Abstract
Mast cells play critical roles in the regulation of inflammation. One characteristic feature of mast cells is their relatively long lifespan in vivo. Members of the Bcl-2 protein family are regulators of cell survival and apoptosis, where the BH3-only proteins are critical proapoptotic proteins. In this study we investigated the role of the BH3-only proteins Noxa, Bad, Bim, Bmf, Bid, and Puma in apoptosis of mucosal-like mast cells (MLMCs) and connective tissue–like mast cells (CTLMCs). We demonstrate that Puma is critical for the induction of mast-cell death following cytokine deprivation and treatment with the DNA-damaging agent etoposide in MLMCs and CTLMCs. Using p53−/− mast cells, we found that cytokine deprivation–induced apoptosis, in contrast to that elicited by etoposide, is p53-independent. Interestingly, mast cells deficient in FOXO3a, previously proposed as a transcription factor for Puma induction in response to growth factor deprivation, were markedly resistant to cytokine withdrawal compared with wild-type cells. Moreover, overexpression of phosphorylation-deficient, constitutively active FOXO3a caused an up-regulation of Puma. In conclusion, our data demonstrate a pivotal role for Puma in the regulation of cytokine deprivation–induced mast-cell apoptosis and suggest a plausible role for Puma in the regulation of mast cell numbers in vivo.
Introduction
Mast cells are of hematopoietic origin, derived from CD34+ progenitor cells in the bone marrow.1 In general, 2 distinct mast-cell populations with different phenotypical and functional characteristics are recognized. Human mast cells are classified into mast cells containing tryptase (MCT) and mast cells containing both tryptase and chymase (MCTC),2 whereas rodent mast cells are divided into connective tissue mast cells (CTMCs) and mucosal mast cells (MMCs), based on their staining properties and tissue distribution.3 Mast cells play a versatile and important role in immunity against certain infections, allergy, and inflammation.4–6 Their differentiation, proliferation, and survival are highly regulated by stem cell factor (SCF) and various other cytokines.7,8 Mast cells generally have a long lifespan in vivo and can survive for several months situated in the tissue.9,10 Mucosal mast-cell numbers are kept relatively constant under normal conditions but increase remarkably during inflammation, due to the action of T-cell–derived cytokines.11–13 In contrast, connective tissue mast cells appear not to depend on T-cell–derived cytokines but primarily require SCF for their persistence.14,15
It is known for many hematopoietic cell types that cytokines promote their survival through regulation of members of the Bcl-2 family of proteins.16 The Bcl-2 protein family contains members that promote apoptosis, as well as proteins that safeguard cell survival. The Bcl-2 family members are characterized by Bcl-2 homology (BH) domains. The antiapoptotic members (Bcl-2, Bcl-xL, Bcl-w, A1, and Mcl-1) contain up to 4 BH domains. The proapoptotic members can be subdivided into 2 groups: BH3 domain–only proteins (BH3-only), which share with each other only the BH3 region and Bax/Bak-like proteins, which contain BH1, BH2, and BH3 regions.17,18 In response to developmental signals or experimentally applied stress stimuli, BH3-only proteins are activated and trigger apoptosis by binding, in a selective fashion, to prosurvival Bcl-2 family proteins, which then leads to the activation of Bax and Bak, causing mitochondrial release of apoptogenic factors (eg, cytochrome c), activation of the caspase cascade and, ultimately, cell destruction. It has been demonstrated that individual BH3-only proteins differ in their ability to bind to the antiapoptotic Bcl-2 family members and that this specificity governs the function of these molecules.19,20 Given that proapoptotic and antiapoptotic Bcl-2 family proteins can bind to each other suggests that the relative concentrations of these proteins determine cell fate.
Bcl-2 interacting mediator of cell death (Bim, also called Bod) has a prominent role among the BH3-only proteins since it binds with high affinity to all antiapoptotic Bcl-2 family proteins,19 including A1, which appears to have an important function in mast- cell survival.21 Studies with gene-targeted mice have shown that Bim is essential for developmentally programmed death and stress-induced apoptosis in numerous cell types, including lymphocytes, myeloid cells, and neurons.22–25 We have previously shown that SCF regulates mast cell survival (at least in part) through inactivation of the Forkhead transcription factor FOXO3a and down-regulation and phosphorylation of its target Bim, subsequently leading to the ubiquitination and proteasomal degradation of Bim.26 Moreover, Bim-deficient mast cells are partially resistant to cytokine deprivation–induced apoptosis in culture.27 Since overexpression of Bcl-2 protected mast cells more potently than loss of Bim,27 it appears that other proapoptotic BH3-only proteins, besides Bim, also might be involved in this process.
p53 up-regulated modulator of apoptosis (Puma, also called Bbc3) was first identified as a BH3-only protein that is transcriptionally up-regulated by p53 and activated upon p53-dependent apoptotic stimuli, such as treatment with DNA-damaging drugs.28,29 However, it has been shown that Puma is also up-regulated in response to certain p53-independent apoptotic stimuli, such as growth factor deprivation or treatment with glucocorticoids or phorbol ester.30 Experiments with gene-targeted mice have shown that Puma is required for apoptosis of lymphoid cells and fibroblasts in response to p53-dependent stimuli (eg, γ-radiation, etoposide) and also certain p53-independent ones, such as cytokine deprivation, treatment with glucocorticoids, or phorbol ester.31,32 It was demonstrated recently that the transcription factor FOXO3a increases Puma expression in response to growth factor deprivation in lymphoid cells and mouse embryonic fibroblasts, suggesting that Puma together with Bim may have overlapping functions as FOXO3a downstream targets following removal of survival factors.33
To examine the importance of BH3-only proteins in the regulation of mast-cell apoptosis, we compared the survival of in vitro–differentiated bone marrow–derived mucosal-like mast cells (MLMCs) and connective tissue–like mast cells (CTLMCs) from Bad−/−, Bid−/−, Bmf−/−, Noxa−/−, Puma−/−, or wild-type (wt) mice following cytokine deprivation or treatment with the topoisomerase inhibitor etoposide. The BH3-only protein Puma was of particular interest, since it has been demonstrated that Puma plays a critical role in cytokine deprivation–induced apoptosis of thymocytes and myeloid progenitors.31,34 In this study we demonstrate for the first time that Puma is involved in the induction of mast-cell death following cytokine deprivation. In addition, we found that phosphorylation-deficient FOXO3a induces both Bim and Puma expression and that FOXO3a deficiency protects mast cells from cytokine deprivation–induced apoptosis. This indicates a FOXO3a-dependent regulation of cytokine deprivation–induced apoptosis in mast cells. Our results also show that mast cells are sensitive to etoposide and that mast cells lacking Puma or p53 as well as those overexpressing Bcl-2 are abnormally resistant to this apoptotic stimulus.
Materials and methods
Mice
The mice bearing gene deletions for FOXO3a, Bid, Bad, Bim, Puma, Noxa, or those expressing the vav-Bcl-2− transgene and the generation of Bim−/−/Puma−/− double-deficient mice have been published previously.22,31,35–39 Bmf-deficient mice (A.V. and A.S., manuscript in preparation) have been generated by conventional gene-targeting methods. p53−/− mice were a kind gift from Dr Monica Nistér (Karolinska Institutet, Department of Oncology-Pathology). All experiments with animals were performed according to the guidelines of the Royal Melbourne Hospital Research Foundation Animal Ethics Committee or the Animal Ethics Committee in Stockholm.
Cell culture
Bone marrow cells were differentiated into either a connective tissue–like phenotype (CTLMCs) or a mucosal-like phenotype (MLMCs) as described.40 The viability, maturity, and purity of the cells were examined by counting cells using staining with trypan blue or toluidine blue as well as by flow cytometric analysis for expression of Kit and FcϵRI, using fluorescein isothiocyanate (FITC)–conjugated antimouse CD117 (Kit) monoclonal antibody 2B8 or FITC-conjugated rat IgG2b isotype control antibody (both from PharMingen, San Diego, CA), FITC-conjugated antimouse FcϵRI-α monoclonal antibody MAR-1, or FITC-conjugated Armenian Hamster IgG isotype control (both from eBioscience, San Diego, CA).
Analysis of apoptosis
To monitor apoptosis, cells were stained with propidium iodide (PI, 2 μg/mL) plus FITC-conjugated annexin V (0.3 μg/mL) and analyzed in a FACScan (Becton Dickinson, San Jose, CA). The total number of viable cells after re-adding cytokines after 96 hours of deprivation was counted by adding, in addition to PI and annexin V, a known number of beads (Flow Cytometry Reference Beads, Molecular Probes, Eugene, OR), followed by FACS analysis and calculation of viable mast cell number from the ratio of beads to viable cells (PI− and annexin V−).
Mast cells were deprived of cytokines but otherwise kept in medium supplemented with 10% fetal bovine serum (FBS) as previously described for cell cultures. One μg/mL of etoposide (Sigma Chemicals, St Louis, MO) was added to mast cells kept in fully supplemented medium. Mast cells kept in fully supplemented medium were exposed to 30 Gy γ-radiation. All cells were kept at a concentration of 1 × 106 cells/mL.
Mast-cell activation
In experiments where activation-induced survival after FcϵRI crosslinking was measured, cells were deprived of cytokines and kept in medium supplemented with 10% FBS during sensitization and activation.21 Cells were sensitized with a monoclonal mouse antitrinitrophenyl (TNP) IgE-antibody (IgEl-b4, American Type Culture Collection, Rockville, MD), used as a 15% hybridoma supernatant before activated by 100 ng/mL TNP-BSA (Biosearch Technologies, San Francisco, CA) with a coupling ratio of 9:1.
Western blot analysis
Cells were lysed in sodium dodecyl sulfate (SDS) sample buffer (125 mM Tris-HCl (pH 6.8), 4% w/v SDS, 20% glycerol, 0.02% w/v bromphenol blue, and 50 mM dithiothreitol, added just before use) before sonicated on ice. Western blotting was performed by using NuPAGE Bis-Tris Western gels (Invitrogen, Carlsbad, CA). After electrophoresis, proteins were electro-blotted onto nitro-cellulose membranes (Hybond ECL, Amersham Biosciences, Uppsala, Sweden). After transfer, membranes were blocked before incubated overnight at 4°C with the primary antibody, either anti-Puma (NT) antibody (ProSci, Poway, CA), anti-Bim antibody (Assay Designs, Ann Arbor, MI, or Affinity BioReagents, Golden, CO), anti-Erk antibody (Santa Cruz Biotechnology, Santa Cruz, CA), anti–b-actin antibody (Sigma), or anti-Hsp70 antibody (gift from Dr R. Anderson, Peter MacCallum Cancer Research Center, Melbourne, Australia), washed, and subsequently incubated with horseradish peroxidase–conjugated secondary antibody. The proteins were visualized using enhanced chemiluminescence (ECL) system LumiGLO and exposure to a Hybond ECL film (Amersham Biosciences).
Retroviral vectors and infections
The retroviral infection of BMMCs (15% WEHI-3–enriched RPMI 1640 medium) was performed as previously described.41 Phoenix-Eco cells were transfected with either pBabe-puro containing 4-hydroxy tamoxifen (4-OHT)–inducible mutated human FOXO3(A3) (pBabe-Puro/FOXO3(A3):ER) (kindly provided by Dr P. Coffer, Utrecht, The Netherlands)42 or pBabe-puro only. The supernatants were harvested, filtered, and supplemented with protamine sulfate at 4 μg/mL and used for transduction of GP + E86 cells to establish a polyclonal producer cell line. BMMCs were then cultured with filtered supernatant from the producer cell line in the presence of 4 μg/mL protamine sulfate followed by selection in puromycin, at 2 μg/mL for 3 days and at 1 μg/mL for 5 days.
Statistical analysis
Statistical analysis was performed using the Student t test. P values of less than .05 were considered to indicate statistically significant differences.
Results
The BH3-only proteins Noxa, Bad, Bid, Bmf, and Puma all have been previously implicated in growth factor withdrawal–induced apoptosis.18 To study their involvement in cytokine deprivation–induced apoptosis in MLMCs and CTLMCs, mast cells were cultured from mice lacking these BH3-only proteins or, as controls, from wt mice or mice expressing a Bcl-2 transgene in all hemopoietic cells.36 We also generated MLMCs and CTLMCs from p53- and FOXO3a-deficient mice to establish the influence these transcription factors have on cytokine deprivation–induced apoptosis. The cultured cells resembled primary mast cells, as confirmed by toluidine blue staining of cytoplasmic granules, expression of the high-affinity IgE receptor (FcϵRI), and the receptor for SCF, c-Kit (data not shown). In these respects, wt mast cells and mast cells lacking any of the aforementioned BH3-only proteins, p53, FOXO3a, or those overexpressing Bcl-2, were indistinguishable.
Loss of Puma protects mast cells from apoptosis induced by cytokine deprivation
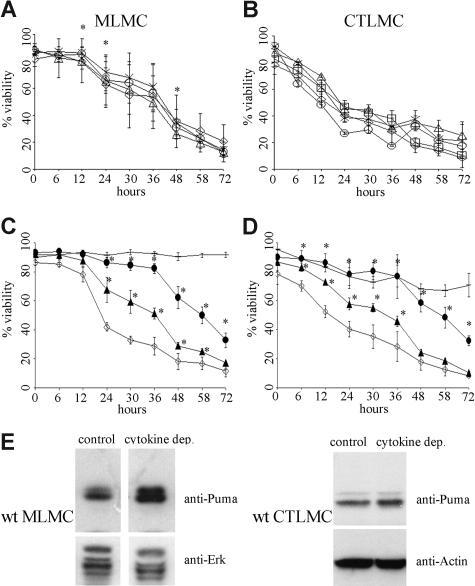
The cytokine deprivation–induced apoptosis of mast cells was first assessed in short-term survival assays by binding of annexin V and PI exclusion. Wt MLMCs deprived of cytokines died rapidly and at a similar rate as wt CTLMCs. Interestingly, MLMCs lacking Noxa or Bid were not protected from cytokine deprivation–induced apoptosis, whereas the absence of Bad offered a small but statistically significant protection (P < .004, Figure 1A). In the case of CTLMCs, loss of Noxa, Bad, Bid, or Bmf offered no protection against cytokine deprivation–induced apoptosis (Figure 1B) but, as previously shown,27 expression of the Bcl-2 transgene potently inhibited this death (Figure 1C,D). Remarkably, loss of Puma conferred substantial protection from apoptosis induced by cytokine deprivation, in fact nearly as potent as did Bcl-2 overexpression. After 36 hours approximately 30% of the wt mast cells remained viable (PI- and annexin V–negative), while approximately 80% of the mast cells lacking Puma and approximately 90% of those overexpressing Bcl-2 remained viable. In addition, using mast cells derived from mice lacking only one allele of Puma, we could see a clear gene dosage effect, since Puma+/− mast cells showed approximately 45% to 50% viability after 36 hours of cytokine deprivation (Figure 1C,D). These results demonstrate that the BH3-only protein Puma plays an essential role in cytokine deprivation–induced apoptosis in mast cells.
Figure 1.
Loss of the BH3-only protein Puma protects mast cells from apoptosis induced by cytokine deprivation. Survival of mast cells in the absence of cytokines was measured by staining with PI plus annexin V-FITC (A) MLMCs where ◇ indicates wild-type; ▵, Noxa−/−; ×, Bad−/−; and ○, Bid−/−. N = 4-12 (B) CTLMCs where ◇ indicates wild-type; ▵, Noxa−/−; ×, Bad−/−; ○, Bid−/−; and □, Bmf−/−. N = 2-6 (C) MLMCs where ◇ indicates wild-type; −, vav-Bcl2; ●, Puma−/−; ▴, Puma+/−. N = 6 (D) CTLMCs where ◇ indicates wild-type; −, vav-Bcl2; ●, Puma−/−; ▴, Puma+/−. N = 3-4 and results are presented as mean (± SD). (E) Expression of Puma was determined by Western blotting of lysates from wt MLMCs and CTLMCs following cytokine deprivation for 10 hours. Probing for Erk or Actin served as a loading control. The results shown are representative of at least 3 independent experiments.
Mast cells up-regulate Puma in response to cytokine deprivation
To determine whether wt mast cells increased the expression of Puma in response to cytokine deprivation, we probed Western blots of protein lysates derived from MLMCs and CTLMCs deprived of cytokines for 10 hours with an antibody against Puma. We observed an up-regulation of Puma in both MLMCs and CTLMCs in response to cytokine deprivation (Figure 1E).
Loss of Puma promotes clonogenic survival of cytokine-deprived mast cells
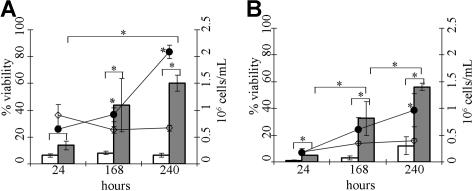
Next we investigated whether loss of Puma would allow mast cells to survive cytokine deprivation in the longer term and to remain functional. Puma−/− and wt mast cells were deprived of cytokines for 96 hours, and cytokines were then added back to these cultures. Under these conditions all wt mast cells were permeable to PI and bound annexin V, and no increase in the numbers of viable cells could be detected over 5 days. In contrast, upon re-addition of cytokines, mast cells lacking Puma resumed proliferation, and their numbers increased over time (Figure 2). These results show that loss of Puma promotes long-term, clonogenic survival of mast cells deprived of their requisite growth factors.
Figure 2.
Loss of Puma allows long-term survival of cytokine-deprived mast cells. Cytokine-induced proliferation of wild-type (wt) and Puma−/− MLMCs after re-addition of cytokines following 96 hours of cytokine deprivation. (A) Total cell number and viability were determined using trypan blue staining at 24, 168, and 240 hours after re-addition of cytokines, where □ indicates viable wt MLMCs and ■ indicates viable Puma−/− MLMCs, whereas ◇ indicates total cell number of wt and ●, Puma−/− MLMCs. (B) Total cell number and viability were determined using staining with PI plus annexin V-FITC and addition of a known number of beads followed by FACS analysis at 24, 168, and 240 hours after re-addition of cytokines, where □ indicates viable wt MLMCs and ■ indicates viable Puma−/− MLMCs, whereas ◇ indicates total cell number of wt and ●, Puma−/− MLMCs. N = 4 and results are presented as mean (± SD).
Loss of the BH3-only protein Puma protects mast cells from cytokine deprivation more potently than loss of Bim
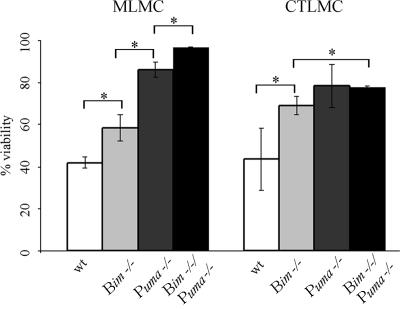
Since we have previously shown that the BH3-only protein Bim also plays a role in cytokine deprivation–induced mast-cell apoptosis,26,27 we directly compared side-by-side the viability of mast cells lacking either Bim or Puma and also cells lacking both of these proteins after 24 hours of cytokine deprivation. The results demonstrate that both Bim and Puma play an important role in apoptosis induced by cytokine deprivation; however, the viability of MLMCs lacking Puma was significantly higher than that seen for cells lacking Bim (P < .008 for Puma−/− vs Bim−/−), and the loss of both Bim and Puma further increased the viability compared with loss of either BH3-only protein alone (P < .001 for Puma−/− vs Bim−/−/Puma−/− and Bim−/− vs Bim−/−/Puma−/−). Although CTLMCs lacking Puma also appeared to have an increased viability compared with cells lacking Bim, this difference was not statistically significant. The loss of both Bim and Puma increased the viability compared with loss of Bim (P < .004 for Bim−/− vs Bim−/−/Puma−/−), whereas the viability seen for Puma−/− and Bim−/−/Puma−/− CTLMCs was comparable (Figure 3).
Figure 3.
Loss of Puma protects mast cells from apoptosis induced by cytokine deprivation more potently than loss of Bim. Wt, Bim−/−, Puma−/− or Bim−/−/Puma−/− MLMCs and CTLMCs were deprived of cytokines, and cell viability was measured by staining with PI plus annexin V-FITC at 24 hours. N = 3-6 and results are presented as mean (± SD).
Mast cells require Puma for etoposide-induced apoptosis
Since Puma has been shown to be essential for p53-mediated apoptosis in lymphocytes and fibroblasts,31,34 we examined its role in DNA damage–induced killing of mast cells. Upon treatment with the DNA-damaging chemotherapeutic agent etoposide, MLMCs were not protected by loss of Noxa, Bad, or Bid (Figure 4A), and CTLMCs lacking Noxa, Bid, or Bmf were as sensitive as wt cells. In contrast, the absence of Bad afforded CTMLCs with a small but significant protection (P < .045, Figure 4B). Moreover, Puma deficiency, even loss of one allele of Puma, protected both MLMCs and CTLMCs profoundly against treatment with etoposide (Figure 4C,D).
Figure 4.
Loss of the BH3-only protein Puma protects mast cells from apoptosis induced by treatment with etoposide, a p53-dependent death stimulus. Survival of mast cells treated with 1 μg/mL of etoposide was measured by staining with PI plus annexin V-FITC. (A) MLMCs, where ◇ indicates wt; ρ, Noxa−/−; ×, Bad−/−; and ○, Bid−/−. N = 2-6. (B) CTLMCs, where ◇ indicates wt; ▵, Noxa−/−; ×, Bad−/−; ○, Bid−/−; and □, Bmf−/−. N = 2-4. (C) MLMCs, where ◇ indicates wt; −, vav-Bcl2; ●, Puma−/−; ▴, Puma+/−. N = 6. (D) CTLMCs, where ◇ indicates wt; −, vav-Bcl2; ●, Puma−/−; ▴, Puma+/−. N = 3-4, and results are presented as mean (± SD). (E, top) MLMCs, where ◇ indicates wt and ◆, p53−/−. N = 6, and results are presented as mean (± SD). (E, bottom) Expression of Puma was determined by Western blotting of lysates from wt and p53−/− MLMCs following etoposide treatment for 10 hours. The results shown are representative of at least 3 independent experiments. (F, top) CTLMCs, where ◇ indicates wt and ◆, p53−/−. N = 3-4 and results are presented as mean (± SD). (F, bottom) Expression of Puma was determined by Western blotting of lysates from wt and p53−/− CTLMCs following etoposide treatment for 10 hours. The results shown are representative of at least 3 independent experiments.
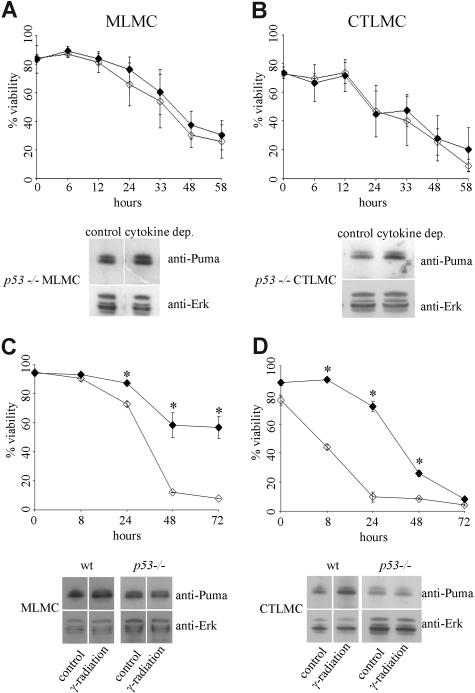
To examine if all p53-mediated apoptosis or only a part of it can be accounted for by Puma we cultured wt and p53−/− mast cells, treated them with etoposide, and measured their survival. Etoposide-treated MLMCs and CTLMCs lacking p53 exhibited increased viability compared with their corresponding wt cell populations, and this increased viability mimicked the protection conferred by loss of Puma. Furthermore, Puma protein levels were up-regulated in wt MLMCs and CTLMCs but not in MLMCs and CTLMCs lacking p53 in response to etoposide treatment (Figure 4E,F). This demonstrates that most etoposide-mediated apoptosis in CTLMCs and MLMCs is mediated through Puma.
Cytokine deprivation–induced apoptosis in mast cells mediated by the BH3-only protein Puma is p53-independent
Puma was first identified as a BH3-only protein transcriptionally regulated by p53 and was shown to be activated by p53-dependent stimuli.28,29 It has, however, also been shown that Puma is critical for apoptosis induced by a range of p53-independent stress stimuli, such as growth factor deprivation or treatment with glucocorticoids.31 To examine whether cytokine deprivation triggers mast-cell apoptosis by a p53-dependent or p53-independent mechanism, we cultured wt and p53−/− mast cells in the presence or absence of cytokines and measured their survival. Both cytokine-deprived MLMCs and CTLMCs lacking p53 exhibited no increased viability compared with their corresponding wt cell populations, and in response to cytokine deprivation Puma protein levels were normally up-regulated in both MLMCs and CTLMCs lacking p53 (Figure 5A,B). As a comparison, we also exposed MLMCs and CTLMCs to 30 Gy γ-radiation, which kills mast cells by a process known to be p53-dependent.43 γ-irradiated MLMCs and CTLMCs lacking p53 exhibited increased viability compared with their corresponding wt cell populations, and in response to γ-radiation Puma protein levels were up-regulated in wt MLMCs and CTLMCs but not in MLMCs and CTLMCs lacking p53 (Figure 5C,D). These results demonstrate that cytokine deprivation kills mast cells by a Puma-dependent but p53-independent mechanism, whereas γ-radiation kills mast cells by a process requiring p53-mediated induction of Puma.
Figure 5.
Mast-cell apoptosis following cytokine deprivation is a p53-independent process. Survival of wt or p53−/− mast cells in the absence of cytokines or following 30 Gy γ-radiation was measured by staining with PI plus annexin V-FITC (A, top) cytokine-deprived MLMCs, where ◇ indicates wt and ◆, p53−/−. N = 4 and results are presented as mean (± SD). (A, bottom) Expression of Puma was determined by Western blotting of lysates from wt and p53−/− MLMCs following cytokine deprivation for 10 hours; the results shown are representative of at least 3 independent experiments. (B, top) Cytokine-deprived CTLMCs, where ◇ indicates wt and ◆, p53−/−. N = 4 and results are presented as mean (± SD). (B, bottom) Expression of Puma was determined by Western blotting of lysates from wt and p53−/− CTLMCs following cytokine deprivation for 10 hours; the results shown are representative of at least 3 independent experiments. (C, top) γ-irradiated MLMCs, where ◇ indicates wt and ◆, p53−/−. N = 4 and results are presented as mean (± SD). (C, bottom) Expression of Puma was determined by Western blotting of lysates from wt and p53−/− MLMCs following γ-radiation after 10 hours; the results shown are representative of at least 3 independent experiments. (D, top) γ-irradiated CTLMCs, where ◇ indicates wt and ◆, p53−/−. N = 4 and results are presented as mean (± SD). (D, bottom) Expression of Puma was determined by Western blotting of lysates from wt and p53−/− CTLMCs following γ-radiation after 10 hours; the results shown are representative of at least 3 independent experiments.
FOXO3a-dependent regulation of cytokine deprivation–induced apoptosis in mast cells
The transcription factor FOXO3a has been shown to regulate the BH3-only protein Bim44 and also was recently described to control Puma expression in response to growth factor deprivation in lymphoid cells and mouse embryonic fibroblasts.33 We have previously shown that SCF prevents cytokine deprivation–induced mast-cell apoptosis by inhibiting Bim expression via inactivation/phosphorylation of the Forkhead transcription factors FOXO1a and FOXO3a.26 To investigate if FOXO3a regulates cytokine deprivation–induced apoptosis in mast cells, we cultured wt and FOXO3a−/− mast cells in the presence or absence of cytokines and measured their survival. Cytokine-deprived MLMCs and CTLMCs lacking FOXO3a exhibited increased viability compared with wt cells. This demonstrates that FOXO3a is critical for cytokine deprivation–induced apoptosis of mast cells (Figure 6A,B). However, the increased viability conferred by the loss of FOXO3a was not comparable to the protection offered by loss of either Bim or Puma.
Figure 6.
Regulation of cytokine deprivation–induced apoptosis in mast cells is FOXO3a dependent. Survival of wt or FOXO3a−/− mast cells in the absence of cytokines was measured by staining with PI plus annexin V-FITC. (A) MLMCs, where ◇ indicates wt and ■, FOXO3a−/−. (B) CTLMCs, where ◇ indicates wt and ■, FOXO3a−/−. N = 3-6 and results are presented as mean (± SD). (C) Expression of an inducible, dominant-active mutant of FOXO3 results in up-regulation of Puma and Bim. BMMCs were infected with a retrovirus encoding FOXO3(A3):ER and incubated with 4-hydroxy tamoxifen (4-OHT) (+) or left untreated (−) in the presence of SCF for 48 hours. The level of Puma or Bim was analyzed by Western blotting. As a control, uninfected cells were also treated with 4-OHT. Probing for Erk served as a loading control. (D) Expression of Puma and Bim was determined by Western blotting of lysates from FOXO3a−/− MLMCs and CTLMCs following cytokine deprivation for 10 hours. Probing for Hsp70 (left panel) or beta-Actin (right panel) served as a loading control. The results shown (C,D) are representative of at least 3 independent experiments.
We next infected BMMCs with a retrovirus encoding an inducible human FOXO3a-estrogen receptor (ER) fusion protein, FOXO3(A3):ER.42 Upon addition of 4-hydroxy tamoxifen (4-OHT), this protein is released from its chaperone, heat shock protein 90, and transported into the nucleus where it can induce transcription of target genes. We have previously demonstrated increased apoptosis in FOXO3(A3):ER-expressing mast cells upon 4-OHT treatment.26 The treatment with 4-OHT induced expression of Bim and Puma in FOXO3(A3):ER-infected BMMCs but not in control cells (Figure 6C). This indicates that FOXO3a is involved not only in regulating the transcription of Bim, as previously described,26 but also identifies Puma as a FOXO3a downstream target in mast cells.
Since cytokine-deprived MLMCs and CTLMCs lacking FOXO3a exhibited increased viability compared with wt cells (Figure 6A,B), although not as prominent as mast cells lacking Puma or Bim, we next determined whether FOXO3a−/− mast cells were still able to up-regulate Puma and Bim in response to cytokine deprivation. Probing Western blots of protein lysates from FOXO3a−/− MLMCs and CTLMCs deprived of cytokines for 10 hours with antibodies against Puma and Bim indicated that Puma and Bim were still up-regulated (Figure 6D), and densitometric measurements showed an induction of 11.4 plus or minus 3.4 for Puma and 18.7 plus or minus 3.3 for Bim protein levels in MLMCs, whereas a more modest induction in CTLMCs with 1.25 plus or minus 0.15 for Puma and 1.47 plus or minus 0.23 for Bim protein levels were observed. This indicates that transcription factors apart from FOXO3a might also be involved in the regulation of Puma and Bim following cytokine deprivation.
Mast cells do not up-regulate Puma in response to FcϵRI crosslinking
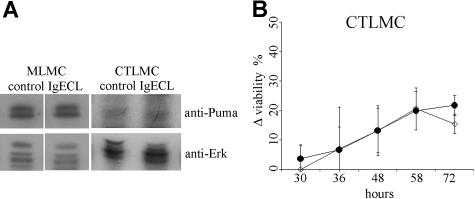
We have previously shown that the expression of both proapoptotic Bim as well as antiapoptotic Bcl-xL and A1 are increased upon FcϵRI crosslinking,21,27 and it has been postulated that cell fate is determined by the balance between these proteins.45 We therefore investigated whether Puma could also be regulated by FcϵRI crosslinking. Western blot analysis of protein lysates from wt MLMCs and CTLMCs activated through FcϵRI crosslinking for 10 hours did not reveal any up-regulation of Puma in response to FcϵRI crosslinking in wt mast cells (Figure 7A). This, together with the observation that Puma deficiency did not further increase the survival of mast cells mediated by FcϵRI crosslinking (Figure 7B), suggests that Puma does not play a critical role in this antiapoptotic pathway.
Figure 7.
Puma plays no role in mast-cell survival induced by FcϵRI crosslinking. (A) Expression of Puma was determined by Western blotting of lysates from wt MLMCs and CTLMCs following FcϵRI crosslinking (IgECL) for 10 hours. Probing for Erk served as a loading control. The results shown are representative of at least 3 independent experiments. (B) Survival of wt or Puma−/− CTLMCs after FcϵRI crosslinking in the absence of cytokines was measured by staining with PI plus annexin V-FITC. The percentage viable wt cells after FcϵRI crosslinking (following cytokine deprivation) were deducted from viable wt cells after cytokine deprivation and compared with the identically derived results for Puma−/− CTLMCs. ◇ indicates wt, whereas ● indicates Puma−/− CTLMCs. N = 3-4 and results are presented as mean (± SD).
Discussion
The proapoptotic and antiapoptotic members of the Bcl-2 protein family are critical regulators of cell survival, and the balance between the 2 subgroups determines whether a cell will survive or die.18 It has been shown that individual BH3-only proteins differ in their ability to bind to the antiapoptotic Bcl-2 family members and that this specificity governs the function of these molecules.19 We have previously demonstrated that Bim-deficient mast cells are partially resistant to cytokine deprivation–induced apoptosis and mast cells that express a Bcl-2 transgene are almost completely resistant.27 These findings indicated an important role for Bim in regulating mast-cell apoptosis but also suggested that other proapoptotic BH3-only proteins besides Bim might be involved in this process.
In the present study we demonstrate that the BH3-only protein Puma plays an essential role in cytokine deprivation–induced apoptosis of mast cells. Puma previously has been shown to play a critical role in cytokine deprivation–induced apoptosis of lymphoid cells and normal as well as transformed myeloid progenitors.31,34,46 As our results demonstrate, MLMCs and CTLMCs lacking the BH3-only protein Puma are highly resistant to apoptosis caused by cytokine deprivation, protecting cells almost as potently as overexpression of antiapoptotic Bcl-2. Mast cells lacking only one allele of Puma also showed significantly increased viability compared with wt cells; the degree of protection being similar to that obtained with complete loss of Bim. Bim and Puma cooperate in mediating apoptosis in response to both p53-dependent and -independent apoptotic stimuli in lymphocytes,37 and our data also suggest an overlapping function of Bim and Puma in regulating mast-cell survival following cytokine deprivation. Most likely Puma and Bim cooperate so potently in apoptosis signaling because they are the only BH3-only proteins that bind with high affinity to all prosurvival Bcl-2 family members.19
When MLMCs were tested for their ability to proliferate upon re-addition of cytokines, Puma-deficient mast cells exhibited an ability to proliferate compared with wt mast cells that were already committed to apoptosis. Only MLMCs were tested for their ability to proliferate upon re-addition since CTLMCs are mostly postmitotic.40 Puma protein levels were increased in both MLMCs and CTLMCs in response to cytokine deprivation. Taken together, these results identify Puma as the most important BH3-only protein involved in cytokine deprivation–induced apoptosis of MLMCs and CTLMCs.
In contrast to Puma, we found no requirement for the BH3-only proteins Noxa, Bad, Bid, or Bmf following cytokine deprivation in CTLMCs. The same was observed in MLMCs, with the minor exception that loss of Bad offered a small yet significant degree of protection. Interestingly, we recently observed a minor role for Bad in imatinib (Gleevec)–induced killing of Bcr-Abl–transformed hemopoietic progenitors,47 a pathway to cell death thought to be similar to growth factor withdrawal–induced apoptosis. Bad has been implicated as a key mediator of cytokine deprivation–induced apoptosis.48,49 However, there have been reports showing that cytokine deprivation does not correlate with the phosphorylation status of Bad50 and that Bad is not required for cytokine deprivation–induced killing of interleukin 3 (IL-3)–dependent immortalized myeloid progenitor cell lines.46 In accordance, we found that the protection afforded by loss of Bad was not comparable to the one seen for MLMCs lacking either Puma or Bim. The minor importance of Bad is consistent with the observation that it binds only to antiapoptotic Bcl-2, Bcl-xL, and Bcl-w but not to Mcl-1 or A1, whereas Bim and Puma, which play more prominent roles, bind with high affinity to all of these proteins.19
Puma was first identified as a BH3-only protein that is transcriptionally up-regulated by p53 and activated in response to p53-dependent apoptotic stimuli.28,29 Puma, however, also is up-regulated in response to certain p53-independent stimuli and required for their ability to kill lymphoid cells.30,32 Cytokine deprivation in mast cells was found to be a p53-independent stimulus, since cells lacking p53 died in response to cytokine deprivation with similar kinetics as wt mast cells. This finding is supported by a report stating that IL-3–dependent bone marrow–derived mast cells (BMMCs) lacking p53 undergo apoptosis normally upon cytokine deprivation.51 The normal up-regulation of Puma in cytokine-deprived p53-deficient MLMCs and CTLMCs indicates that another transcription factor is critical for this process. In lymphoid cells and mouse embryonic fibroblasts it recently has been shown that the transcription factor FOXO3a induces Puma expression in response to growth factor deprivation.33
Here we report the involvement of FOXO3a in the regulation of cytokine deprivation–induced apoptosis and the expression of Puma. FOXO3a−/− mast cells showed increased survival compared with wt mast cells following cytokine deprivation and BMMCs transfected with an inducible FOXO3a protein identifies Puma as a FOXO3a downstream target in mast cells. However, we believe that additional factors must be involved in cytokine deprivation–induced Puma up-regulation and apoptosis of mast cells given that loss of FOXO3a did not confer as much protection from apoptosis as loss of Puma or Bim. Moreover, although Puma and Bim are FOXO3a targets in mast cells, their protein levels were increased in FOXO3a−/− mast cells upon cytokine deprivation, comparable to that seen in wt cells. Members of the Forkhead transcription factor family play important roles in biological processes, such as control of the cell cycle, apoptosis, and response to stress.52,53 Although the FOXO members have diverse functions,54 they also appear to have some downstream transcriptional targets in common.55,56 Previously, we have shown that FOXO1a and FOXO3a are both phosphorylated in mast cells upon treatment with SCF, thereby down-regulating the extent of Bim.26 We therefore hypothesize that there is redundancy between FOXO3a and other FOXO members57 in Bim and Puma up-regulation during mast-cell apoptosis. It is also possible, however, that other (unrelated) transcription factors play a role in this process.
Protection from apoptosis through loss of Puma was not restricted to cytokine deprivation, but was seen also when Puma-deficient MLMCs and CTLMCs were treated with etoposide. In addition, p53-deficient MLMCs and CTLMCs exhibited increased viability and no up-regulation of Puma protein levels compared with corresponding wt mast-cell populations upon etoposide treatment. This identifies etoposide-induced apoptosis in mast cells as a process that is dependent on p53-mediated induction of Puma. Puma is also essential for p53-mediated apoptosis in several other cell types, including lymphoid as well as myeloid cells and fibroblasts,31,32,34 but other BH3-only proteins, such as Noxa31,58 and Bim22,59 also have been implicated. We found no requirement for the BH3-only proteins Noxa, Bad, or Bid for etoposide-induced apoptosis in MLMCs. The same was observed in CTLMCs, apart from the finding that loss of Bad offered a small yet significant protection. How Bad is activated in response to etoposide treatment in mast cells is presently unclear, but so far no p53 binding sites have been detected within the Bad gene. In contrast to 2 previous reports,60,61 we found that both thymocytes and mouse embryonic fibroblasts lacking Bid are normally sensitive to p53-dependent stimuli38 and in agreement with this, we detected no requirement for Bid in etoposide-treated mast cells. It has been demonstrated that etoposide induces apoptosis in mast cells at a concentration of 10 μg/mL and that loss of either Apaf-1 or caspase-9 protects mast cells against this death stimulus.62
There is considerable heterogeneity among tissue mast cells when comparing their phenotype, function, and survival.6,63 Their functions are often associated with the crosslinking of their high-affinity IgE receptor (FcϵRI), the subsequent release of prestored inflammatory mediators and de novo synthesis of cytokines.64,65 We and others have previously demonstrated that mast-cell viability increases upon FcϵRI crosslinking,21,66,67 and this was associated with the increased levels of both antiapoptotic A1/Bfl-1 and Bcl-xL, although, curiously, proapoptotic Bim also was induced.21,27,68 The up-regulation of Bim might play a critical role in this setting, since it can bind with high affinity to all antiapoptotic Bcl-2 family members, including A1, which has an essential antiapoptotic function in mast cells.21 A recent report described that the interaction between A1 and Bim stabilizes the A1 protein, thereby strongly reducing A1 turnover and perhaps amplifying its antiapoptotic effect.69 However, knowledge of the binding of A1 to proapoptotic Bcl-2 family members in mast cells is limited, and Bim is not the only BH3-only protein that can bind to A1; for example Puma also can do this.19,70Therefore we investigated whether the levels of Puma were altered in response to FcϵRI stimulation, but our results indicated that Puma protein levels remain unchanged following FcϵRI crosslinking in MLMCs or CTLMCs. Since we have shown previously that FcϵRI crosslinking prolongs the survival of cytokine-deprived CTLMCs,40 we also examined the survival induced by FcϵRI crosslinking in CTLMCs lacking Puma. We found that the lack of Puma did not affect the prolonged survival of cytokine-deprived CTLMCs upon FcϵRI crosslinking. These results indicate that regulation of Puma does not play an important role in the survival effect seen after FcϵRI crosslinking.
Mast cells are distributed throughout the tissues, and their differentiation, proliferation, and survival are highly regulated.7,8 To investigate whether Puma deficiency compromises the normal development and regulation of mast-cell numbers in vivo, we compared tissue sections of wt and Puma-deficient mice determining the mast-cell numbers. However, we found no indication that loss of Puma alone promoted abnormal accumulation of mast cells in tissues (unpublished results, M.E., May 2006). During nematode infections in the gut, mast cells differentiate, become activated, and hyperplasia occurs before their numbers decline as the infection resolves.15,71,72 This resolution of the hyperplasia involves both apoptosis and recirculation back to the spleen for elimination,73 and in this setting, Puma deficiency might lead to an increased accumulation of mast cells and a delay in clearance.
In conclusion, our results demonstrate an important role for Puma in p53-dependent as well as p53-independent mast-cell apoptosis where removal of only one allele of this BH3-only gene protects mast cells from apoptosis more sufficiently than complete removal of any of the other BH3-only proteins investigated.
Acknowledgments
We thank Dr P. Coffer for 4-OHT–inducible mutated human FOXO3(A3), Dr H. Martin and P. Morgan for gifts of SCF, Dr H. Karasuyama for IL-3–producing cells, Drs L. O'Reilly and D. Huang for antibodies, and Drs P. Bouillet, N. Danial, P. Kelly, J. Adams, and S. Cory for providing genetically modified mice.
This work was supported by grants from the International Cancer Technology Transfer Fellowship by the International Union Against Cancer (UICC; no. ICR/04/172; 2004); the Swiss National Science Foundation and the Novartis Foundation (formerly Ciba-Geigy-Jubilaeumsstiftung) (all from Switzerland); the Austrian Science Fund (FWF; START and SFB021); the Erik and Edith Fernströms foundation for Medical Research, Karolinska Institutet; the Swedish Research Council-Medicine; the Swedish Cancer Foundation, Cancer and Allergy foundation; Ellen, Walter and Lennart Hesselmans Foundation for scientific research; Consul Th C Berghs Foundation; Ollie and Elof Ericsson's Foundation; and King Gustav V's 80-years Foundation (all from Sweden), as well as the National Health and Medical Research Council (Canberra, Australia; program #257502 and #461221), the Juvenile Diabetes Research Foundation/National Health and Medical Research Council program grant (Australia), the National Cancer Institute (NIH; CA 80188 and 43540), and the Leukemia and Lymphoma Society of America Specialized Center of Research (SCOR; grant #7015).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.E. designed and performed research, analyzed data, and wrote the paper; T.K. performed research and analyzed data; M.E. performed research; N.M. contributed with valuable reagents; A.V. contributed with valuable reagents; J.I.J. designed and performed research; A.S. designed research, analyzed data, and wrote the paper; G.N. designed research, analyzed data, and wrote the paper; and A.S. and G.N. contributed equally to this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gunnar Nilsson, Karolinska Institutet, Department of Medicine, Clinical Immunology and Allergy Unit, KS L2:04, SE-171 76 Stockholm, Sweden; e-mail: gunnar.p.nilsson@ki.se.
References
- 1.Kirshenbaum AS, Kessler SW, Goff JP, Metcalfe DD. Demonstration of the origin of human mast cells from CD34+ bone marrow progenitor cells. J Immunol. 1991;146:1410–1415. [PubMed] [Google Scholar]
- 2.Irani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LB. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci U S A. 1986;83:4464–4468. doi: 10.1073/pnas.83.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enerback L. Mast cells in rat gastrointestinal mucosa, I: effects of fixation. Acta Pathol Microbiol Scand. 1966;66:289–302. doi: 10.1111/apm.1966.66.3.289. [DOI] [PubMed] [Google Scholar]
- 4.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 5.Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. 2004;4:787–799. doi: 10.1038/nri1460. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson G, Costa JJ, Metcalfe DD. Mast Cells and Basophils. In: Gallin JI, Snyderman R, editors. Inflammation: Basic Principles and Clinical Correlates. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1999. pp. 97–117. [Google Scholar]
- 7.Gurish MF, Austen KF. The diverse roles of mast cells. J Exp Med. 2001;194:F1–F5. doi: 10.1084/jem.194.1.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piliponsky AM, Levi-Schaffer F. Regulation of apoptosis in mast cells. Apoptosis. 2000;5:435–441. doi: 10.1023/a:1009680500988. [DOI] [PubMed] [Google Scholar]
- 9.Padawer J. Mast cells: extended lifespan and lack of granule turnover under normal in vivo conditions. Exp Mol Pathol. 1974;20:269–280. doi: 10.1016/0014-4800(74)90059-8. [DOI] [PubMed] [Google Scholar]
- 10.Blenkinsopp WK. Mast cell proliferation in adult mice. Nature. 1967;214:930–931. doi: 10.1038/214930a0. [DOI] [PubMed] [Google Scholar]
- 11.Mayrhofer G, Fisher R. Mast cells in severely T-cell depleted rats and the response to infestation with Nippostrongylus brasiliensis. Immunology. 1979;37:145–155. [PMC free article] [PubMed] [Google Scholar]
- 12.Faulkner H, Humphreys N, Renauld JC, Van Snick J, Grencis R. Interleukin-9 is involved in host protective immunity to intestinal nematode infection. Eur J Immunol. 1997;27:2536–2540. doi: 10.1002/eji.1830271011. [DOI] [PubMed] [Google Scholar]
- 13.Madden KB, Urban JF, Jr, Ziltener HJ, Schrader JW, Finkelman FD, Katona IM. Antibodies to IL-3 and IL-4 suppress helminth-induced intestinal mastocytosis. J Immunol. 1991;147:1387–1391. [PubMed] [Google Scholar]
- 14.Irani AM, Craig SS, DeBlois G, Elson CO, Schechter NM, Schwartz LB. Deficiency of the tryptase-positive, chymase-negative mast cell type in gastrointestinal mucosa of patients with defective T lymphocyte function. J Immunol. 1987;138:4381–4386. [PubMed] [Google Scholar]
- 15.Gurish MF, Boyce JA. Mast cell growth, differentiation, and death. Clin Rev Allergy Immunol. 2002;22:107–118. doi: 10.1385/CRIAI:22:2:107. [DOI] [PubMed] [Google Scholar]
- 16.Marsden VS, Strasser A. Control of apoptosis in the immune system: Bcl-2, BH3-only proteins and more. Annu Rev Immunol. 2003;21:71–105. doi: 10.1146/annurev.immunol.21.120601.141029. [DOI] [PubMed] [Google Scholar]
- 17.Adams JM, Cory S. Life-or-death decisions by the Bcl-2 protein family. Trends Biochem Sci. 2001;26:61–66. doi: 10.1016/s0968-0004(00)01740-0. [DOI] [PubMed] [Google Scholar]
- 18.Huang DC, Strasser A. BH3-only proteins–essential initiators of apoptotic cell death. Cell. 2000;103:839–842. doi: 10.1016/s0092-8674(00)00187-2. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Willis SN, Wei A, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 20.Kuwana T, Bouchier-Hayes L, Chipuk JE, et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Xiang Z, Ahmed AA, Moller C, Nakayama K, Hatakeyama S, Nilsson G. Essential role of the prosurvival bcl-2 homologue A1 in mast cell survival after allergic activation. J Exp Med. 2001;194:1561–1569. doi: 10.1084/jem.194.11.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouillet P, Metcalf D, Huang DC, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 23.Putcha GV, Moulder KL, Golden JP, et al. Induction of BIM, a proapoptotic BH3-only BCL-2 family member, is critical for neuronal apoptosis. Neuron. 2001;29:615–628. doi: 10.1016/s0896-6273(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 24.Bouillet P, Purton JF, Godfrey DI, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 25.Villunger A, Scott C, Bouillet P, Strasser A. Essential role for the BH3-only protein Bim but redundant roles for Bax, Bcl-2, and Bcl-w in the control of granulocyte survival. Blood. 2003;101:2393–2400. doi: 10.1182/blood-2002-07-2132. [DOI] [PubMed] [Google Scholar]
- 26.Möller C, Alfredsson J, Engström M, et al. Stem cell factor promotes mast cell survival via inactivation of FOXO3a-mediated transcriptional induction and MEK-regulated phosphorylation of the proapoptotic protein Bim. Blood. 2005;106:1330–1336. doi: 10.1182/blood-2004-12-4792. [DOI] [PubMed] [Google Scholar]
- 27.Alfredsson J, Puthalakath H, Martin H, Strasser A, Nilsson G. Proapoptotic Bcl-2 family member Bim is involved in the control of mast cell survival and is induced together with Bcl-X(L) upon IgE-receptor activation. Cell Death Differ. 2005;12:136–144. doi: 10.1038/sj.cdd.4401537. [DOI] [PubMed] [Google Scholar]
- 28.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 29.Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell. 2001;7:673–682. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
- 30.Han J, Flemington C, Houghton AB, et al. Expression of bbc3, a pro-apoptotic BH3-only gene, is regulated by diverse cell death and survival signals. Proc Natl Acad Sci U S A. 2001;98:11318–11323. doi: 10.1073/pnas.201208798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villunger A, Michalak EM, Coultas L, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 32.Erlacher M, Michalak EM, Kelly PN, et al. BH3-only proteins Puma and Bim are rate-limiting for gamma-radiation- and glucocorticoid-induced apoptosis of lymphoid cells in vivo. Blood. 2005;106:4131–4138. doi: 10.1182/blood-2005-04-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.You H, Pellegrini M, Tsuchihara K, et al. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med. 2006;203:1657–1663. doi: 10.1084/jem.20060353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeffers JR, Parganas E, Lee Y, et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4:321–328. doi: 10.1016/s1535-6108(03)00244-7. [DOI] [PubMed] [Google Scholar]
- 35.Ranger AM, Zha J, Harada H, et al. Bad-deficient mice develop diffuse large B cell lymphoma. Proc Natl Acad Sci U S A. 2003;100:9324–9329. doi: 10.1073/pnas.1533446100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogilvy S, Metcalf D, Print CG, Bath ML, Harris AW, Adams JM. Constitutive Bcl-2 expression throughout the hematopoietic compartment affects multiple lineages and enhances progenitor cell survival. Proc Natl Acad Sci U S A. 1999;96:14943–14948. doi: 10.1073/pnas.96.26.14943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erlacher M, Labi V, Manzl C, et al. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J Exp Med. 2006;203:2939–2951. doi: 10.1084/jem.20061552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaufmann T, Tai L, Ekert PG, et al. The BH3-only protein bid is dispensable for DNA damage- and replicative stress-induced apoptosis or cell-cycle arrest. Cell. 2007;129:423–433. doi: 10.1016/j.cell.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 39.Miyamoto K, Araki KY, Naka K, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Ekoff M, Strasser A, Nilsson G. FcepsilonRI aggregation promotes survival of connective tissue-like mast cells but not mucosal-like mast cells. J Immunol. 2007;178:4177–4183. doi: 10.4049/jimmunol.178.7.4177. [DOI] [PubMed] [Google Scholar]
- 41.Engstrom M, Karlsson R, Jonsson JI. Inactivation of the forkhead transcription factor FoxO3 is essential for PKB-mediated survival of hematopoietic progenitor cells by kit ligand. Exp Hematol. 2003;31:316–323. doi: 10.1016/s0301-472x(03)00002-x. [DOI] [PubMed] [Google Scholar]
- 42.Dijkers PF, Medema RH, Pals C, et al. Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27(KIP1). Mol Cell Biol. 2000;20:9138–9148. doi: 10.1128/mcb.20.24.9138-9148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yee NS, Paek I, Besmer P. Role of kit-ligand in proliferation and suppression of apoptosis in mast cells: basis for radiosensitivity of white spotting and steel mutant mice. J Exp Med. 1994;179:1777–1787. doi: 10.1084/jem.179.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dijkers PF, Medemadagger RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10:1201–1204. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- 45.Alfredsson J, Moller C, Nilsson G. IgE-receptor activation of mast cells regulates phosphorylation and expression of forkhead and Bcl-2 family members. Scand J Immunol. 2006;63:1–6. doi: 10.1111/j.1365-3083.2006.01704.x. [DOI] [PubMed] [Google Scholar]
- 46.Ekert PG, Jabbour AM, Manoharan A, et al. Cell death provoked by loss of interleukin-3 signaling is independent of Bad, Bim, and PI3 kinase, but depends in part on Puma. Blood. 2006;108:1461–1468. doi: 10.1182/blood-2006-03-014209. [DOI] [PubMed] [Google Scholar]
- 47.Kuroda J, Puthalakath H, Cragg MS, et al. Bim and Bad mediate imatinib-induced killing of Bcr/Abl+ leukemic cells, and resistance due to their loss is overcome by a BH3 mimetic. Proc Natl Acad Sci U S A. 2006;103:14907–14912. doi: 10.1073/pnas.0606176103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3–induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 49.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14–3-3 not BCL-X(L). Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 50.Hinton HJ, Welham MJ. Cytokine-induced protein kinase B activation and Bad phosphorylation do not correlate with cell survival of hemopoietic cells. J Immunol. 1999;162:7002–7009. [PubMed] [Google Scholar]
- 51.Silva A, Wyllie A, Collins MK. p53 is not required for regulation of apoptosis or radioprotection by interleukin-3. Blood. 1997;89:2717–2722. [PubMed] [Google Scholar]
- 52.Burgering BM, Medema RH. Decisions on life and death: FOXO Forkhead transcription factors are in command when PKB/Akt is off duty. J Leukoc Biol. 2003;73:689–701. doi: 10.1189/jlb.1202629. [DOI] [PubMed] [Google Scholar]
- 53.Tran H, Brunet A, Griffith EC, Greenberg ME. The many forks in FOXO's road. Sci STKE. 2003;172:RE5. doi: 10.1126/stke.2003.172.re5. [DOI] [PubMed] [Google Scholar]
- 54.Hosaka T, Biggs WH, 3rd, Tieu D, et al. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci U S A. 2004;101:2975–2980. doi: 10.1073/pnas.0400093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 56.Furuyama T, Nakazawa T, Nakano I, Mori N. Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem J. 2000;349:629–634. doi: 10.1042/0264-6021:3490629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paik JH, Kollipara R, Chu G, et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shibue T, Takeda K, Oda E, et al. Integral role of Noxa in p53-mediated apoptotic response. Genes Dev. 2003;17:2233–2238. doi: 10.1101/gad.1103603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O'Connor L, Strasser A, O'Reilly LA, et al. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998;17:384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kamer I, Sarig R, Zaltsman Y, et al. Proapoptotic BID is an ATM effector in the DNA-damage response. Cell. 2005;122:593–603. doi: 10.1016/j.cell.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 61.Zinkel SS, Hurov KE, Ong C, Abtahi FM, Gross A, Korsmeyer SJ. A role for proapoptotic BID in the DNA-damage response. Cell. 2005;122:579–591. doi: 10.1016/j.cell.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 62.Marsden VS, Kaufmann T, O'Reilly LA, Adams JM, Strasser A. Apaf-1 and caspase-9 are required for cytokine withdrawal–induced apoptosis of mast cells but dispensable for their functional and clonogenic death. Blood. 2006;107:1872–1877. doi: 10.1182/blood-2005-05-2160. [DOI] [PubMed] [Google Scholar]
- 63.Nilsson G, Schwartz LB. Mast-cell heterogeneity: structure and mediators. In: Busse WW, Holgate ST, editors. Asthma and rhinitis. Cambridge, MA: Blackwell Science, Inc; 1995. pp. 195–208. [Google Scholar]
- 64.Kinet JP. The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annu Rev Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 65.Williams CM, Galli SJ. The diverse potential effector and immunoregulatory roles of mast cells in allergic disease. J Allergy Clin Immunol. 2000;105:847–859. doi: 10.1067/mai.2000.106485. [DOI] [PubMed] [Google Scholar]
- 66.Kitaura J, Xiao W, Maeda-Yamamoto M, Kawakami Y, Lowell CA, Kawakami T. Early divergence of Fc epsilon receptor I signals for receptor up-regulation and internalization from degranulation, cytokine production, and survival. J Immunol. 2004;173:4317–4323. doi: 10.4049/jimmunol.173.7.4317. [DOI] [PubMed] [Google Scholar]
- 67.Yoshikawa H, Nakajima Y, Tasaka K. Glucocorticoid suppresses autocrine survival of mast cells by inhibiting IL-4 production and ICAM-1 expression. J Immunol. 1999;162:6162–6170. [PubMed] [Google Scholar]
- 68.Xiang Z, Möller C, Nilsson G. IgE-receptor activation induces survival and Bfl-1 expression in human mast cells but not basophils. Allergy. 2006;61:1040–1046. doi: 10.1111/j.1398-9995.2006.01024.x. [DOI] [PubMed] [Google Scholar]
- 69.Herold MJ, Zeitz J, Pelzer C, et al. The stability and anti-apoptotic function of A1 are controlled by its C terminus. J Biol Chem. 2006;281:13663–13671. doi: 10.1074/jbc.M600266200. [DOI] [PubMed] [Google Scholar]
- 70.Willis SN, Adams JM. Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol. 2005;17:617–625. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miller HR. Mucosal mast cells and the allergic response against nematode parasites. Vet Immunol Immunopathol. 1996;54:331–336. doi: 10.1016/s0165-2427(96)05696-6. [DOI] [PubMed] [Google Scholar]
- 72.Woodbury RG, Miller HR, Huntley JF, Newlands GF, Palliser AC, Wakelin D. Mucosal mast cells are functionally active during spontaneous expulsion of intestinal nematode infections in rat. Nature. 1984;312:450–452. doi: 10.1038/312450a0. [DOI] [PubMed] [Google Scholar]
- 73.Friend DS, Gurish MF, Austen KF, Hunt J, Stevens RL. Senescent jejunal mast cells and eosinophils in the mouse preferentially translocate to the spleen and draining lymph node, respectively, during the recovery phase of helminth infection. J Immunol. 2000;165:344–352. doi: 10.4049/jimmunol.165.1.344. [DOI] [PubMed] [Google Scholar]