Abstract
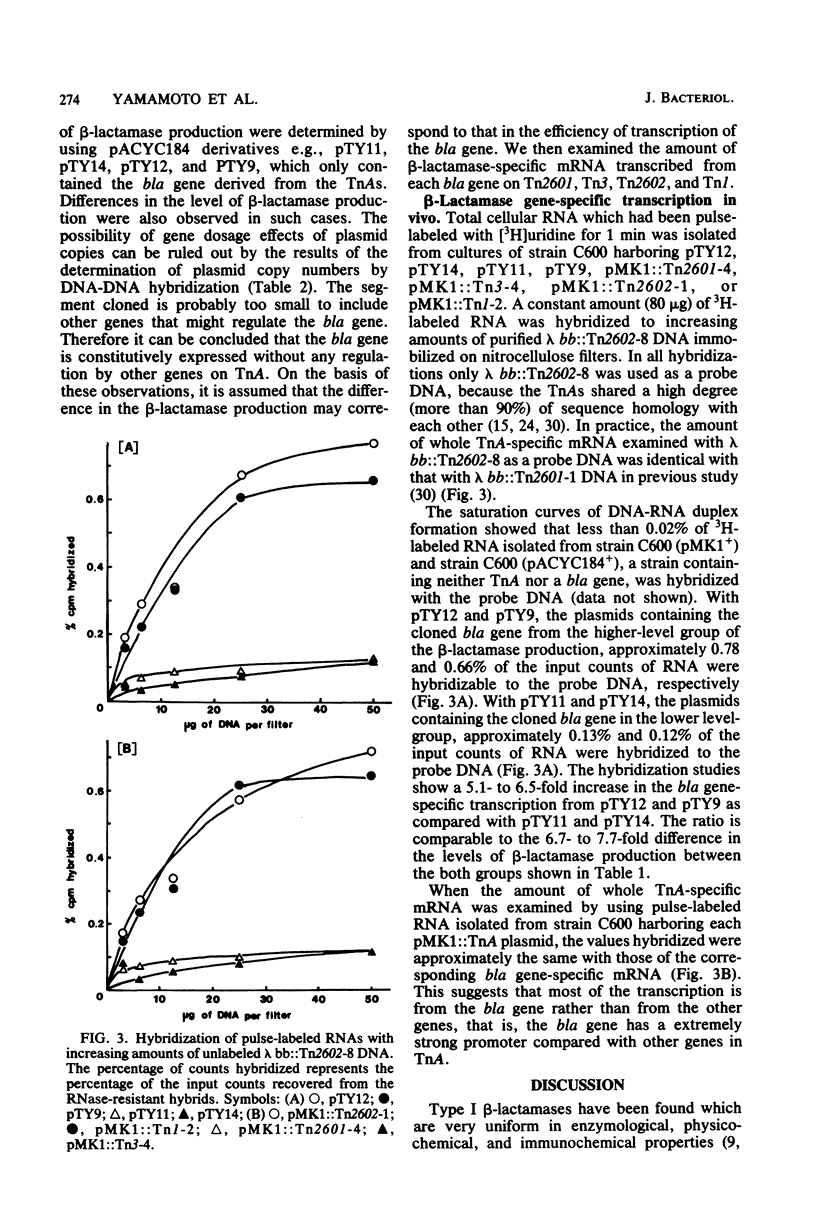
The beta-lactamase gene from four kinds of ampicillin transposons, Tn2601, Tn3, Tn2602 and Tn1, specifying the type I (or TEM type, alternatively) beta-lactamase was cloned onto plasmid pACYC184, and the level of in vivo transcription from each beta-lactamase gene was determined by DNA-RNA hybridization. Type I beta-lactamase is very uniform enzymologically, but heterogeneous in absolute levels of enzyme activity. The results demonstrated that the heterogeneity can be explained by the efficiency of transcription of each beta-lactamase gene, suggesting a difference in its promoter efficiency. A comparison of the levels of transcription of the beta-lactamase gene and the whole ampicillin transposon suggested that the beta-lactamase gene has the strongest promoter all of the genes in the ampicillin transposon.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calame K. L., Yamada Y., Shanblatt S. H., Nakada D. Location of promoter sites on plasmid NTP1 which contains the ampicillin resistance transposon Tn1701. J Mol Biol. 1979 Feb 5;127(4):397–409. doi: 10.1016/0022-2836(79)90229-8. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J., Casadaban M. J., Lemaux P. G., Cohen S. N. Identification and characterization of a self-regulated repressor of translocation of the Tn3 element. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4020–4024. doi: 10.1073/pnas.76.8.4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J., Lemaux P. G., Casadaban M. J., Cohen S. N. Transposition protein of Tn3: identification and characterisation of an essential repressor-controlled gene product. Nature. 1979 Dec 20;282(5741):801–806. doi: 10.1038/282801a0. [DOI] [PubMed] [Google Scholar]
- Crowlesmith I., Howe T. G. Quantitative correlation between penicillin resistance and beta-lactamase activity specified by the R plasmids R1, R1 bla-45, and RP1 in Escherichia coli K-12. Antimicrob Agents Chemother. 1980 Nov;18(5):675–679. doi: 10.1128/aac.18.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Shaw E. J., Sykes R. B., Richmond M. H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R., Vapnek D. In vivo transcription of R-plasmid deoxyribonucleic acid in Escherichia coli strains with altered antibiotic resistance levels and/or conjugal proficiency. J Bacteriol. 1976 Mar;125(3):1148–1155. doi: 10.1128/jb.125.3.1148-1155.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill R. E., Heffron F., Falkow S. Identification of the protein encoded by the transposable element Tn3 which is required for its transposition. Nature. 1979 Dec 20;282(5741):797–801. doi: 10.1038/282797a0. [DOI] [PubMed] [Google Scholar]
- Grinsted J., Saunders J. R., Ingram L. C., Sykes R. B., Richmond M. H. Properties of a R factor which originated in Pseudomonas aeruginosa 1822. J Bacteriol. 1972 May;110(2):529–537. doi: 10.1128/jb.110.2.529-537.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Datta N., Kontomichalou P., Smith J. T. Molecular specificities of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol. 1974 Jan;117(1):56–62. doi: 10.1128/jb.117.1.56-62.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Jacob A. E. Transposition of ampicillin resistance from RP4 to other replicons. Mol Gen Genet. 1974;132(1):31–40. doi: 10.1007/BF00268228. [DOI] [PubMed] [Google Scholar]
- Heffron F., Sublett R., Hedges R. W., Jacob A., Falkow S. Origin of the TEM-beta-lactamase gene found on plasmids. J Bacteriol. 1975 Apr;122(1):250–256. doi: 10.1128/jb.122.1.250-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecko D. J., Cohen S. N. Site specific recA--independent recombination between bacterial plasmids: involvement of palindromes at the recombinational loci. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1373–1377. doi: 10.1073/pnas.72.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matthew M., Hedges R. W. Analytical isoelectric focusing of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol. 1976 Feb;125(2):713–718. doi: 10.1128/jb.125.2.713-718.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell E., Datta N. The relation of resistance transfer factors to the F-factor (sex-factor) of Escherichia coli K12. Genet Res. 1966 Feb;7(1):134–140. doi: 10.1017/s0016672300009538. [DOI] [PubMed] [Google Scholar]
- Ooka T., Hashimoto H., Mitsuhashi S. Comparison of penicillinases produced by R factors isolated from ampicillin-resistant gram-negative bacteria. Jpn J Microbiol. 1970 Mar;14(2):123–128. doi: 10.1111/j.1348-0421.1970.tb00499.x. [DOI] [PubMed] [Google Scholar]
- Rubens C., Heffron F., Falkow S. Transposition of a plasmid deoxyribonucleic acid sequence that mediates ampicillin resistance: independence from host rec functions and orientation of insertion. J Bacteriol. 1976 Oct;128(1):425–434. doi: 10.1128/jb.128.1.425-434.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Takahashi I., Yamagishi S. Iodometric assay method for beta-lactamase with various beta-lactam antibiotics as substrates. Antimicrob Agents Chemother. 1978 Jun;13(6):910–913. doi: 10.1128/aac.13.6.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Takahashi K., Yamagishi S., Mitsuhashi S. Variant of penicillinase mediated by an R factor in Escherichia coli. J Bacteriol. 1970 Nov;104(2):620–629. doi: 10.1128/jb.104.2.620-629.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Katoh R., Shimazu A., Yamagishi S. Gene expression of ampicillin resistance transposons, Tn2601 and Tn2602. Microbiol Immunol. 1980;24(6):479–494. doi: 10.1111/j.1348-0421.1980.tb02852.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Yamagata S., Hashimoto Y., Yamagishi S. Restriction endonuclease cleavage maps of the ampicillin transposons Tn2601 and Tn2602. Microbiol Immunol. 1980;24(12):1139–1149. doi: 10.1111/j.1348-0421.1980.tb02919.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Yokota T. Construction of a physical map of a kanamycin (Km) transposon, Tn5, and a comparison to another Km transposon, Tn903. Mol Gen Genet. 1980 Apr;178(1):77–83. doi: 10.1007/BF00267215. [DOI] [PubMed] [Google Scholar]




