Abstract
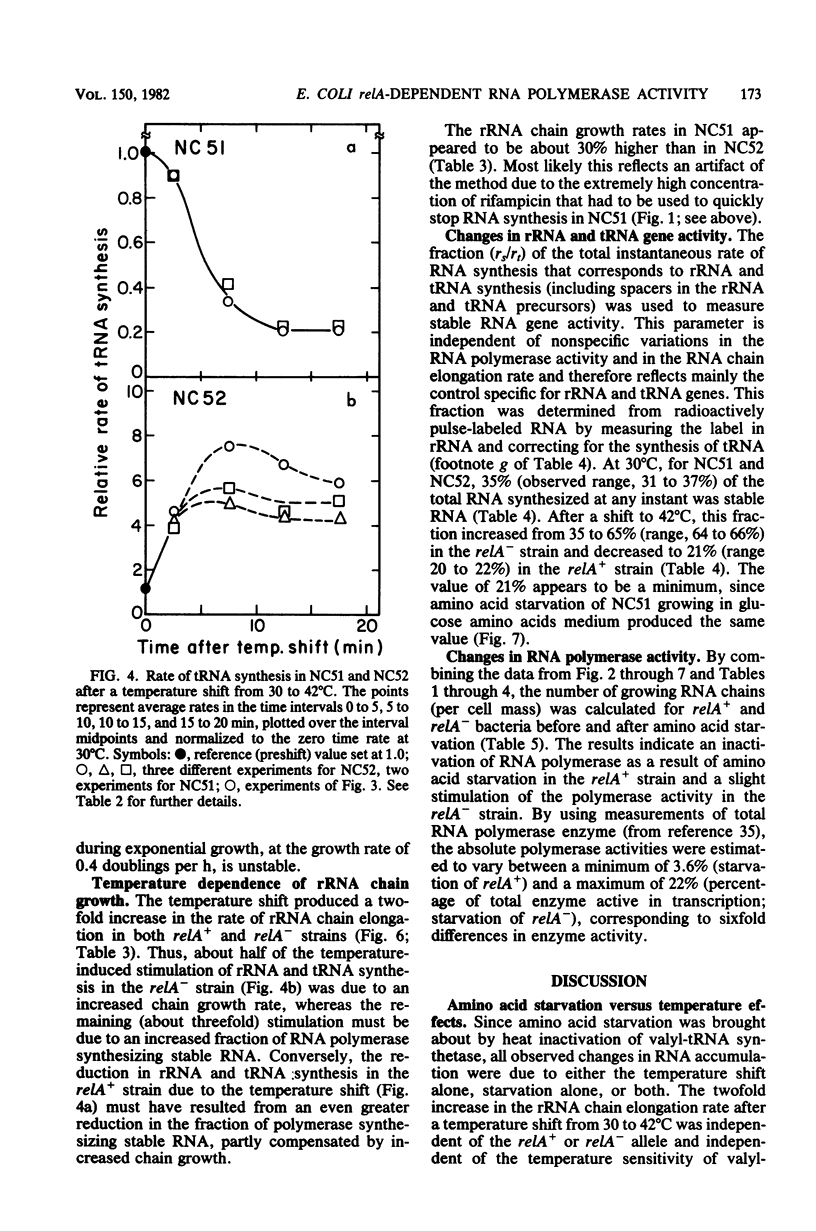
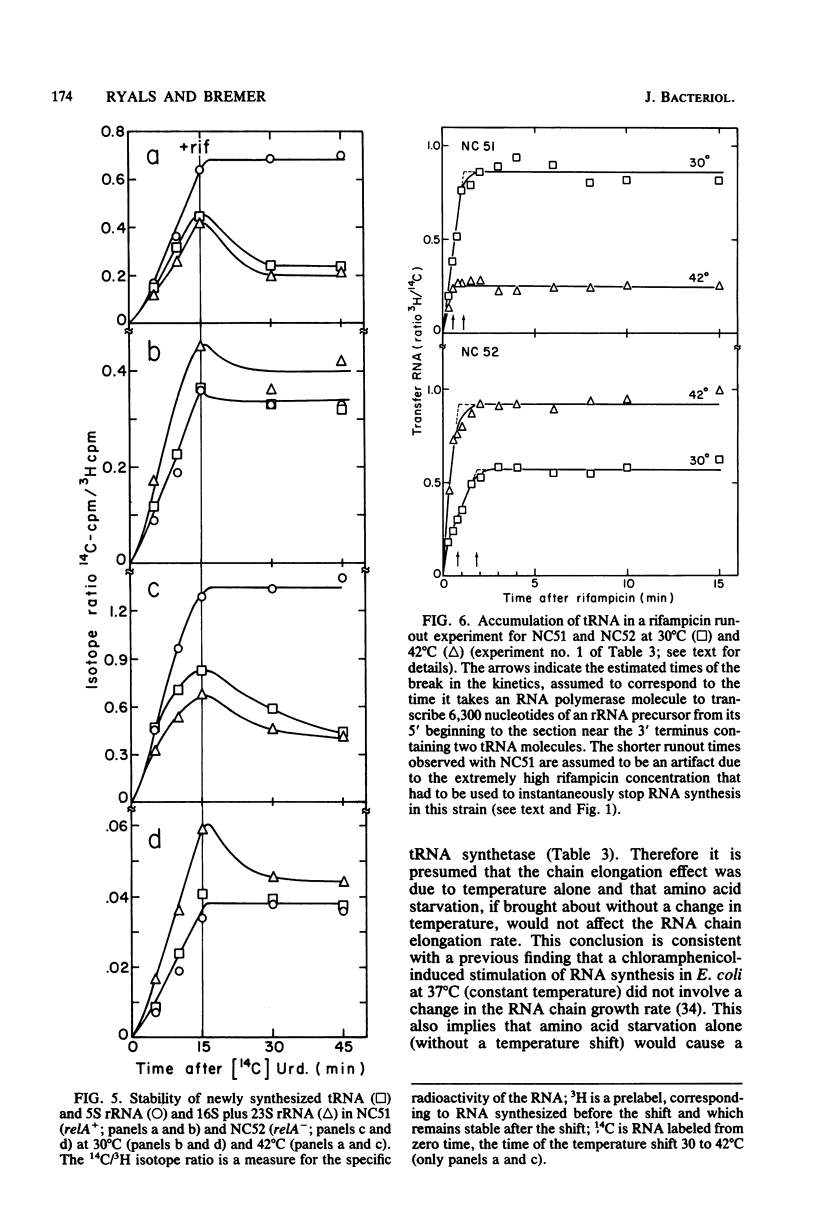
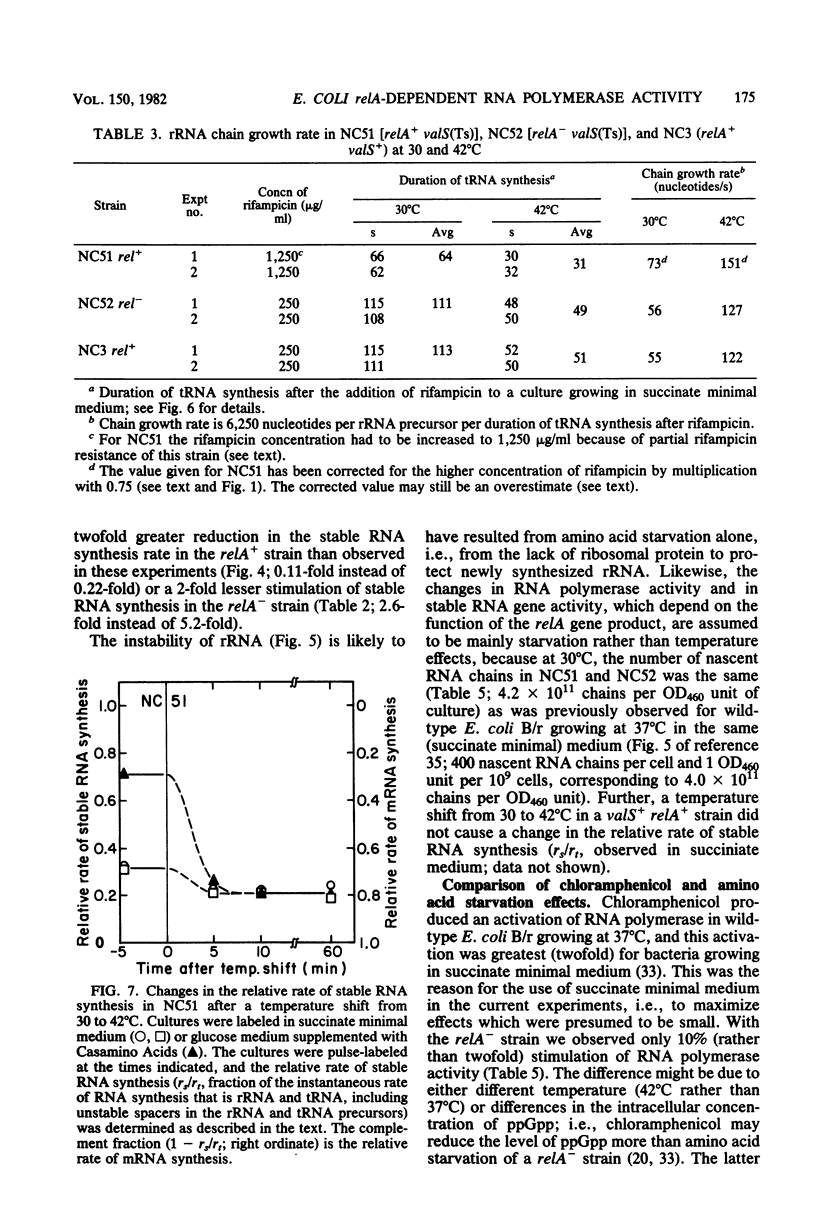
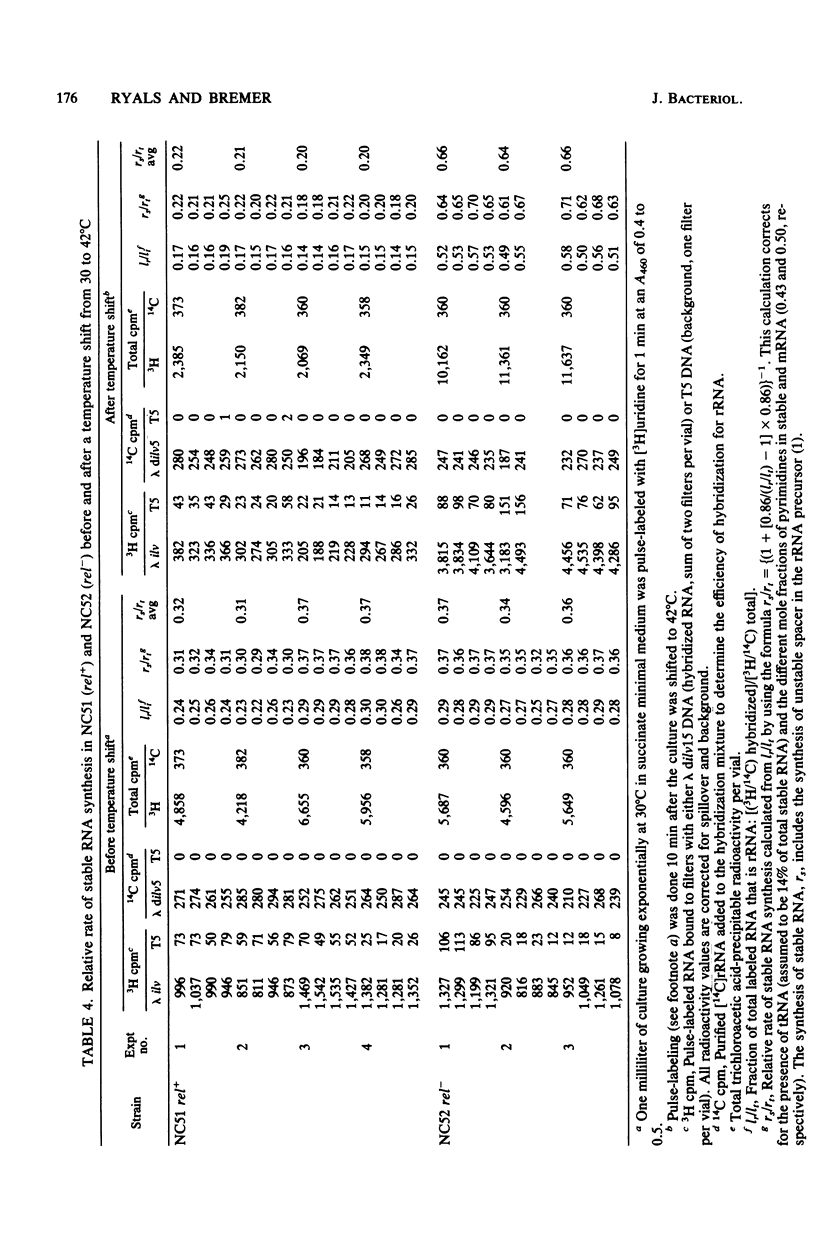
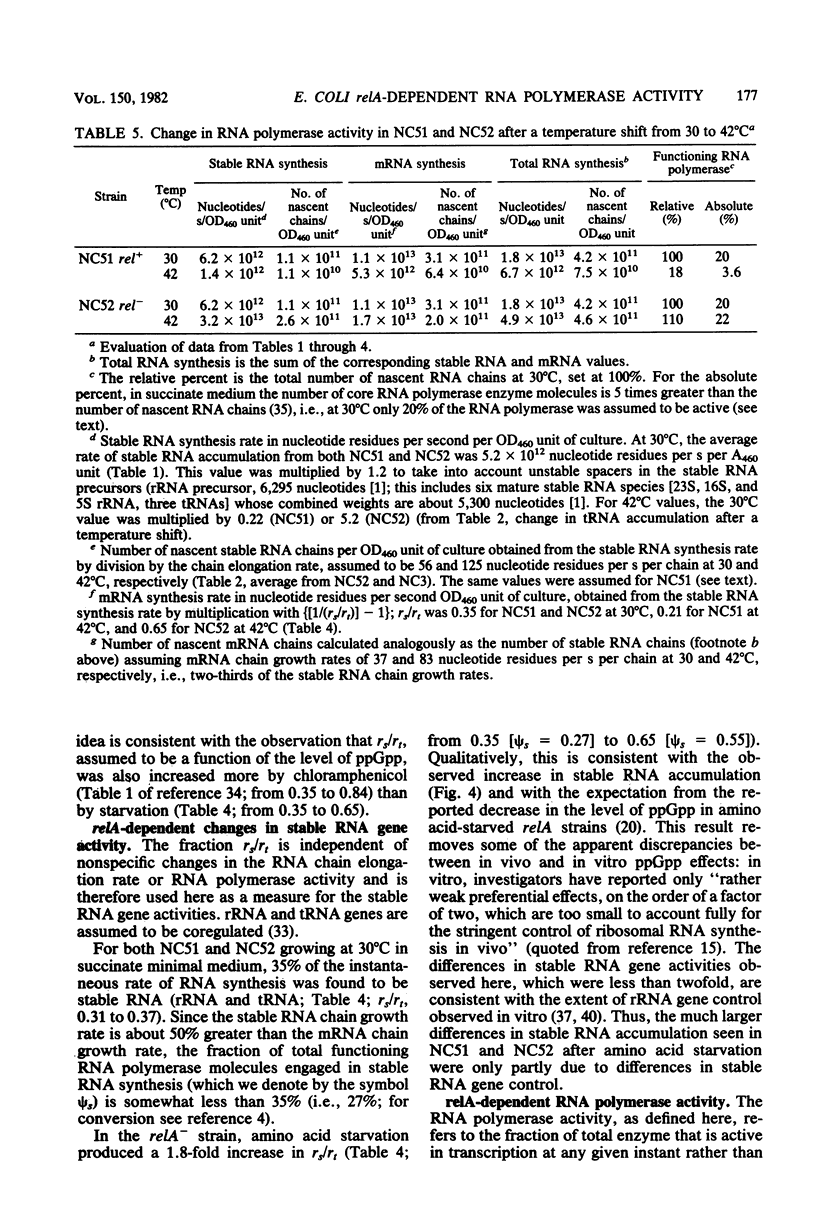
Parameters relating to RNA synthesis were measured after a temperature shift from 30 to 42 degrees C, in a relA+ and relA- isogenic pair of Escherichia coli strains containing a temperature-sensitive valyl tRNA synthetase. The following results were obtained: (i) the rRNA chain growth rate increased 2-fold in both strains; (ii) newly synthesized rRNA became unstable in both strains; (iii) the stable RNA gene activity (rRNA and tRNA, measured as stable RNA synthesis rate relative to the total instantaneous rate of RNA synthesis) decreased 1.7-fold in the relA+ strain and increased 1.9-fold in the relA mutant; and (iv) the RNA polymerase activity (measured by the percentage of total RNA polymerase enzyme active in transcription an any instant) decreased from 20 to 3.6% in the relA+ strain and remained unchanged (or increased at most to 22%) in the relA mutant. It is suggested that both rRNA gene activity and the RNA polymerase activity depend on the intracellular concentration of guanosine tetraphosphate, whereas the altered chain elongation rate and stability of rRNA are temperature or amino acid starvation effects, respectively, without involvement of relA function.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bremer H., Berry L., Dennis P. P. Regulation of ribonucleic acid synthesis in Escherichia coli B-r: an analysis of a shift-up. II. Fraction of RNA polymerase engaged in the synthesis of stable RNA at different steady-state growth rates. J Mol Biol. 1973 Mar 25;75(1):161–179. doi: 10.1016/0022-2836(73)90536-6. [DOI] [PubMed] [Google Scholar]
- Bremer H., Stent G. S. Binding of E. coli transfer RNA to E. coli RNA polymerase. Acta Biochim Pol. 1966;13(4):367–377. [PubMed] [Google Scholar]
- Bremer H., Yegian C., Konrad M. Inactivation of purified Escherichia coli RNA polymerase by transfer RNA. J Mol Biol. 1966 Mar;16(1):94–103. doi: 10.1016/s0022-2836(66)80265-6. [DOI] [PubMed] [Google Scholar]
- Bremer H., Yuan D. RNA chain growth-rate in Escherichia coli. J Mol Biol. 1968 Dec 14;38(2):163–180. doi: 10.1016/0022-2836(68)90404-x. [DOI] [PubMed] [Google Scholar]
- Cashel M., Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969 Mar 1;221(5183):838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- Dalbow D. G. Synthesis of RNA polymerase in Escherichia coli B-r growing at different rates. J Mol Biol. 1973 Mar 25;75(1):181–184. doi: 10.1016/0022-2836(73)90537-8. [DOI] [PubMed] [Google Scholar]
- Dennis P. P., Nordan D. H. Characterization of the hybridization between purified 16S and 23S ribosomal ribonucleic acid and ribosomal deoxyribonucleic acid from Escherichia coli. J Bacteriol. 1976 Oct;128(1):28–34. doi: 10.1128/jb.128.1.28-34.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant J. A. Stringent control in E. coli. Annu Rev Genet. 1979;13:393–415. doi: 10.1146/annurev.ge.13.120179.002141. [DOI] [PubMed] [Google Scholar]
- Gausing K. Regulation of ribosome production in Escherichia coli: synthesis and stability of ribosomal RNA and of ribosomal protein messenger RNA at different growth rates. J Mol Biol. 1977 Sep 25;115(3):335–354. doi: 10.1016/0022-2836(77)90158-9. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Block R. Synthesis of guanosine tetra- and pentaphosphate requires the presence of a codon-specific, uncharged transfer ribonucleic acid in the acceptor site of ribosomes. Proc Natl Acad Sci U S A. 1973 May;70(5):1564–1568. doi: 10.1073/pnas.70.5.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle D. C., Chamberlin M. J. Studies of the binding of Escherichia coli RNA polymerase to DNA. II. The kinetics of the binding reaction. J Mol Biol. 1972 Sep 28;70(2):187–195. doi: 10.1016/0022-2836(72)90532-3. [DOI] [PubMed] [Google Scholar]
- Lagosky P. A., Chang F. N. Influence of amino acid starvation on guanosine 5'-diphosphate 3'-diphosphate basal-level synthesis in Escherichia coli. J Bacteriol. 1980 Nov;144(2):499–508. doi: 10.1128/jb.144.2.499-508.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra U., Barash F. DNA-dependent synthesis of RNA by Escherichia coli RNA polymerase: release and reinitiation of RNA chains from DNA templates. Proc Natl Acad Sci U S A. 1969 Oct;64(2):779–786. doi: 10.1073/pnas.64.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzura H., Hansen B. S., Zeuthen J. Biosynthesis of the beta and beta' subunits of RNA polymerase in Escherichia coli. J Mol Biol. 1973 Feb 15;74(1):9–20. doi: 10.1016/0022-2836(73)90350-1. [DOI] [PubMed] [Google Scholar]
- Mueller K., Bremer H. Heterogeneous initiation and termination of enzymically synthesized ribonucleic acid. J Mol Biol. 1969 Jul 14;43(1):89–107. doi: 10.1016/0022-2836(69)90081-3. [DOI] [PubMed] [Google Scholar]
- Mueller K. The function of the -factor of Escherichia coli RNA polymerase in template site selection. Mol Gen Genet. 1971;111(3):273–296. doi: 10.1007/BF00433112. [DOI] [PubMed] [Google Scholar]
- Nierlich D. P. Amino acid control over RNA synthesis: a re-evaluation. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1345–1352. doi: 10.1073/pnas.60.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramabhadran T. V., Jagger J. Mechanism of growth delay induced in Escherichia coli by near ultraviolet radiation. Proc Natl Acad Sci U S A. 1976 Jan;73(1):59–63. doi: 10.1073/pnas.73.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. P. Initiation of transcription by Escherichia coli RNA polymerase from supercoiled and non-supercoiled bacteriophage PM2 DNA. J Mol Biol. 1975 Feb 5;91(4):477–487. doi: 10.1016/0022-2836(75)90274-0. [DOI] [PubMed] [Google Scholar]
- Shen V., Bremer H. Chloramphenicol-induced changes in the synthesis of ribosomal, transfer, and messenger ribonucleic acids in Escherichia coli B/r. J Bacteriol. 1977 Jun;130(3):1098–1108. doi: 10.1128/jb.130.3.1098-1108.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen V., Bremer H. Rate of ribosomal ribonucleic acid chain elongation in Escherichia coli B/r during chloramphenicol treatment. J Bacteriol. 1977 Jun;130(3):1109–1116. doi: 10.1128/jb.130.3.1109-1116.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd N. S., Churchward G., Bremer H. Synthesis and activity of ribonucleic acid polymerase in Escherichia coli. J Bacteriol. 1980 Mar;141(3):1098–1108. doi: 10.1128/jb.141.3.1098-1108.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TISSIERES A., BOURGEOIS S., GROS F. Inhibition of RNA polymerase by RNA. J Mol Biol. 1963 Jul;7:100–103. doi: 10.1016/s0022-2836(63)80024-8. [DOI] [PubMed] [Google Scholar]
- Travers A. A. A tRNATyr promoter with an altered in vitro response to ppgpp. J Mol Biol. 1980 Jul 25;141(1):91–97. doi: 10.1016/s0022-2836(80)80030-1. [DOI] [PubMed] [Google Scholar]
- Travers A. A., Buckland R., Goman M., Le Grice S. S., Scaife J. G. A mutation affecting the sigma subunit of RNA polymerase changes transcriptional specificity. Nature. 1978 Jun 1;273(5661):354–358. doi: 10.1038/273354a0. [DOI] [PubMed] [Google Scholar]
- Travers A. Modulation of RNA polymerase specificity by ppGpp. Mol Gen Genet. 1976 Aug 19;147(2):225–232. doi: 10.1007/BF00267575. [DOI] [PubMed] [Google Scholar]
- van Ooyen A. J., Gruber M., Jorgensen P. The mechanism of action of ppGpp on rRNA synthesis in vitro. Cell. 1976 May;8(1):123–128. doi: 10.1016/0092-8674(76)90193-8. [DOI] [PubMed] [Google Scholar]



