Abstract
Matrix metalloproteinases (MMPs) are expressed by T cells and macrophages, but there is a paucity of evidence for their role in immune responses. We have studied mice with deficiencies of stromelysin-1 (MMP-3) or gelatinase B (MMP-9) in a dinitrofluorobenzene (DNFB)-induced model of contact hypersensitivity (CHS). Stromelysin-1-deficient mice showed a markedly impaired CHS response to topical DNFB, although they responded normally to cutaneously applied phenol, an acute irritant. Lymphocytes from lymph nodes of DNFB-sensitized stromelysin-1-deficient mice did not proliferate in response to specific soluble antigen dinitrobenzenesulfonic acid, but did proliferate identically to lymph node lymphocytes from wild-type mice when presented with the mitogen Con A. An intradermal injection of stromelysin-1 immediately before DNFB sensitization rescued the impaired CHS response to DNFB in stromelysin-1-deficient mice. Unlike stromelysin-1-deficient mice, gelatinase B-deficient mice exhibited a CHS response comparable to wild-type controls at 1 day postchallenge, but the response persisted beyond 7 days in contrast to the complete resolution observed in wild-type mice by 7 days. However, gelatinase B-deficient mice had a normal rate of resolution of acute inflammation elicited by cutaneous phenol. Gelatinase B-deficient mice failed to show IL-10 production at the site of CHS, an essential feature of resolution in control mice. These results indicate that stromelysin-1 and gelatinase B serve important functions in CHS. Stromelysin-1 is required for initiation of the response, whereas gelatinase B plays a critical role in its resolution.
Contact hypersensitivity (CHS) is a classic experimental model for the study of antigen-specific, T cell-mediated immune responses (1–4). In CHS, typified by poison ivy dermatitis, initial sensitization occurs by topical application of an immunizing antigen, processing of antigen by skin dendritic cells (i.e., Langerhans cells), migration of antigen-bearing Langerhans cells to regional lymph nodes, and stimulation of naive T cells (5, 6). A second exposure of the same antigen to skin then results in local influx of antigen-specific T cells, which release cytokines that attract other inflammatory cells to the site, dilate cutaneous blood vessels, and cause dermal edema (7). Typically, the resultant swelling is used to quantify the immune response.
Matrix metalloproteinases (MMPs) are a family of zinc-containing, extracellular, matrix-degrading enzymes that share common structural and functional properties (8). There are 14 well characterized human MMPs: three collagenases, three stromelysins, two gelatinases, metalloelastase, matrilysin, and four cell-associated or “membrane-type” MMPs. Immune cells, including T cells, Langerhans cells, and macrophages, produce MMPs such as stromelysin-1 and gelatinase B. Stromelysin-1 has broad substrate specificity, attacking laminin, collagen IV, fibronectin, entactin, and proteoglycans (9). Gelatinase B (MMP-9) is highly efficient in the cleavage of denatured collagens of all genetic types, but also degrades collagen IV, fibronectin, and elastin (8).
Although MMP expression by immune cells has been well documented (10), a specific role for MMPs in immune responses in vivo has not been demonstrated. To address this issue, we studied stromelysin-1- and gelatinase B-deficient mice in a DNFB-induced model of CHS. Our results demonstrate that stromelysin-1 is essential for the initiation of CHS and that gelatinase B is necessary for its resolution.
MATERIALS AND METHODS
Mice.
Stromelysin-1-deficient mice were generated by targeted mutagenesis (11) and maintained on a B10.RIII background. Gelatinase B-deficient mice were generated by targeted mutagenesis (12) and maintained on a 129/Sv background. Matrilysin-deficient mice were kindly provided by Carole L. Wilson (Washington University School of Medicine) (13). MMP-deficient mice and their wild-type controls were 8–12 weeks old at the time of experimental manipulation.
CHS.
CHS was induced in mice with DNFB (4, 14). Briefly, 20 μl of 0.5% DNFB (Sigma) in 4:1 acetone/olive oil was applied topically over an area of 3–4 cm2 on the shaved abdomen. This procedure was repeated 24 h later. Five days afterward, the mice were challenged by spreading 20 μl of 0.2% DNFB on the dorsal surface of each ear. Ear thickness was used to quantify CHS and was measured with a digital micrometer (Mitutoyo, Japan) immediately before DNFB challenge (baseline) and 1, 2, 3, 5, and 7 days after challenge. Four to six mice were used in each experimental group. Ear swelling in sensitized mice was corrected for the small “irritant” effect produced by application of 0.2% DNFB. This irritant effect was determined in naive gene-deficient and wild-type mice and was 3% at day 1, 2% at day 2, and less than 1% thereafter.
For stromelysin-1 rescue experiments, we used truncated, recombinant stromelysin-1 containing the enzyme’s catalytic domain (kindly provided by Vijay Baragi, Parke-Davis) (15). Truncated stromelysin has the same substrate specificity as the full-length active enzyme (15). Ten micrograms (70 μl) of stromelysin-1 or normal saline (70 μl) was injected intradermally at the sensitization site on the abdomen 10 min before the application of DNFB. Both the injection of stromelysin-1 and DNFB sensitization were repeated 1 day later. Ear challenge with DNFB was performed 5 days later.
Irritant Dermatitis.
To induce an acute irritant dermatitis, 10 μl of 15% phenol in absolute ethanol (vol/vol) was painted on the dorsal surface of the mouse ear (4). Ear thickness was measured before application and 4, 24, and 72 h afterward. Four to six mice were used in each experimental group.
Lymphocyte Proliferation.
The cervical, axillary, and inguinal lymph nodes from animals that had been sensitized with DNFB and subjected to ear challenge were excised immediately after determination of ear thickness, and the lymphocytes were isolated (16). Lymphocytes from five stromelysin-1-deficient mice or five wild-type control mice were pooled, and 5 × 105 cells were plated in triplicate on 96-well flat-bottom microtiter plates containing 100 μl RPMI 1640 medium with 10% FBS/2 mM glutamine/0.1% 2-mercaptoethanol/5 units/ml of penicillin/streptomycin. Cells were cultured in the presence or absence of 25–200 μg/ml 2,4-dinitrobenzenesulfonic acid (DNBS; Sigma), a soluble analog of DNFB, or 1–4 μg/ml of Con A (Sigma). After 24 h, 1 μCi of [3H]thymidine (DuPont/NEN) was added to each well, and 24 h later, cells were collected by using a cell harvester. The incorporation of radioactivity in each well, a measurement of cell proliferation, was determined by liquid scintillation counting (17).
Tissue IL-10.
At various times after DNFB challenge, mice were killed and the ears were excised and submerged immediately into liquid N2. The frozen tissue from gelatinase B-deficient mice and their wild-type controls was pulverized and dissolved in 1 ml PBS containing 0.1% Tween 20, followed by homogenization for 30–60 sec. Samples then were refrozen quickly in liquid N2, thawed at 37°C, sonicated for 15 sec, and centrifuged for 5 min at 12,000 rpm. Supernatants were removed and IL-10 was quantified by ELISA (R & D Systems). The detection limit for the assay was 4 pg/ml, and the antibody was a polyclonal derived against the entire IL-10 protein. Results were normalized to total protein as determined by the bicinchoninic acid protein assay (Pierce).
Flow Cytometry.
Single-cell suspensions from spleen were prepared from an 8-week-old stromelysin-1-deficient mouse and its wild-type control and an 8-week-old gelatinase B-deficient mouse and its wild-type control. Erythrocytes were removed by incubating at 37°C for 10 min in 5 ml Tris lysing buffer (0.16 M Tris/0.16 M NH4Cl, pH 7.35) and centrifuging at 1,000 rpm for 15 min. Cells were washed in flow cytometry buffer (PBS, pH 7.35, containing 0.1% BSA and 0.01% sodium azide), and 1.0 × 106 cells were incubated for 30 min at 4°C with the following antibody–receptor conjugates: phycoerythrin-conjugated anti-mouse CD3, CD4, and CD8 (PharMingen), FITC-conjugated anti-mouse IgM (PharMingen), and FITC-conjugated anti-mouse T cell antigen receptor (PharMingen). Cells were washed again with flow cytometry buffer, fixed in PBS containing 1% paraformaldehyde, and analyzed on a Becton Dickinson FACScan by using the lysis i program.
RESULTS
Stromelysin-1-Deficient Mice Exhibit Impaired Contact Hypersensitivity.
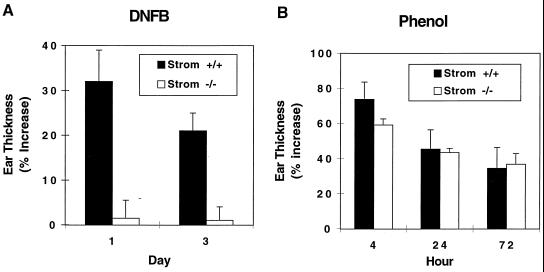
Stromelysin-1-deficient mice and their wild-type controls were studied in a DNFB-induced model of CHS. As shown in Fig. 1A, ear thickness in wild-type mice was increased >30% at 24 h after DNFB challenge and remained increased by 20% at 72 h. In contrast, stromelysin-1-deficient mice exhibited <2% increase in ear thickness at 24 h and 72 h post-DNFB challenge. Histologic evaluation of the ears disclosed an exuberant inflammatory cell infiltrate in sensitized wild-type mice, but essentially no detectable inflammation in sensitized stromelysin-1-deficient mice (not shown). These data indicate a significant defect in the ability of stromelysin-1-deficient mice to exhibit CHS.
Figure 1.
Impaired CHS response and normal irritant dermatitis in stromelysin-1-deficient mice. (A) Eight- to 12-week-old stromelysin-1-deficient mice and their wild-type controls were painted on shaved abdominal skin with 20 μl of 0.5% DNFB on successive days. Five days later, the mice were challenged with 20 μl of 0.2% DNFB applied topically to the dorsal surface of both ears. (B) Eight- to 12-week-old stromelysin-1-deficient mice and their wild-type controls were painted on the dorsal surface of ears with 10 μl of 15% phenol. In both A and B, ear thickness was measured at the indicated time points as described in Materials and Methods. Solid bars, wild-type mice; open bars, stromelysin-1-deficient mice. Values are mean ± SEM, n = 8–12.
To determine whether stromelysin-1 is required for nonimmune-mediated cutaneous inflammation, we applied 15% phenol to the ears of stromelysin-1-deficient and wild-type mice. Phenol increased ear thickness 60–70% at 4 h, which declined to 35% at 72 h in both stromelysin-1-deficient and wild-type mice (Fig. 1B). Accordingly, stromelysin-1 is not required to initiate irritant, chemical-induced cutaneous inflammation.
Lymphocytes of DNFB-Sensitized Stromelysin-1-Deficient Mice Exhibit a Muted Proliferative Response to DNBS.
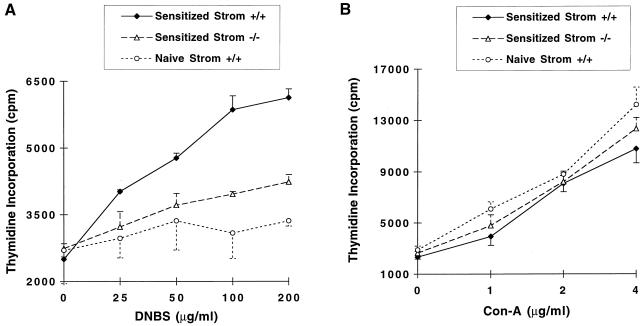
To study the basis for impaired CHS in stromelysin-1-deficient mice, we determined the populations of splenic lymphocytes by flow cytometry. Wild-type and stromelysin-1-deficient mice did not differ in their numbers of CD4+, CD8+, CD3+, or IgM-positive cells (data not shown). Next, we examined the proliferative capacity of lymphocytes from the draining lymph nodes of sensitized wild-type and stromelysin-1-deficient mice in response to DNBS, a soluble form of the contact antigen. A dose-dependent proliferative response was observed with cells obtained from DNFB-sensitized wild-type mice (Fig. 2A). In contrast, the proliferative response in lymphocytes from stromelysin-1-deficient mice was minimal and similar to the response with lymphocytes from nonsensitized (i.e., naive) wild-type mice. Nevertheless, lymphocytes from stromelysin-1-deficient mice exhibited normal lymphocyte proliferation when challenged with the mitogen Con A (Fig. 2B). Together, these results indicate that stromelysin-1-deficient lymphocytes do not become sensitized to DNFB when using an in vivo regimen that does sensitize lymphocytes from wild-type mice.
Figure 2.
Reduced lymphocyte proliferative response to soluble contact antigen and normal proliferative response to Con A are seen in stromelysin-1-deficient mice. Lymphocytes were isolated from the cervical, axillary, and inguinal lymph nodes of animals sensitized and subjected to ear challenge with DNFB. (A) In vitro proliferative response to soluble form of the contact antigen, DNBS, was measured as described in Materials and Methods. (B) In vitro proliferative response to the mitogen Con A was measured as described in Materials and Methods. ♦, Wild-type mice; ▵, stromelysin-1-deficient mice; ○, naive mice. Each graph represents results from an experiment with n = 3; values are mean ± SEM. Each experiment was repeated three times with similar results.
Intradermal Injection of Stromelysin-1 Restores Normal CHS in Stromelysin-1-Deficient Mice.
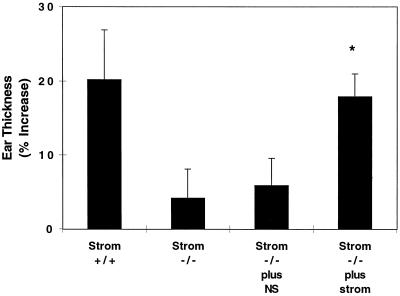
Because our results indicated a defect in sensitization of lymphocytes from stromelysin-1-deficient mice, we tested whether intradermal injection of stromelysin-1 immediately before DNFB sensitization would restore normal CHS. We injected either stromelysin-1 (10 μg) or normal saline intradermally into the shaved abdominal wall just before sensitization with DNFB. As shown in Fig. 3, exogenous stromelysin-1 restored CHS to DNFB challenge 5 days later. These findings lend further support to a specific role for stromelysin-1 in the sensitization phase of the CHS response.
Figure 3.
Normal CHS is restored in stromelysin-1-deficient mice by intradermal injection of stromelysin-1. Ten micrograms of recombinant stromelysin-1 in 70 μl normal saline or 70 μl normal saline (NS) was injected intradermally at the site of sensitization 10 min before application of DNFB to stromelysin-1-deficient mice on 2 successive days. The mice then were challenged with DNFB 5 days later. Two days after challenge, ear swelling was measured. Values are mean ± SEM, n = 8–12. ∗, P < 0.05, compared to either Strom −/− or Strom −/− plus NS.
Gelatinase B-Deficient Mice Exhibit Prolonged CHS.
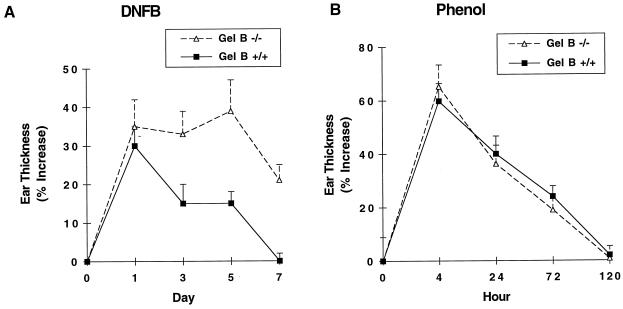
Wild-type and gelatinase B-deficient mice were sensitized and challenged with DNFB 5 days later. Both genotypes exhibited a 30–35% increase in ear thickness at 24 h after DNFB challenge, but the resolution of the responses differed markedly (Fig. 4A). In wild-type mice, ear thickness decreased to 15% at 72 h and returned to baseline by day 7. Gelatinase B-deficient mice, in contrast, exhibited a prolonged response, with 40% increased ear thickness at day 5 and 20% increased ear thickness 7 days after challenge. Histologic evaluation at 7 days postchallenge confirmed the persistence of inflammatory cell infiltration in the gelatinase B-deficient mice and its absence in wild-type mice (not shown).
Figure 4.
Persistent CHS response and normal resolution of irritant dermatitis in gelatinase B-deficient mice. (A) Eight- to 12-week-old gelatinase B-deficient mice and their wild-type controls were painted on shaved abdominal skin with 20 μl of 0.5% DNFB on successive days. Five days later, the mice were challenged with topically applied 20 μl of 0.2% DNFB to the dorsal surface of both ears. (B) Eight- to 12-week-old gelatinase B-deficient mice and their wild-type controls were painted on the dorsal surface of both ears with 10 μl of 15% phenol. In both A and B, ear thickness was measured at the indicated time points. ■, Wild-type mice; ▵, gelatinase B-deficient mice. Values are mean ± SEM, n = 8–12.
Although there was delayed resolution of DNFB-induced contact dermatitis, gelatinase B deficiency did not affect the rate of resolution of inflammation elicited by phenol (Fig. 4B). Thus, gelatinase B is not required for the resolution of an acute, nonspecific, irritant dermatitis.
Gelatinase B-Deficient Mice Have Impaired Production of IL-10 in CHS Response.
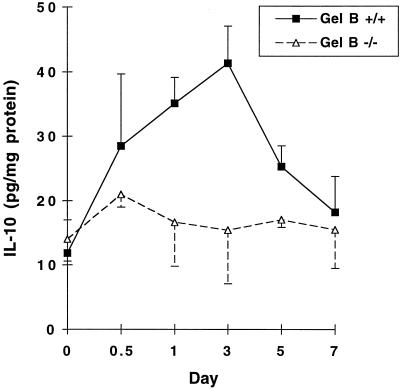
IL-10 has an important antiinflammatory role in the resolution of CHS (18). Accordingly, we speculated that the reason for delayed resolution of CHS in gelatinase B-deficient mice might involve IL-10 production. We measured IL-10 protein in ear tissue extracts of DNFB-sensitized and subsequently challenged wild-type and gelatinase B-deficient mice. In wild-type mice, IL-10 levels were increased significantly by 12 h after challenge with DNFB, peaked at 72 h at 4-fold over baseline, and returned to baseline by 7 days (Fig. 5). In contrast, IL-10 levels remained at baseline throughout the entire course of the CHS response in gelatinase B-deficient mice. Failure to have a local increase in IL-10 would appear to be involved in the prolonged CHS response found in gelatinase B deficiency. Of importance, spleen cells of gelatinase B-deficient mice and their wild-type controls exhibited similar fold-increases of IL-10 after being exposed to lipopolysaccharide in vitro (data not shown), indicating that gelatinase B-deficient mice are capable of IL-10 production.
Figure 5.
IL-10 protein levels in ear tissue do not increase in gelatinase-B-deficient mice after DNFB challenge. Gelatinase B-deficient mice and their wild-type controls were sensitized and challenged as described in Materials and Methods. At various times after DNFB challenge, mice ears were excised and used for protein extraction. IL-10 in ear homogenate supernatants was quantified by ELISA. ■, Wild-type mice; ▵, gelatinase B-deficient mice. Values are mean ± SEM, n = 3.
Matrilysin (MMP-7)-Deficient Mice Have Normal CHS.
To assess whether MMP deficiencies in general alter CHS, we tested CHS in mice with a null mutation of matrilysin. In contrast to stromelysin-1- and gelatinase B-deficient mice, matrilysin-deficient mice showed a similar CHS response to DNFB as their wild-type controls (data not shown). Accordingly, not all MMPs affect the CHS response.
DISCUSSION
The principal finding in this study is that stromelysin-1 and gelatinase B are necessary for a normal CHS response in mice. Stromelysin-1 activity is necessary for initiation of the CHS response, whereas gelatinase B is required for its timely resolution. These in vivo data implicate MMPs in immune-mediated reactions.
Our findings indicate the requirement for stromelysin-1 in initiation of CHS. Many steps are involved in CHS sensitization, most of them dependent on the antigen-presenting cells of skin, the Langerhans cells (LC). LC, which reside in the suprabasal portion of the epidermis, capture and process antigens in the skin. LC that have processed antigen migrate to T cell-rich areas in the draining lymph nodes and present processed antigen in association with MHC products to naive T cells (5, 19, 20). To reach the draining lymph nodes, LC must detach from adjacent keratinocytes, cross the basement membrane at the dermo-epidermal junction, and then migrate through dermal matrix. One or more of these steps may require stromelysin-1.
Our finding that injecting stromelysin-1 into the skin-sensitization site restored a normal CHS response in stromelysin-1-deficient mice suggests a local requirement for stromelysin-1 activity. In this regard, E-cadherin, a homophilic adhesion molecule, is expressed in the epidermis by both LC and keratinocytes (21, 22). Interestingly, when stromelysin-1 was overexpressed in mammary epithelial cells in culture, cell detachment occurred that correlated with stromelysin-1 activity and was associated with release of E-cadherin fragments (23). Thus, stromelysin-1 may be required for E-cadherin degradation and dissociation of LC from adjacent keratinocytes. Recently, gelatinase B has been reported to be expressed by LC (24); however, the normal development of CHS exhibited in gelatinase B-deficient mice suggests that gelatinase B is not necessary for releasing LC from their epidermal attachments or for LC migration.
To identify potential mechanisms of how gelatinase B deficiency leads to prolongation of the CHS response, we measured IL-10 protein at the site of antigen challenge because IL-10 is an important down-regulator of delayed-type hypersensitivity reactions (18). Indeed, sensitized mice given neutralizing IL-10 antibody at the time of challenge exhibit a markedly prolonged inflammatory response (29). IL-10 is produced by CD4+ T helper 2 (Th2) cell clones that inhibit the proliferation of CD8+ Th1 cell clones, which release proinflammatory modulators such as IFN-γ and IL-2 (25, 26). IL-10 is also made by a variety of other cell types, including keratinocytes (27), macrophages (28), B lymphocytes (29), and mast cells (30). In addition to promoting CHS resolution at the site of challenge, IL-10 is released during the induction phase of CHS and has been shown to convert epidermal LC from potent inducers of primary immune responses to specifically tolerizing cells (27, 31).
We found that IL-10 levels in challenged ears of sensitized wild-type mice were increased 4-fold over baseline, whereas they remained at baseline in ears of challenged gelatinase B-deficient mice. IL-10 levels were measured in ear extract supernatants that were prepared by repeated homogenizations and freeze-thawings in the presence of detergent to ensure release of this cytokine from the tissue. IL-10 levels were quantified by ELISA with a polyclonal antiserum directed against the entire mature protein. The N-terminal 18 aa of IL-10 indicate the presence of a secretion-leader sequence (32), mouse and human cDNA clones are readily expressed as secreted proteins in monkey COS cells (32), and there is no evidence for protein processing of IL-10 in any system. Thus, we believe that our observations indicate that gelatinase B-deficient mice fail to induce IL-10 production, and this leads to an exaggerated and prolonged CHS response.
We found that spleen cells from naive gelatinase B-deficient mice and wild-type mice provide similar induction of IL-10 upon exposure to LPS. Therefore, gelatinase B-deficient mice can produce IL-10 outside the context of a CHS response. Possible “links” between gelatinase B and IL-10 production include: (i) Th2, but not Th1 cells, require gelatinase B to enter the site of antigen challenge; (ii) gelatinase B stimulates keratinocytes and/or macrophages directly to produce IL-10; or (iii) a product of extracellular matrix is produced by gelatinase B that stimulates IL-10 production by keratinocytes and/or macrophages.
In summary, stromelysin-1 and gelatinase B play important but different roles in CHS in mice. Stromelysin-1 is involved in initiation or induction of the CHS response. In contrast, gelatinase B is required for timely resolution of the response, at least in part, through increasing the local expression of IL-10. These studies are further evidence that functions of MMPs extend beyond the remodeling of extracellular matrix.
Acknowledgments
We express our appreciation to Hui Zhang for conducting the flow cytometry studies and to Dr. Carole L. Wilson, for providing matrilysin-deficient mice. This work was supported by U.S. Public Health Service Grants HL47328 (R.M.S.) and AR35805 (H.G.W.) and the Alan A. and Edith L. Wolff Charitable Trust (R.M.S.). The work also was supported by the Washington University/Monsanto–Searle Biomedical Research Agreement.
ABBREVIATIONS
- MMP
matrix metalloproteinase
- DNFB
2,4-dinitrofluorobenzene
- DNBS
2,4-dinitrobenzenesulfonic acid
- CHS
contact hypersensitivity
- LC
Langerhans cell
References
- 1.Macher E, Chase M W. J Exp Med. 1969;129:81–102. doi: 10.1084/jem.129.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimber I, Botham P, Rattray N, Walsh S. Int Arch Allergy Appl Immunol. 1986;81:258–264. doi: 10.1159/000234144. [DOI] [PubMed] [Google Scholar]
- 3.Ptak W, Janeway C A J, Marcinkiewicz J, Flood P M. Cell Immunol. 1991;132:400–410. doi: 10.1016/0008-8749(91)90037-c. [DOI] [PubMed] [Google Scholar]
- 4.Kondo S, Beissert S B, Wang B, Fujisawa H, Kooshesh F, Stratigos A, Granstein R D, Mak T W, Sauder D N. J Invest Dermatol. 1996;106:993–1000. doi: 10.1111/1523-1747.ep12338505. [DOI] [PubMed] [Google Scholar]
- 5.Kripke M L, Munn C C, Jeevan A, Tang J M, Bucana C. J Immunol. 1990;145:2833–2838. [PubMed] [Google Scholar]
- 6.Cumberbatch M, Illingworth I, Kimber I. Immunology. 1991;74:139–145. [PMC free article] [PubMed] [Google Scholar]
- 7.Hopkins T, Clark R A F. In: Current Practice of Medicine, Vol. 1, Dermatology. Callen J P, editor. Philadelphia: Current Medicine; 1995. pp. 68–76. [Google Scholar]
- 8.Birkedal-Hansen H, Moore W G, Bodden M K, Windsor L J, Birkedal-Hansen B, DeCarlo A, Engler J A. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 9.Matrisian L M. BioEssays. 1992;14:455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- 10.Goetzl E J, Banda M J, Leppert D. J Immunol. 1996;156:1–4. [PubMed] [Google Scholar]
- 11.Mudgett J S, Hutchinson N I, Chartrain N A, Forsyth A I, McDonnell J, Singer I I, Bayne E K, Flanagan J, Kawka D, Shen C F, et al. Arthritis Rheum. 1998;41:110–121. doi: 10.1002/1529-0131(199801)41:1<110::AID-ART14>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 12.Vu T H, Shipley J M, Bergers G, Berger J E, Helms J A, Hanahan D, Shapiro S D, Senior R M, Werb Z. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson C L, Heppner K J, Labosky P A, Hogan B L M, Matrisian L M. Proc Natl Acad Sci USA. 1997;94:1402–1407. doi: 10.1073/pnas.94.4.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phanuphak P, Moorhead J W, Claman H N. J Immunol. 1974;112:115–123. [PubMed] [Google Scholar]
- 15.Ye Q Z, Johnson L L, Hupe D J, Baragi V. Biochemistry. 1992;31:11231–11235. doi: 10.1021/bi00160a038. [DOI] [PubMed] [Google Scholar]
- 16.Shornick L P, Togni P D, Mariathasan S, Goellner J, Strauss-Schoenberger J, Karr R W, Ferguson T A, Chaplin D D. J Exp Med. 1996;183:1427–1436. doi: 10.1084/jem.183.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferguson T A, Mizutani H, Kupper T S. Proc Natl Acad Sci USA. 1991;88:8072–8076. doi: 10.1073/pnas.88.18.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferguson T A, Dube P, Griffith T S. J Exp Med. 1994;179:1597–1604. doi: 10.1084/jem.179.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinman R M. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 20.Macatonia S E, Edwards A J, Knight S C. Immunology. 1986;59:509–514. [PMC free article] [PubMed] [Google Scholar]
- 21.Tang A, Amagai M, Granger L G, Stanley J R, Udey M C. Nature (London) 1993;361:82–85. doi: 10.1038/361082a0. [DOI] [PubMed] [Google Scholar]
- 22.Blauvelt A, Katz S I, Udey M C. J Invest Dermatol. 1995;104:293–296. doi: 10.1111/1523-1747.ep12612830. [DOI] [PubMed] [Google Scholar]
- 23.Lochter A, Galosy S, Muschlter J, Freeman N, Werb Z, Bissell M J. J Cell Biol. 1997;139:1861–1872. doi: 10.1083/jcb.139.7.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi Y, Headquarters R D. Immunology. 1997;90:496–501. doi: 10.1046/j.1365-2567.1997.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiorentno D F, Bond M W, Mosmann T R. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandez-Botran R, Sanders V M, Mosmann T R, Vitetta E S. J Exp Med. 1988;168:543–558. doi: 10.1084/jem.168.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enk A H, Katz S I. J Immunol. 1992;149:92–95. [PubMed] [Google Scholar]
- 28.Howard M, O’Garra A. Immunol Today. 1992;13:198–200. doi: 10.1016/0167-5699(92)90153-X. [DOI] [PubMed] [Google Scholar]
- 29.Go N F, Castle B E, Barrett R, Kastelein R, Dang W, Mosmann T R, Moore K W, Howard M. J Exp Med. 1990;172:1625–1631. doi: 10.1084/jem.172.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompsin-Snipes L, Dhar V V, Bond M W, Mosmann T R, Moore K W, Rennick D M. J Exp Med. 1991;173:507–510. doi: 10.1084/jem.173.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Enk A H, Angeloni V L, Udey M C, Katz S I. J Immunol. 1993;151:2390–2398. [PubMed] [Google Scholar]
- 32.Mosmann T R. Adv Immunol. 1994;56:1–26. [PubMed] [Google Scholar]