Abstract
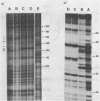
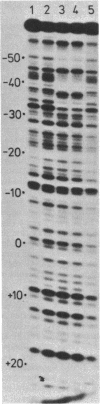
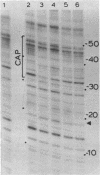
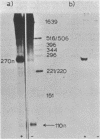
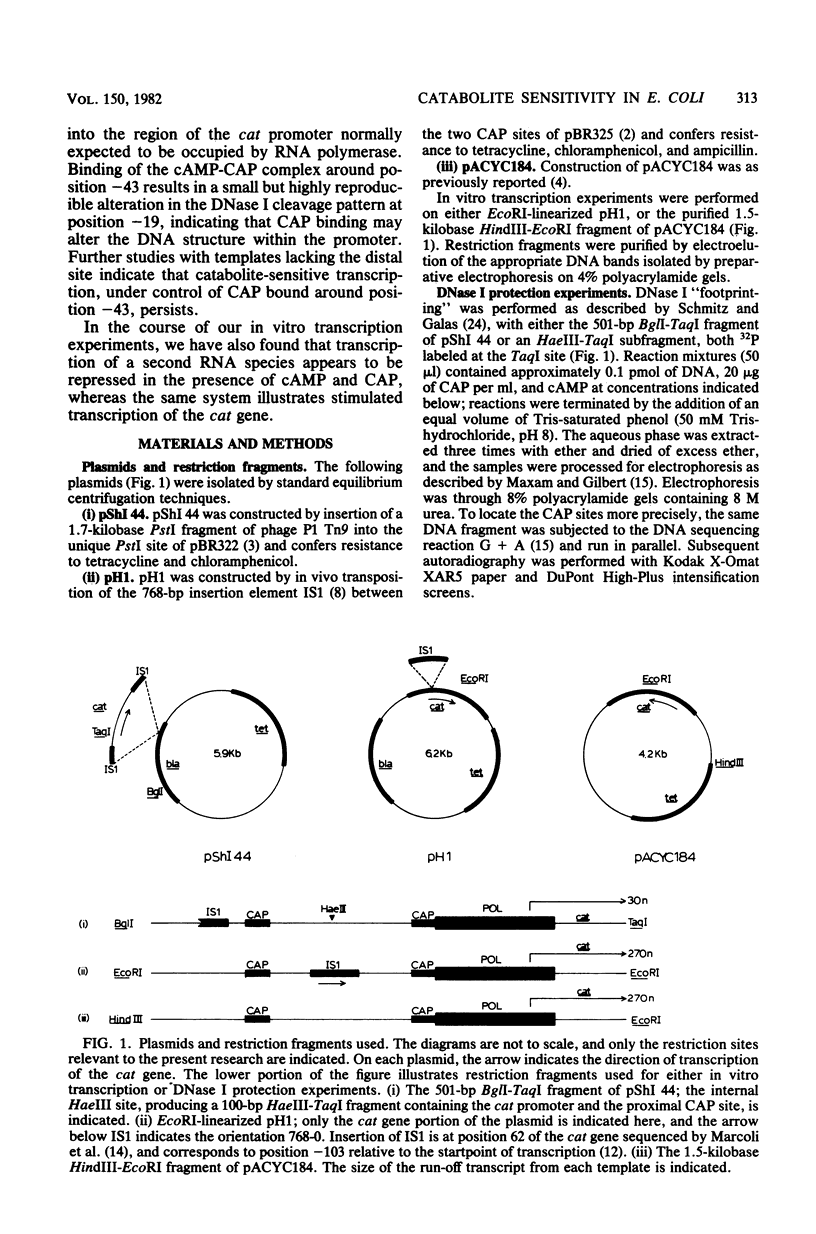
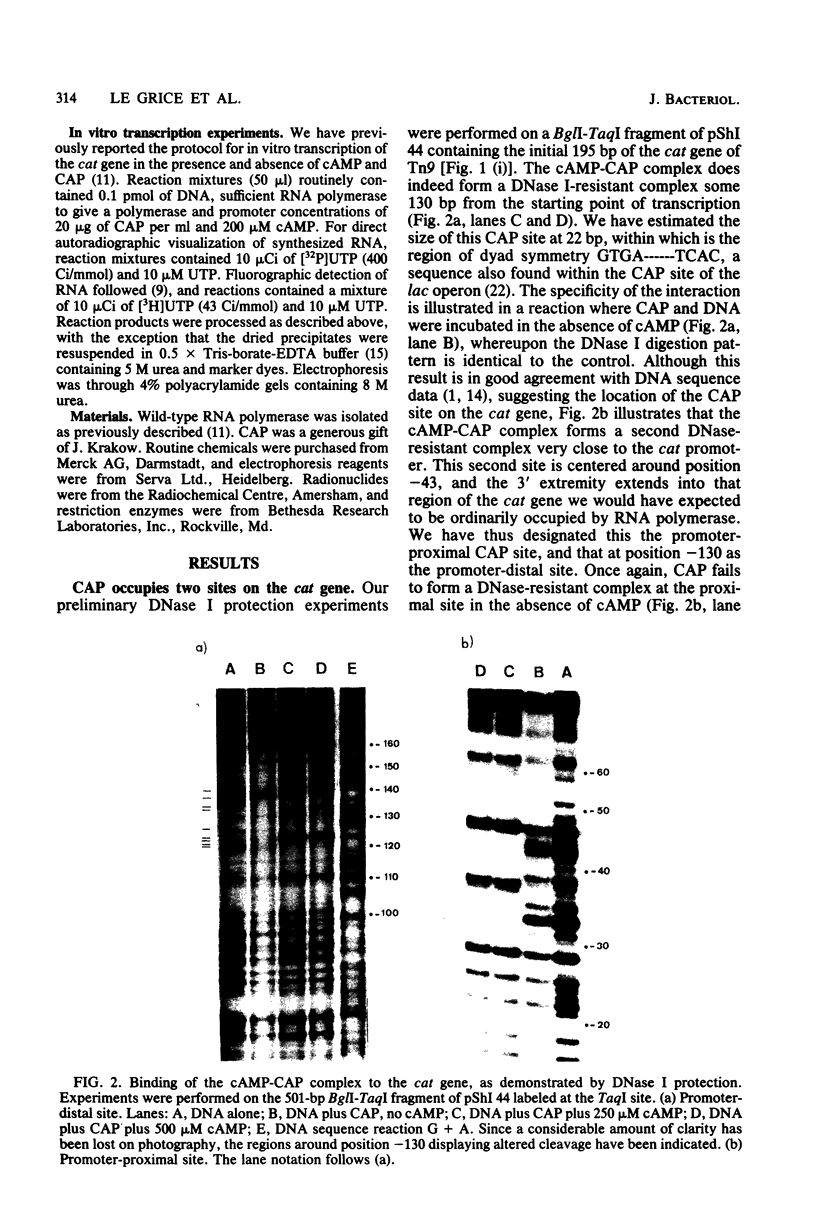
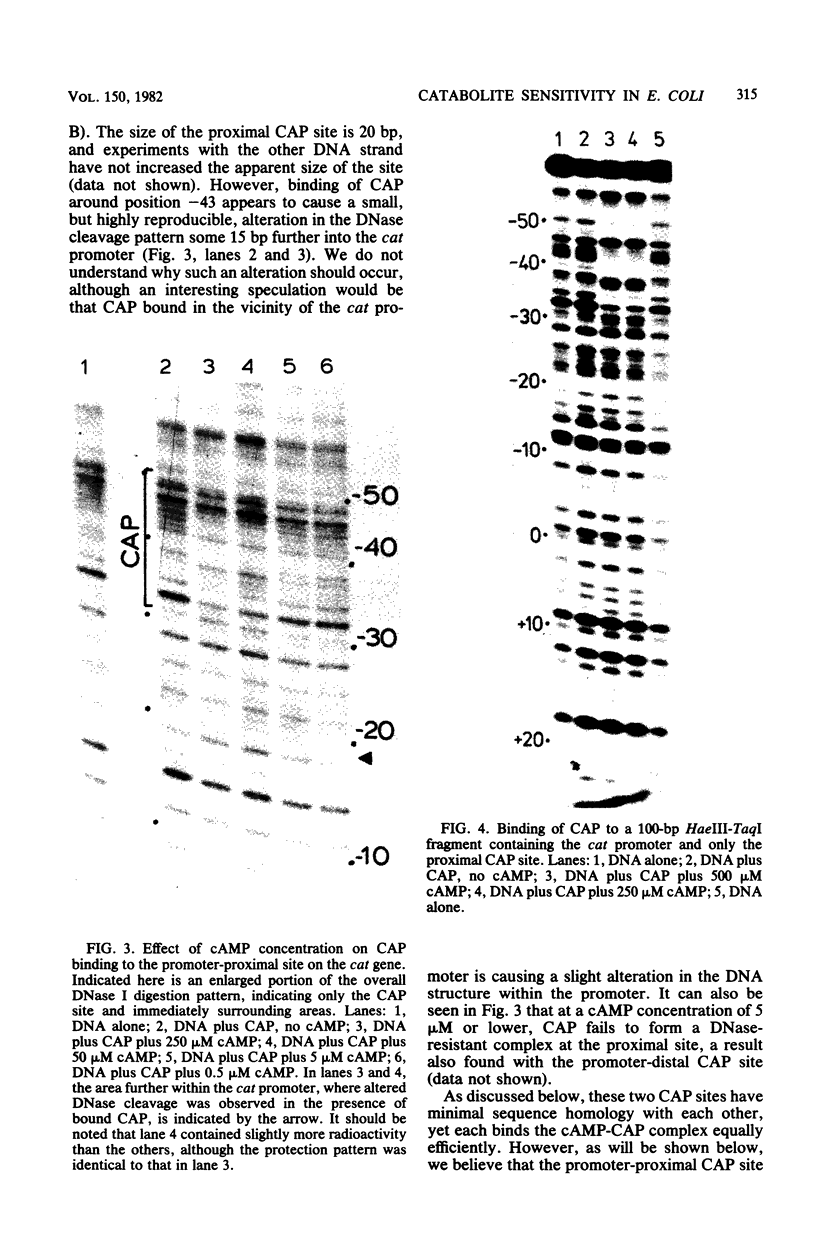
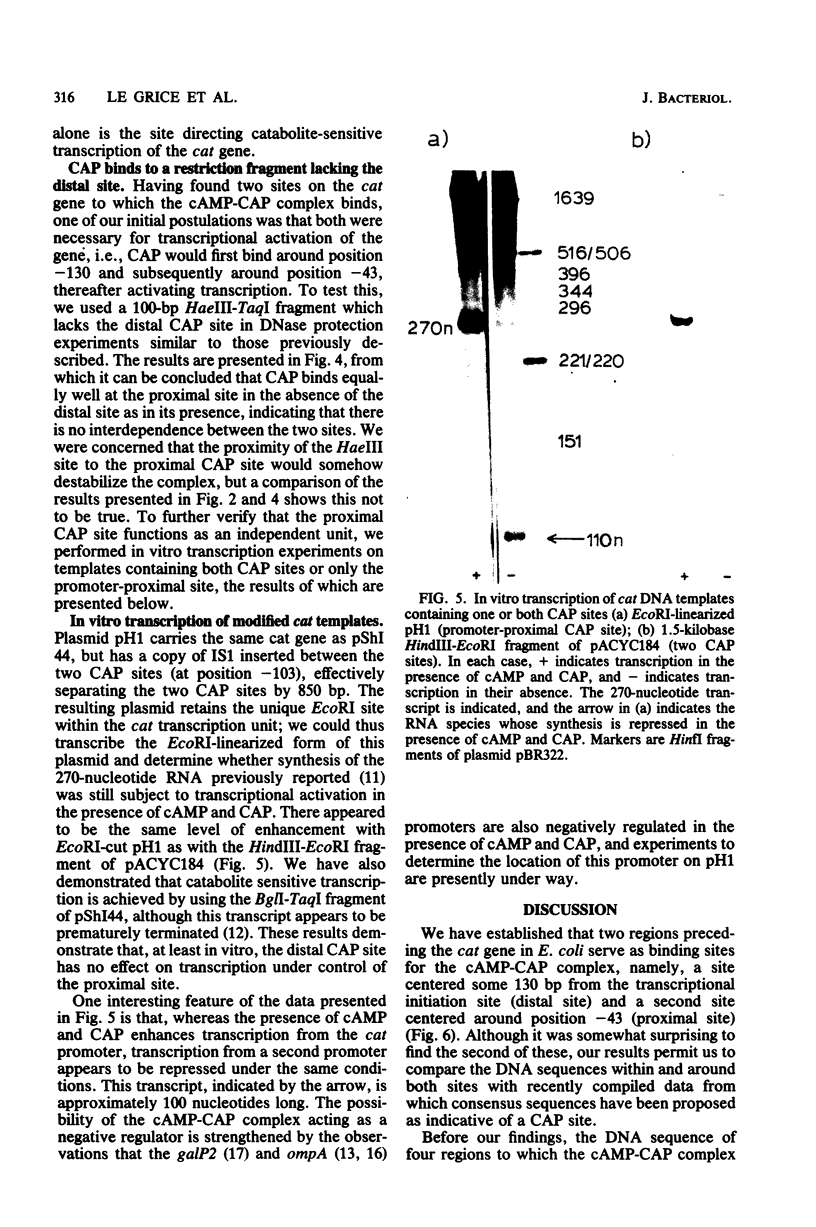
DNase I protection experiments have indicated that the cyclic AMP-catabolite gene activator protein complex binds to two regions preceding the chloramphenicol acetyl transferase (cat) gene in Escherichia coli. One of these lies adjacent to the RNA polymerase binding site, whereas the second lies approximately 130 base pairs upstream from the starting point of transcription. Additional DNase protection experiments and in vitro transcription experiments with modified templates indicate that the catabolite gene activator protein site proximal to the cat promoter functions independently of the distal site, indicating that in vitro the second of these sites is not required for transcriptional activation of the cat gene.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton N. K., Vapnek D. Nucleotide sequence analysis of the chloramphenicol resistance transposon Tn9. Nature. 1979 Dec 20;282(5741):864–869. doi: 10.1038/282864a0. [DOI] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. C., Abelson J., Johnson P. Nucleotide sequence changes produced by mutations in the lac promoter of Escherichia coli. J Mol Biol. 1977 Mar 25;111(1):65–75. doi: 10.1016/s0022-2836(77)80132-0. [DOI] [PubMed] [Google Scholar]
- Ebright R. H., Wong J. R. Mechanism for transcriptional action of cyclic AMP in Escherichia coli: entry into DNA to disrupt DNA secondary structure. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4011–4015. doi: 10.1073/pnas.78.7.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsrud L. DNA sequence of the transposable element IS1. Mol Gen Genet. 1979 Jan 31;169(2):213–218. doi: 10.1007/BF00271673. [DOI] [PubMed] [Google Scholar]
- Laskey R. A. The use of intensifying screens or organic scintillators for visualizing radioactive molecules resolved by gel electrophoresis. Methods Enzymol. 1980;65(1):363–371. doi: 10.1016/s0076-6879(80)65047-2. [DOI] [PubMed] [Google Scholar]
- Le Grice S. F., Matzura H. Binding of RNA polymerase and the catabolite gene activator protein within the cat promoter in Escherichia coli. J Mol Biol. 1981 Aug 5;150(2):185–196. doi: 10.1016/0022-2836(81)90448-4. [DOI] [PubMed] [Google Scholar]
- Le Grice S. F., Matzura H. Localisation of the transcription initiation site of the chloramphenicol resistance gene on plasmid pAC184. FEBS Lett. 1980 Apr 21;113(1):42–46. doi: 10.1016/0014-5793(80)80490-x. [DOI] [PubMed] [Google Scholar]
- Lee N. L., Gielow W. O., Wallace R. G. Mechanism of araC autoregulation and the domains of two overlapping promoters, Pc and PBAD, in the L-arabinose regulatory region of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Feb;78(2):752–756. doi: 10.1073/pnas.78.2.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallick U., Herrlich P. Regulation of synthesis of a major outer membrane protein: cyclic AMP represses Escherichia coli protein III synthesis. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5520–5523. doi: 10.1073/pnas.76.11.5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcoli R., Iida S., Bickle T. A. The DNA sequence of an IS/-flanked transposon coding for resistance to chloramphenicol and fusidic acid. FEBS Lett. 1980 Jan 28;110(1):11–14. doi: 10.1016/0014-5793(80)80011-1. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Movva R. N., Green P., Nakamura K., Inouye M. Interaction of cAMP receptor protein with the ompA gene, a gene for a major outer membrane protein of Escherichia coli. FEBS Lett. 1981 Jun 15;128(2):186–190. doi: 10.1016/0014-5793(81)80077-4. [DOI] [PubMed] [Google Scholar]
- Musso R. E., Di Lauro R., Adhya S., de Crombrugghe B. Dual control for transcription of the galactose operon by cyclic AMP and its receptor protein at two interspersed promoters. Cell. 1977 Nov;12(3):847–854. doi: 10.1016/0092-8674(77)90283-5. [DOI] [PubMed] [Google Scholar]
- O'Neill M. C., Amass K., de Crombrugghe B. Molecuar model of the DNA interaction site for the cyclic AMP receptor protein. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2213–2217. doi: 10.1073/pnas.78.4.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Adhya S. Cyclic adenosine 5'-monophosphate in Escherichia coli. Bacteriol Rev. 1976 Sep;40(3):527–551. doi: 10.1128/br.40.3.527-551.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C., Rosenberg M. A promoter of pBR322 activated by cAMP receptor protein. Nucleic Acids Res. 1981 Jul 24;9(14):3365–3377. doi: 10.1093/nar/9.14.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A. Cyclic AMP receptor proteins interacts with lactose operator DNA. Nucleic Acids Res. 1981 Jan 24;9(2):277–292. doi: 10.1093/nar/9.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A., Galas D. J. The interaction of RNA polymerase and lac repressor with the lac control region. Nucleic Acids Res. 1979 Jan;6(1):111–137. doi: 10.1093/nar/6.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Simpson R. B., Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- Simpson R. B. Interaction of the cAMP receptor protein with the lac promoter. Nucleic Acids Res. 1980 Feb 25;8(4):759–766. [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T., O'Neill M., de Crombrugghe B. Interaction site of Escherichia coli cyclic AMP receptor protein on DNA of galactose operon promoters. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5090–5094. doi: 10.1073/pnas.76.10.5090. [DOI] [PMC free article] [PubMed] [Google Scholar]