Abstract
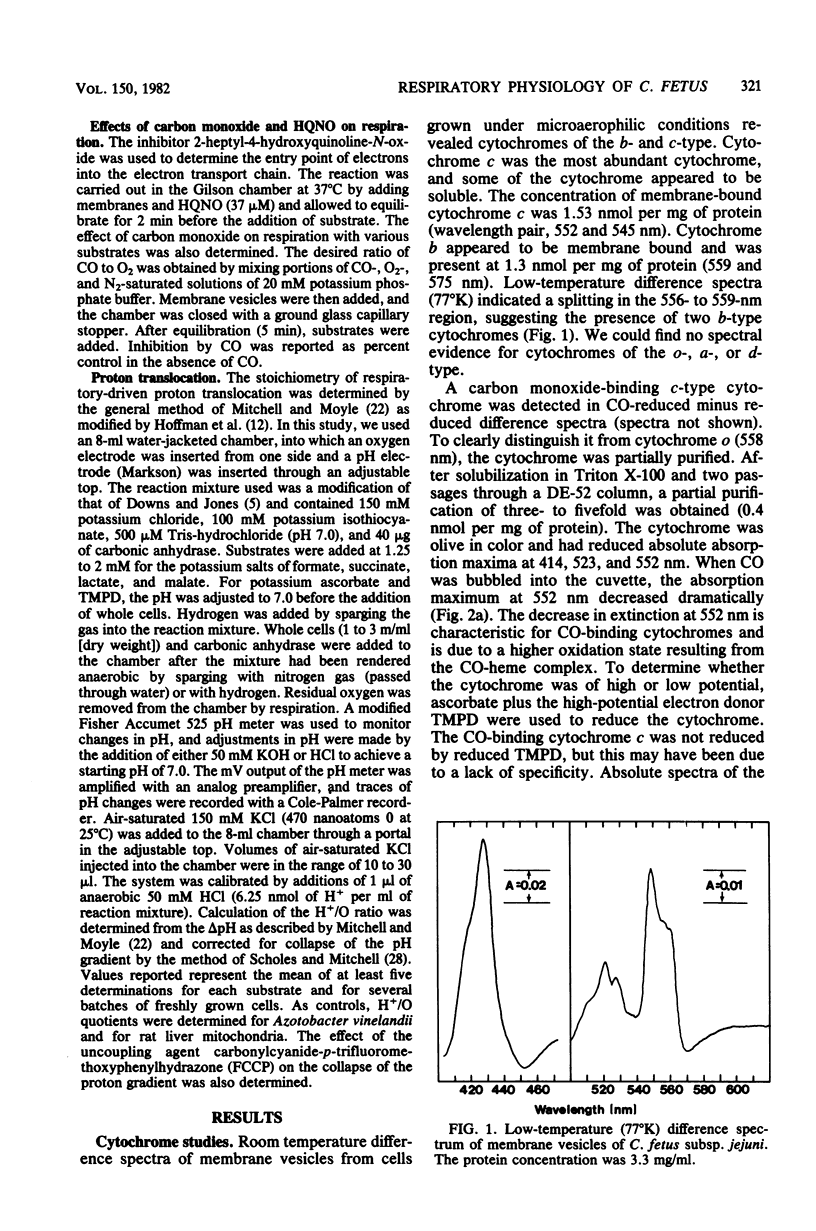
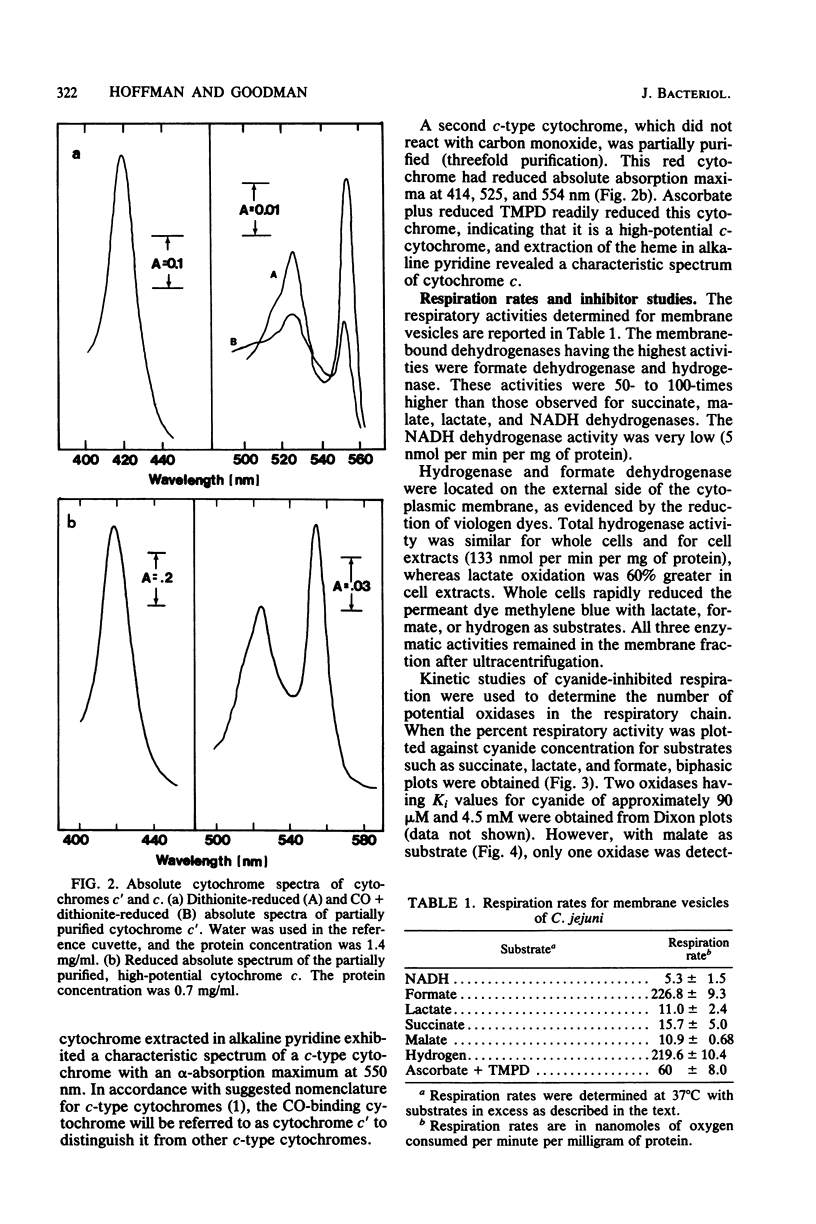
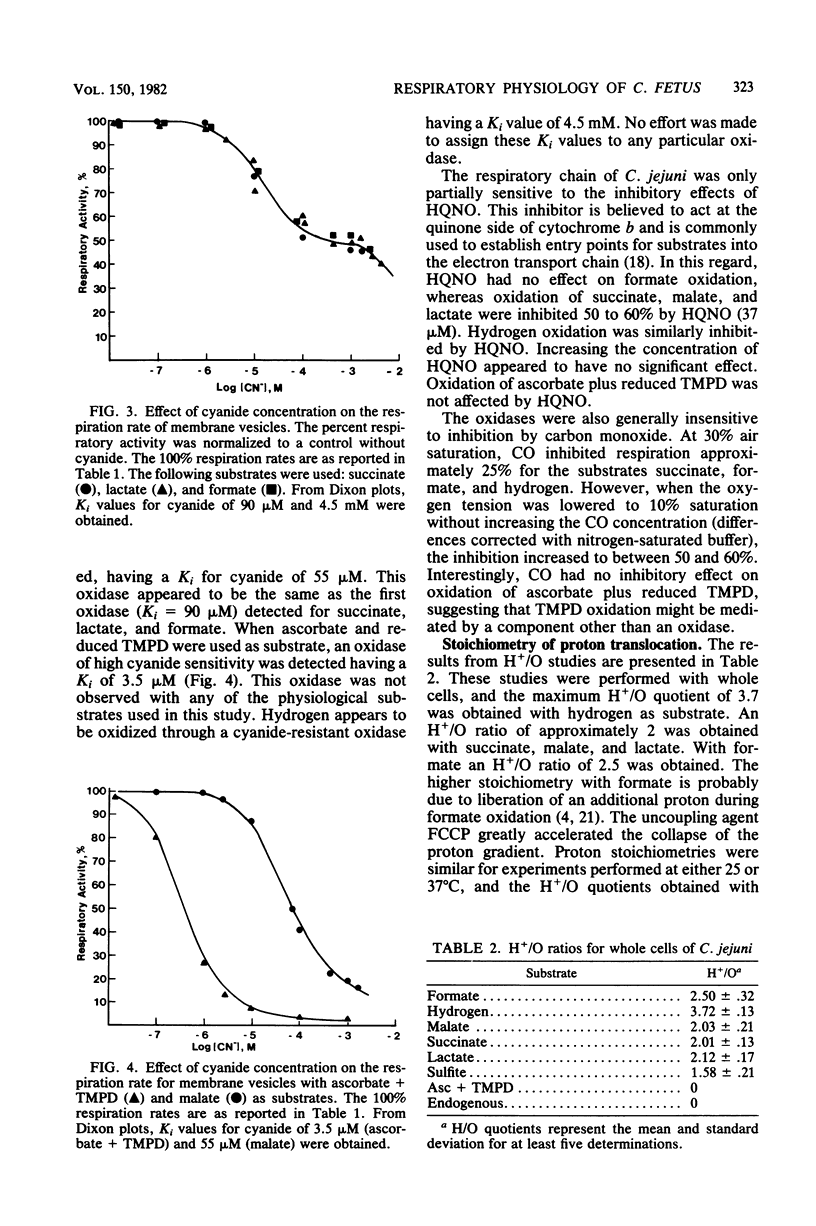
A study of the electron transport chain of the human intestinal pathogen Campylobacter jejuni revealed a rich complement of b- and c-type cytochromes. Two c-type cytochromes were partially purified: one, possibly an oxidase, bound carbon monoxide whereas the other, of high potential was unreactive with carbon monoxide. Respiratory activities determined with membrane vesicles were 50- to 100-fold higher with formate and hydrogen than with succinate, lactate, malate, or NADH as substrates. Evidence for three terminal respiratory components was obtained from respiratory kinetic studies employing cyanide, and the following Ki values for cyanide were determined from Dixon plots: ascorbate + reduced N,N,N', N'-tetramethyl-p-phenylenediamine, K1 + 3.5 muM; malate, K1 = 55 muM; and hydrogen, K1 = 4.5 muM. Two oxidases (K1 = 90 muM, 4.5 mM) participated in the oxidation of succinate, lactate, and formate. Except with formate, 37 muM HQNO inhibited respiration by approximately 50%. Carbon monoxide had little inhibitory effect on respiration except under low oxygen tension (less than 10% air saturation). The stoichiometry of respiratory-driven proton translocation (H+/O) determined with whole cells was approximately 2 for all substrates examined except hydrogen (H+/) = 3.7) and formate (H+/O = 2.5). The higher stoichiometries observed with hydrogen and formate are consistent with their respective dehydrogenase being located on the periplasmic face of the cytoplasmic membrane. The results of this study suggest that the oxidation of hydrogen and formate probably serves as the major sources of energy for growth.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartsch R. G. Bacterial cytochromes. Annu Rev Microbiol. 1968;22:181–200. doi: 10.1146/annurev.mi.22.100168.001145. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Berkowitz I. D., LaForce F. M., Cravens J., Reller L. B., Wang W. L. Campylobacter enteritis: clinical and epidemiologic features. Ann Intern Med. 1979 Aug;91(2):179–185. doi: 10.7326/0003-4819-91-2-179. [DOI] [PubMed] [Google Scholar]
- Campbell W. H., Orme-Johnson W. H., Burris R. H. A comparison of the physical and chemical properties of four cytochromes c from Azotobacter vinelandii. Biochem J. 1973 Dec;135(4):617–630. doi: 10.1042/bj1350617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs A. J., Jones C. W. Respiration-linked proton translocation in Azotobacter vinelandii. FEBS Lett. 1975 Dec 1;60(1):42–46. doi: 10.1016/0014-5793(75)80414-5. [DOI] [PubMed] [Google Scholar]
- George H. A., Hoffman P. S., Smibert R. M., Krieg N. R. Improved media for growth and aerotolerance of Campylobacter fetus. J Clin Microbiol. 1978 Jul;8(1):36–41. doi: 10.1128/jcm.8.1.36-41.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey S., Lascelles J. Respiratory systems and cytochromes in Campylobacter fetus subsp. intestinalis. J Bacteriol. 1980 Dec;144(3):917–922. doi: 10.1128/jb.144.3.917-922.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M. F., Vignais P. M. Induction by cyanide of cytochrome d in the plasma membrane of Paracoccus denitrificans. FEBS Lett. 1979 Apr 1;100(1):41–46. doi: 10.1016/0014-5793(79)81127-8. [DOI] [PubMed] [Google Scholar]
- Hoffman P. S., George H. A., Krieg N. R., Smibert R. M. Studies of the microaerophilic nature of Campylobacter fetus subsp. jejuni. II. Role of exogenous superoxide anions and hydrogen peroxide. Can J Microbiol. 1979 Jan;25(1):8–16. doi: 10.1139/m79-002. [DOI] [PubMed] [Google Scholar]
- Hoffman P. S., Krieg N. R., Smibert R. M. Studies of the microaerophilic nature of Campylobacter fetus subsp. jejuni. I. Physiological aspects of enhanced aerotolerance. Can J Microbiol. 1979 Jan;25(1):1–7. doi: 10.1139/m79-001. [DOI] [PubMed] [Google Scholar]
- Hoffman P. S., Morgan T. V., DerVartanian D. V. Respiratory-chain characteristics of mutants of Azotobacter vinelandii negative to tetramethyl-p-phenylenediamine oxidase. Eur J Biochem. 1979 Oct;100(1):19–27. doi: 10.1111/j.1432-1033.1979.tb02029.x. [DOI] [PubMed] [Google Scholar]
- Hoffman P. S., Morgan T. V., Dervartanian D. V. Respiratory properties of cytochrome-c-deficient mutants of Azotobacter vinelandii. Eur J Biochem. 1980 Sep;110(2):349–354. doi: 10.1111/j.1432-1033.1980.tb04874.x. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Brice J. M., Downs A. J., Drozd J. W. Bacterial respiration-linked proton translocation and its relationship to respiratory-chain composition. Eur J Biochem. 1975 Mar 17;52(2):265–271. doi: 10.1111/j.1432-1033.1975.tb03994.x. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Brice J. M., Edwards C. The effect of respiratory chain composition on the growth efficiencies of aerobic bacteria. Arch Microbiol. 1977 Oct 24;115(1):85–93. doi: 10.1007/BF00427850. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Redfearn E. R. Electron transport in Azotobacter vinelandii. Biochim Biophys Acta. 1966 Mar 7;113(3):467–481. doi: 10.1016/s0926-6593(66)80005-x. [DOI] [PubMed] [Google Scholar]
- Jones C. W. The inhibition of Azotobacter vinelandii terminal oxidases by cyanide. FEBS Lett. 1973 Nov 1;36(3):347–350. doi: 10.1016/0014-5793(73)80407-7. [DOI] [PubMed] [Google Scholar]
- Jurtshuk P., Jr, Mueller T. J., Acord W. C. Bacterial terminal oxidases. CRC Crit Rev Microbiol. 1975 May;3(4):399–468. doi: 10.3109/10408417509108757. [DOI] [PubMed] [Google Scholar]
- KIGGINS E. M., PLASTRIDGE W. N. Effect of gaseous environment on growth and catalase content of Vibrio fetus cultures of bovine origin. J Bacteriol. 1956 Sep;72(3):397–400. doi: 10.1128/jb.72.3.397-400.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger A., Dorrer E., Winkler E. The orientation of the substrate sites of formate dehydrogenase and fumarate reductase in the membrane of Vibrio succinogenes. Biochim Biophys Acta. 1980 Jan 4;589(1):118–136. doi: 10.1016/0005-2728(80)90136-x. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Respiration-driven proton translocation in rat liver mitochondria. Biochem J. 1967 Dec;105(3):1147–1162. doi: 10.1042/bj1051147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niekus H. G., Wouters C. H., de Vries W., Stouthamer A. H. Superoxide dismutase and hydrogen peroxide formation in Campylobacter sputorum subspecies bubulus. Arch Microbiol. 1978 Oct 4;119(1):37–42. doi: 10.1007/BF00407925. [DOI] [PubMed] [Google Scholar]
- Niekus H. G., van Doorn E., Stouthamer A. H. Oxygen consumption by Campylobacter sputorum subspecies Bubulus with formate as substrate. Arch Microbiol. 1980 Sep;127(2):137–143. doi: 10.1007/BF00428017. [DOI] [PubMed] [Google Scholar]
- Odom J. M., Peck H. D., Jr Localization of dehydrogenases, reductases, and electron transfer components in the sulfate-reducing bacterium Desulfovibrio gigas. J Bacteriol. 1981 Jul;147(1):161–169. doi: 10.1128/jb.147.1.161-169.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholes P., Mitchell P. Respiration-driven proton translocation in Micrococcus denitrificans. J Bioenerg. 1971 Sep;1(3):309–323. doi: 10.1007/BF01516290. [DOI] [PubMed] [Google Scholar]
- Skirrow M. B. Campylobacter enteritis: a "new" disease. Br Med J. 1977 Jul 2;2(6078):9–11. doi: 10.1136/bmj.2.6078.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T. Y., Jurtshuk P., Jr Studies on the red oxidase (cytochrome o) of Azotobacter vinelandii. Biochem Biophys Res Commun. 1978 Apr 14;81(3):1032–1039. doi: 10.1016/0006-291x(78)91454-7. [DOI] [PubMed] [Google Scholar]
- Yoch D. C., Carithers R. P. Bacterial iron-sulfur proteins. Microbiol Rev. 1979 Sep;43(3):384–421. doi: 10.1128/mr.43.3.384-421.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries W., Niekus H. G., Boellaard M., Stouthamer A. H. Growth yields and energy generation by Campylobacter sputorum subspecies bubulus during growth in continuous culture with different hydrogen acceptors. Arch Microbiol. 1980 Feb;124(2-3):221–227. doi: 10.1007/BF00427730. [DOI] [PubMed] [Google Scholar]



