Abstract
Recently, activating myeloproliferative leukemia virus oncogene (MPL) mutations, MPLW515L/K, were described in myeloproliferative disorder (MPD) patients. MPLW515L leads to activation of downstream signaling pathways and cytokine-independent proliferation in hematopoietic cells. The adaptor protein Lnk is a negative regulator of several cytokine receptors, including MPL. We show that overexpression of Lnk in Ba/F3-MPLW515L cells inhibits cytokine-independent growth, while suppression of Lnk in UT7-MPLW515L cells enhances proliferation. Lnk blocks the activation of Jak2, Stat3, Erk, and Akt in these cells. Furthermore, MPLW515L-expressing cells are more susceptible to Lnk inhibitory functions than their MPL wild-type (MPLWT)–expressing counterparts. Lnk associates with activated MPLWT and MPLW515L and colocalizes with the receptors at the plasma membrane. The SH2 domain of Lnk is essential for its binding and for its down-regulation of MPLWT and MPLW515L. Lnk itself is tyrosine-phosphorylated following thrombopoietin stimulation. Further elucidating the cellular pathways that attenuate MPLW515L will provide insight into the pathogenesis of MPD and could help develop specific therapeutic approaches.
Introduction
Myeloproliferative disorders (MPDs) are a group of diseases characterized by increased numbers of one or several of the myeloid lineages. The activating JAK2 mutation, JAK2V617F, associated with these disorders, leads to constitutive activation of JAK2 without the need of ligand binding to hematopoietic receptors.1,2 Recently, gain-of-function mutations in the myeloproliferative leukemia virus oncogene (MPL) receptor (Tpo receptor), MPLW515L/K, were described in a subset of MPD patients,3,4 suggesting that proteins implicated in the MPL signaling axis could represent targets for therapeutic interventions in MPD. One such protein may be Lnk, an adaptor protein that is expressed predominantly in hematopoietic tissues and has critical roles in hematopoiesis.5–10 Animal model studies demonstrated that Lnk acts as a broad inhibitor of signaling pathways. Among pathways inhibited by Lnk are Thrombopoietin (Tpo)–induced proliferation and differentiation of megakaryocytes and Tpo-induced self-renewal in hematopoietic stem cells and myeloid progenitors.11,12 Lnk belongs to a family of proteins that share several structural motifs, including a Src homology 2 (SH2) domain, which binds phosphotyrosines in various signal-transducing proteins.13–15 The SH2 domain is essential for Lnk-mediated negative regulation of several cytokine receptors (ie, MPL, EPOR, and c-KIT).8,11,16 In the present study, we asked whether Lnk can attenuate the constitutive activity of MPLW515L
Materials and methods
Mice and cell culture
Lnk-deficient mice were generously provided by T. Pawson (Samuel Lunenfeld Research Institute, Toronto, Canada). Bone marrow cells derived from 6-week-old wild-type (WT) and Lnk-deficient 129/Sv mice were cultured in Iscove modified Dulbecco medium (IMDM) with 5% fetal bovine serum (FBS), interleukin-3 (IL-3) (10 ng/mL) and granulocyte-macrophage colony-stimulating factor (GM-CSF) (10 ng/mL) for 7 days. The cells were then washed and resuspended in IMDM with 5% FBS and Tpo (50 ng/mL) for 3 additional days to enrich the numbers of megakaryocytes. Lysates from 3 × 106 cells were used for immunoprecipitation. A portion of the cells was subjected to FACS analysis with fluorescein isothiocyanate (FITC)-CD41 antibody (1:200; BD Biosciences, Palo Alto, CA), which demonstrated that 40% of Lnk+/+ and 60% of Lnk−/− cells were CD41-postive. UT7/MPLW515L cells were generously provided by R.L. Levine (Harvard Medical School, Boston, MA) and were grown in RPMI medium supplemented with 10% FBS and GM-CSF (1 ng/mL). Ba/F3 cells were grown in RPMI medium 1640 containing 10% FBS and 10% WEHI-3B cell supernatant as a source of IL-3. 293T cells were grown in Dulbecco modified Eagle medium with 10% fetal calf serum (FCS). To generate stable cell lines, Ba/F3 cells were transduced with retroviral supernatant containing either MSCV-MPLWT-GFP or MSCV-MPLW515L-GFP vectors, and then sorted by flow cytometry to isolate green fluorescence protein (GFP)–positive cells. Stable Ba/F3 and UT7 cell lines were electroporated with either expression vectors or siRNA vectors and then selected in 1 mg/mL G418 (Ba/F3 cells) or 1 μg/mL puromycin (UT7 cells) either with or without Tpo (1 ng/mL). The number of viable cells was determined by trypan blue exclusion. 293T cells were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA).
Expression vectors
Human MPL and Lnk cDNAs were cloned into the retroviral murine stem cell virus (MSCV)–GFP and the pcDNA3.1 vectors, respectively. The MPLW515L mutant, Lnk mutant, and GST-Lnk fusion vectors were generated by polymerase chain reaction (PCR). The siRNA vectors targeting Lnk were purchased from Open Biosystems (Huntsville, AL).
RNA and protein analysis
Real-time reverse transcriptase–polymerase chain reaction (RT-PCR) was performed with an iCycler iQ system (Biorad, Hercules, CA). The following antibodies were used for immunoprecipitation and Western blot analysis: anti-Lnk (Serotec, Raleigh, NC); anti-MPL (Upstate Biotechnology, Lake Placid, NY); anti–phospho-JAK2, anti-JAK2, anti–phospho-Akt, and anti–phospho-STAT3 (Cell Signaling Technology, Beverly, MA); anti–phospho-Erk, anti-Akt, and anti-STAT3 (Santa Cruz Biotechnology, Santa Cruz, CA). For coimmunoprecipitation, lysates were incubated with appropriate antibodies and protein A/G agarose beads (Santa Cruz Biotechnology) at 4°C for 16 hours. Glutathione-S–transferase (GST) pull-down assays were carried out with equal amounts of GST and GST-Lnk SH2 proteins immobilized on glutathione-sepharose beads (Amersham Pharmacia Biotech, Piscataway, NJ). Precipitated proteins were washed 3 times with phosphate buffered saline (PBS), eluted with sodium dodecyl sulfate (SDS) sample buffer, and subjected to Western blot analysis.
Confocal microscopy
Cells were fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, and blocked with 3% milk. Fixed cells were incubated with first antibodies, V5 (Invitrogen) and MPL, and then with secondary Alexa 647-conjugated (Invitrogen) and tetramethylrhodamine-5(and 6)-isothiocyanate (TRITC)–conjugated (Sigma, St Louis, MO) antibodies. Signals were detected using a Leica TCS SP confocal microscope system (Leica Microsystems, Mannheim, Germany) equipped with a 63× oil objective (PLAPO 100.0×/1.40 oil UV) and Confocal Software (Leica).
Results
Lnk inhibits cytokine-independent and Tpo-stimulated growth and signaling pathways induced by MPLW515L
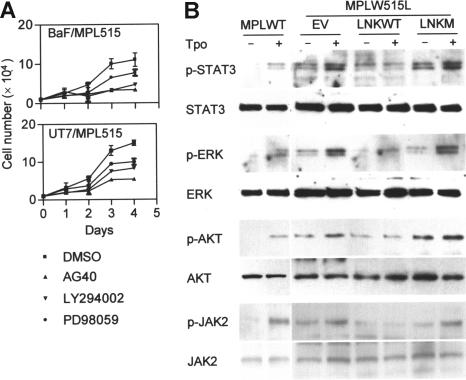
Expression of MPLW515L in hematopoietic cells confers cytokine-independent growth and leads to Tpo hypersensitivity compared with cells expressing wild-type MPL (MPLWT). Since Lnk inhibits the MPLWT signaling, we hypothesized that the high activity of MPLW515L could be attributed, at least in part, to loss of negative regulation by Lnk. To test this, we measured the expression levels of Lnk in cytokine-dependent hematopoietic cell lines, Ba/F3 (murine) and UT7 (human megakaryocytic leukemia), stably expressing either MPLWT or MPLW515L. Real-time PCR (Ba/F3 and UT7) and Western blot (UT7) analysis demonstrated that Lnk levels are actually higher in MPLW515L-expressing cells (Figure 1A-B). Next, we examined whether the constitutive active MPLW515L is still susceptible to negative regulation by Lnk. BaF/MPL515 were transfected with Lnk and then cultured in cytokine-free medium in the presence of antibiotic selection (Figure 1C,E). Lnk expression led to significant growth inhibition compared with control empty vector transfected cells. Lnk also suppressed the growth of BaF/MPL515 and BaF/MPLWT cells cultured with Tpo. Moreover, while the proliferation rate of empty vector transfected BaF/MPLWT and BaF/MPL515 cells was similar, the latter were more sensitive to growth inhibition by Lnk; by day 9 of antibiotic selection, no viable cells could be detected in the BaF/MPL515 culture, whereas in the BaF/MPLWT culture, slow growth continued. A mutation disrupting Lnk SH2 domain, R392E, completely abolished the ability of Lnk to inhibit the proliferation of Ba/F3 cells expressing either the MPLWT or the MPLW515L receptors. siRNA knockdown of Lnk in UT7/MPL515 cells enhanced their proliferation, demonstrating that endogenous expressed Lnk has an important role in attenuating MPLW515L (Figures 1D,E).
Figure 1.
Lnk inhibits cytokine-independent and Tpo-stimulated growth in MPLW515L-expressing cells. (A-B) Real-time PCR (A) and Western blot (B) analysis of Lnk expression in Ba/F3 and UT7 cells stably expressing either MPLWT (WT) or MPLW515L (W515L). Relative mRNA expression levels are expressed in arbitrary units as a ratio of Lnk transcripts/18S transcripts (each value represents the mean (± SD) of 3 measurements of the sample). (C-D) BaF/MPLWT and BaF/MPL515 cells (C) were transfected by electroporation with either empty vector (EV), wild-type Lnk (LNKW), or an SH2 mutant Lnk (LNKM); UT7/MPL515 cells (D) were similarly transfected with either empty vector (EV), siRNA control vector (siC), or siLNK vectors (siL1 and siL2). Two days later, cells were cultured in selection media either with or without Tpo (1 ng/mL). Proliferation was measured by cell counts. Data represent the mean (± SD) of duplicate samples and are representative of 3 independent experiments. (E) Western blot analysis of Lnk expression in transfected Ba/F3 and UT7 cells. β-actin was used to control for equal loading and siRNA specificity.
In MPLW515L-expressing cells, various signaling molecules, including those in the JAK/STAT, MAPK p42/44, and PI3K pathways, are constitutively phosphorylated, and their activation is further enhanced by Tpo.3 The involvement of these pathways in MPLW515L-induced cell growth was examined with specific inhibitors: AG490 (JAK2 inhibitor), LY294002 (PI3K inhibitor), and PD98059 (MAPK inhibitor). All 3 inhibitors reduced the growth rate of BaF/MPL515 and UT7/MPL515 cells (Figure 2A), indicating that these signaling cascades are important for MPLW515L-mediated cell proliferation. To determine whether Lnk inhibition of cell proliferation is associated with JAK/STAT, MAPK, and PI3K signaling, we assessed the phosphorylation levels of Jak2, Stat3, p42/44 Erk, and Akt (downstream PI3K) in BaF/MPL515 cells. Overexpression of Lnk, but not Lnk R392E, inhibited the constitutive, as well as the Tpo-induced, phosphorylation of these molecules (Figure 2B).
Figure 2.
Lnk inhibits signaling pathways induced by MPLW515L. (A) BaF/MPL515 and UT7/MPL515 cells were treated with either 25 μM AG490 (JAK2 inhibitor), 16 μM LY294002 (PI3K inhibitor), 25 μM PD98059 (MAPK inhibitor), or dimethyl sulfoxide (DMSO) (inhibitor solvent). Proliferation was measured by cell counts. Data represent the mean (± SD) of duplicate samples and are representative of 2 independent experiments. (B) Transfected BaF/MPL515 and nontransfected BaF/MPLWT cells were depleted of cytokines for 4 hours and then stimulated with Tpo (1 ng/mL, 30 minutes). Protein lysates were analyzed by Western blot for the phosphorylated and total protein levels of Stat3, Erk, and Akt. Lysates from similarly treated cells were immunoprecipitated with JAK2 antibody and analyzed by Western blot with phospho-JAK2 and JAK2 antibodies.
Lnk via its SH2 domain interacts with MPLWT and MPLW515L
The molecular mechanisms by which Lnk inhibits Tpo signaling are largely unknown. We next wanted to determine whether Lnk can physically interact with the MPLWT and MPLW515L receptors. Murine bone marrow (BM) cells were cultured with Tpo to enrich the population of cells expressing MPL. Immunoprecipitation experiments demonstrated the presence of Lnk in MPL immunocomplexes, while a band corresponding to Lnk was not detected in BM cells form Lnk nullizygous mice (Figure 3A). Lnk also bound to mutant MPLW515L in UT7 cells, and the interaction was stronger following Tpo stimulation (Figure 3B). Similarly, Lnk was found in MPLWT immunocomplexes in Tpo-treated 293T cells overexpressing Lnk and MPLWT (Figure 3C). In contrast, Lnk did not associate with MPLWT in untreated cells. The Lnk-MPL interaction may, therefore, depend on MPL phosphorylation. In support of this, Lnk association with MPLW515L, which is constitutively activated, was readily detected in untreated 293T cells and was enhanced by Tpo. Furthermore, the binding of Lnk to MPLW515L appears to be stronger than its binding to MPLWT. Reciprocal immunoprecipitation with Lnk antibody showed that indeed Lnk immunocomplexes contained more MPLW515L than MPLWT when the receptors were expressed at equal levels. Lnk protein carrying the SH2 domain mutation, R392E, abolishing binding to phosphotyrosine-containing targets, was unable to associate with either WT or mutant receptors. Moreover, in GST pull-down assays, MPLW515L (which is phosphorylated in 293T cells), but not MPLWT (nonphosphorylated in 293T cells), specifically interacted with the Lnk SH2 domain (Figure 3D). Immunoprecipitations with an anti-phosphotyrosine antibody showed that the phosphorylation levels of Lnk itself are higher in 293T cells expressing MPLW515L compared with cells expressing MPLWT, while Lnk R392E was no longer phosphorylated (Figure 3E). The interaction of Lnk with MPLWT and MPLW515L, therefore, requires an active phosphorylated receptor and the SH2 domain of Lnk.
Figure 3.
SH2 domain of Lnk interacts with MPLWT and MPLW515L. (A-C) Bone marrow cells (A) from Lnk+/+ and Lnk−/− mice (6 each) were cultured with Tpo (50 ng/mL, 3 days). UT7/MPL515 cells (B) were cultured either without or with Tpo (1 ng/mL). 293T cells (C) were transfected with combinations of MPLWT, MPLW515L, wild-type Lnk (LW), or the SH2 mutant Lnk (LM) as indicated and were either untreated or treated with Tpo (1 ng/mL, 30 minutes). Lysates were immunoprecipitated (IP) with MPL or Lnk antibodies and analyzed by Western blot as indicated. Total Lnk and MPL levels were analyzed by Western blot (bottom panels). (D) Protein lysates from 293T cells transfected with either MPLWT or MPLW515L were incubated with either GST protein or GST-Lnk-SH2 fusion protein (GST-L-SH2). GST-protein complexes were analyzed by Western blot with MPL antibody. Lysates also were immunoprecipitated with a phosphotyrosine antibody and analyzed by Western blot with MPL antibody (right panel). (E) 293T cells were transfected and treated as described in panel C. Lysates were immunoprecipitated with phosphotyrosine antibody and analyzed by Western blot with Lnk antibody (top panel). Lnk levels in the lysates were analyzed by Western blot (bottom panel).
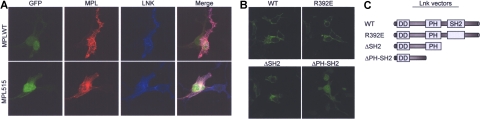
Confocal microscopy analysis demonstrated significant colocalization between V5- tagged-Lnk and both MPLWT and MPLW515L in BaF/3 cells (Figure 4A, and supplementary Figure 1). While Lnk was mostly found in the plasma membrane, in some cells it was present in the cytoplasm (data not shown). Moreover, membrane staining of Lnk also was noted in cells not expressing MPL, demonstrating that Lnk localizes to the membrane independent of its MPL association. To determine which domains of Lnk are required for its membrane expression, we transfected Lnk deletion mutants into 293T cells and analyzed their expression pattern. The point mutant, Lnk R392E, and a deletion mutant lacking the SH2 domain (Δ SH2) retained the capacity to localize to the plasma membrane, while further deletion of the PH domain (Δ PHSH2) abolished it (Figure 4B). In our system, WT Lnk localized mostly to the membrane. Previously, Lnk-GFP expressed in COS-7 cells was found mostly in the juxtanuclear region with some membrane localization.15 The discrepancy could arise from different experimental conditions and vectors used.
Figure 4.
Lnk co-localizes with MPLWT and MPLW515L in the plasma membrane. (A) BaF/3 cells were cotransfected with either GFP-MPLWT or GFP-MPLW515L and V5-tagged Lnk and grown in the presence of Tpo (1 ng/mL). Cells were stained for MPL (MPL antibody, red) and Lnk (V5 antibody, blue). Transfected cells were detected by GFP fluorescence (green). The merged images show co-localization of MPL and Lnk (pink). (B) 293T cells were transfected with V5-wild type Lnk (LNKWT), V5-Lnk R392E (R392E), V5-Δ SH2 (aa 1-307), or V5-Δ PH-SH2 (aa 1-205) and stained with a V5 antibody (green). Stained cells were examined by confocal microscopy. (C) Schematic presentation of Lnk expression vectors.
Discussion
In the present study we show that Lnk is a negative regulator of MPLW515L. Lnk inhibited the proliferation of MPLW515L-expressing cells and down-regulated signaling by JAK/STAT, MAPK, and PI3K, which are abnormally activated by MPLW515L. While these pathways are important for Tpo-mediated megakaryocyte growth, survival, and differentiation, inhibition of each of them alone may not be sufficient to block Tpo effects.17–19 The significance of Lnk as an important inhibitor of cytokine signaling in hematopoietic cells is highlighted by its ability, simultaneously, to down-regulate 3 major signal transduction pathways (references 11, 16, and our data). The molecular mechanism by which Lnk inhibits its target receptors are mostly unknown, yet they do not seem to involve degradation or internalization of the receptors (references 6, 16, and our data [not shown]). The Lnk family member, SH2-B, enhances cytokine signaling by increasing ruffling at membrane microdomains.20 Lnk localizes with MPL at the membrane and could disrupt efficient MPL signal transduction by interfering with regulated functions at specific membrane sites.
The MPLW515L mutation is located in an inhibitory motif (KWQFP) at the cytoplasmic-transmembrane junction of MPL.21 Data have suggested that mutations in this region disrupt the structural inhibition imposed by the KWQFP motif. We show that the interaction of Lnk with both MPLWT and MPLW515L requires an active phosphorylated receptor and the SH2 domain of Lnk. This suggests that Lnk regulates both wild-type and mutant MPL receptors through a common mechanism. In contrast, SOCS3, a known negative regulator of JAK2 and cytokine receptors, is unable to inhibit JAK2V617F.22 Notably, the binding of Lnk to MPLW515L is greater than its binding to MPLWT. This may help explain mechanistically why BaF/3 cells expressing MPLW515L are more susceptible to Lnk inhibition than equivalent cells expressing MPLWT. Interestingly, MPLW515L appears to stimulate not only pro-growth downstream targets (eg, Stats, Akt, and Erk) but also inhibitory ones (eg, Lnk). Blocking MPL by Lnk overexpression could shift the balance between growth-inhibitory and -stimulatory signals leading to cell death. These data are compatible with a recent model suggesting that “oncogene-addicted” cells are uniquely sensitive to oncogene inactivation, causing cell death, as a result of different rates of attenuation of survival and apoptotic pathways.23 Further elucidating the mechanisms by which Lnk and other regulatory proteins constrain MPLW515L will provide insight into the molecular pathophysiology of MPD and could help develop new treatments for this disease.
Supplementary Material
Acknowledgments
This work was supported by NIH grants, UCLA Cancer Gene Medicine Training grant, and also in part by the Parker Hughes Trust, the Inger Foundation, Amgen, and the Mary Barry Foundation.
H. Phillip Koeffler is a member of the UCLA Jonsson Comprehensive Cancer Center and holds the endowed Mark Goodson Chair of Oncology Research at Cedars-Sinai Medical Center/UCLA School of Medicine. Sigal Gery is a Mary Barry Fellow.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.G. designed and performed research, wrote the paper. K.C. and S.G. performed research. N.K. contributed vital cell lines. L.L. contributed vital reagents and scientific discussion. H.P.K. designed research and helped write the paper.
L.L. and H.P.K. were co-last authors.
Conflict-of-interest disclosure: Liqin Liu is employed by Amgen and has stock and stock options in Amgen. The authors declare no competing financial interests.
Correspondence: Sigal Gery, Cedars-Sinai Medical Center, Davis Building 5066, 8700 Beverly Blvd, Los Angeles, CA, 90048; e-mail: gerys@cshs.org.
References
- 1.Campbell PJ, Green AR. The myeloproliferative disorders. N Engl J Med. 2006;355:2452–2466. doi: 10.1056/NEJMra063728. [DOI] [PubMed] [Google Scholar]
- 2.Levine RL, Gilliland DG. JAK-2 mutations and their relevance to myeloproliferative disease. Curr Opin Hematol. 2007;14:43–47. doi: 10.1097/00062752-200701000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3:e270. doi: 10.1371/journal.pmed.0030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardanani AD, Levine RL, Lasho T, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood. 2006;108:3472–3476. doi: 10.1182/blood-2006-04-018879. [DOI] [PubMed] [Google Scholar]
- 5.Takaki S, Sauer K, Iritani BM, et al. Control of B cell production by the adaptor protein lnk: definition of a conserved family of signal-modulating proteins. Immunity. 2000;13:599–609. doi: 10.1016/s1074-7613(00)00060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takaki S, Morita H, Tezuka Y, Takatsu K. Enhanced hematopoiesis by hematopoietic progenitor cells lacking intracellular adaptor, protein, Lnk. J Exp Med. 2002;195:151–160. doi: 10.1084/jem.20011170. Erratum in: J Exp Med. 2002; 195:1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velazquez L, Cheng AM, Fleming HE, et al. Cytokine signaling and hematopoietic homeostasis are disrupted in Lnk-deficient mice. J Exp Med. 2002;195:1599–1611. doi: 10.1084/jem.20011883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nobuhisa I, Takizawa M, Takaki S, et al. Regulation of hematopoietic development in the aorta-gonad-mesonephros region mediated by Lnk adaptor protein. Mol Cell Biol. 2003;23:8486–8494. doi: 10.1128/MCB.23.23.8486-8494.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takaki S, Tezuka Y, Sauer K, et al. Impaired lymphopoiesis and altered B cell subpopulations in mice overexpressing Lnk adaptor protein. J Immunol. 2003;170:703–710. doi: 10.4049/jimmunol.170.2.703. [DOI] [PubMed] [Google Scholar]
- 10.Ema H, Sudo K, Seita J, et al. Quantification of self-renewal capacity in single hematopoietic stem cells from normal and Lnk-deficient mice. Dev Cell. 2005;8:907–914. doi: 10.1016/j.devcel.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Tong W, Lodish HF. Lnk inhibits Tpo-mpl signaling and Tpo-mediated megakaryocytopoiesis. J Exp Med. 2004;200:569–580. doi: 10.1084/jem.20040762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buza-Vidas N, Antonchuk J, Qian H, et al. Cytokines regulate postnatal hematopoietic stem cell expansion: opposing roles of thrombopoietin and LNK. Genes Dev. 2006;20:2018–2023. doi: 10.1101/gad.385606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang X, Li Y, Tanaka K, Moore KG, Hayashi JI. Cloning and characterization of Lnk, a signal transduction protein that links T-cell receptor activation signal to phospholipase C gamma 1, Grb2, and phosphatidylinositol 3-kinase. Proc Natl Acad Sci U S A. 1995;92:11618–11622. doi: 10.1073/pnas.92.25.11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takaki S, Watts JD, Forbush KA, et al. Characterization of Lnk: an adaptor protein expressed in lymphocytes. J Biol Chem. 1997;272:14562–14570. doi: 10.1074/jbc.272.23.14562. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, He X, Schembri-King J, Jakes S, Hayashi J. Cloning and characterization of human Lnk, an adaptor protein with pleckstrin homology and Src homology 2 domains that can inhibit T cell activation. J Immunol. 2000;164:5199–5206. doi: 10.4049/jimmunol.164.10.5199. [DOI] [PubMed] [Google Scholar]
- 16.Tong W, Zhang J, Lodish HF. Lnk inhibits erythropoiesis and Epo-dependent JAK2 activation and downstream signaling pathways. Blood. 2005;105:4604–4612. doi: 10.1182/blood-2004-10-4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Millot GA, Vainchenker W, Dumenil D, Svinarchuk F. Distinct effects of thrombopoietin depending on a threshold level of activated Mpl in BaF-3 cells. J Cell Sci. 2002;115:2329–2337. doi: 10.1242/jcs.115.11.2329. [DOI] [PubMed] [Google Scholar]
- 18.Guerriero R, Parolini I, Testa U, et al. Inhibition of TPO-induced MEK or mTOR activity induces opposite effects on the ploidy of human differentiating megakaryocytes. J Cell Sci. 2006;119:744–752. doi: 10.1242/jcs.02784. [DOI] [PubMed] [Google Scholar]
- 19.Geddis AE, Fox NE, Kaushansky K. Phosphatidylinositol 3-kinase is necessary but not sufficient for thrombopoietin-induced proliferation in engineered Mpl-bearing cell lines as well as in primary megakaryocytic progenitors. J Biol Chem. 2001;276:34473–34479. doi: 10.1074/jbc.M105178200. [DOI] [PubMed] [Google Scholar]
- 20.Herrington J, Diakonova M, Rui L, Gunter DR, Carter-Su C. SH2-B is required for growth hormone-induced actin reorganization. J Biol Chem. 2000;275:13126–13133. doi: 10.1074/jbc.275.17.13126. [DOI] [PubMed] [Google Scholar]
- 21.Staerk J, Lacout C, Sato T, Smith SO, Vainchenker W, Constantinescu SN. An amphipathic motif at the transmembrane-cytoplasmic junction prevents autonomous activation of the thrombopoietin receptor. Blood. 2006;107:1864–1871. doi: 10.1182/blood-2005-06-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hookham MB, Elliott J, Suessmuth Y, et al. The myeloproliferative disorder-associated JAK2 V617F mutant escapes negative regulation by suppressor of cytokine signaling 3. Blood. 2007;109:4924–4929. doi: 10.1182/blood-2006-08-039735. [DOI] [PubMed] [Google Scholar]
- 23.Sharma SV, Gajowniczek P, Way IP, et al. A common signaling cascade may underlie “addiction” to the Src, BCR-ABL, and EGF receptor oncogenes. Cancer Cell. 2006;10:425–435. doi: 10.1016/j.ccr.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.