104:1–6. doi: 10.1016/s0049-3848(01)00331-0. [DOI] [PubMed] [Google Scholar]
- 43.Zeerleder S, Schroeder V, Hack CE, Kohler HP, Wuillemin WA. TAFI and PAI-1 levels in human sepsis. Thromb Res. 2006;118:205–212. doi: 10.1016/j.thromres.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 44.van Gorp EC, Minnema MC, Suharti C, et al. Activation of coagulation factor XI, without detectable contact activation in dengue haemorrhagic fever. Br J Haematol. 2001;113:94–99. doi: 10.1046/j.1365-2141.2001.02710.x. [DOI] [PubMed] [Google Scholar]
- 45.Verbon A, Meijers JC, Spek CA, et al. Effects of IC14, an anti-CD14 antibody, on coagulation and fibrinolysis during low-grade endotoxemia in humans. J Infect Dis. 2003;187:55–61. doi: 10.1086/346043. [DOI] [PubMed] [Google Scholar]
- 46.Bajzar L, Nesheim M, Morser J, Tracy PB. Both cellular and soluble forms of thrombomodulin inhibit fibrinolysis by potentiating the activation of thrombin-activable fibrinolysis inhibitor. J Biol Chem. 1998;273:2792–2798. doi: 10.1074/jbc.273.5.2792. [DOI] [PubMed] [Google Scholar]