Abstract
Peptidoglycan sacculi free of poly-beta-hydroxybutyric acid were prepared from whole cells of four species of Caulobacter and two species of Asticcacaluis and from morphological mutants of Caulobacter crescentus and Caulobacter leidyi. Acid hydrolysates of the sacculi were analyzed quantitatively, and each of the hydrolysates was found to contain significant amounts of only five ninhydrin-reactive compounds: alanine, glutamic acid, alpha , omega-diaminopimelic acid, muramic acid, and glucosamine. Four types of peptidoglycans were distinguishable on the basis of the molar ratios among these five compounds. The respective ratios were as follows: in C. leidyi, 2:1:1:1:0.8; in Asticcacaulis biprosthecum, 1.7:1.6:1.1:0.7; in the cells of the remaining species, 2:1:1:1.2:0.8; and in stalks shed by the abscission mutant 2NY66, 2:1:1:1:1.67. Thus, in addition to some species differences among these caulobacters, it was found that the peptidoglycan sacculus of the stalked C. crescentus cell is chemically differentiated; the cellular peptidoglycan is richer in muramic acid than is the peptidoglycan of typical gram-negative bacteria, and the peptidoglycan of the stalk is correspondingly rich in glucosamine. Empirical formulas for the repeating units of the peptidoglycans have been inferred on the basis of the molar ratios of their amino components.
Full text
PDF



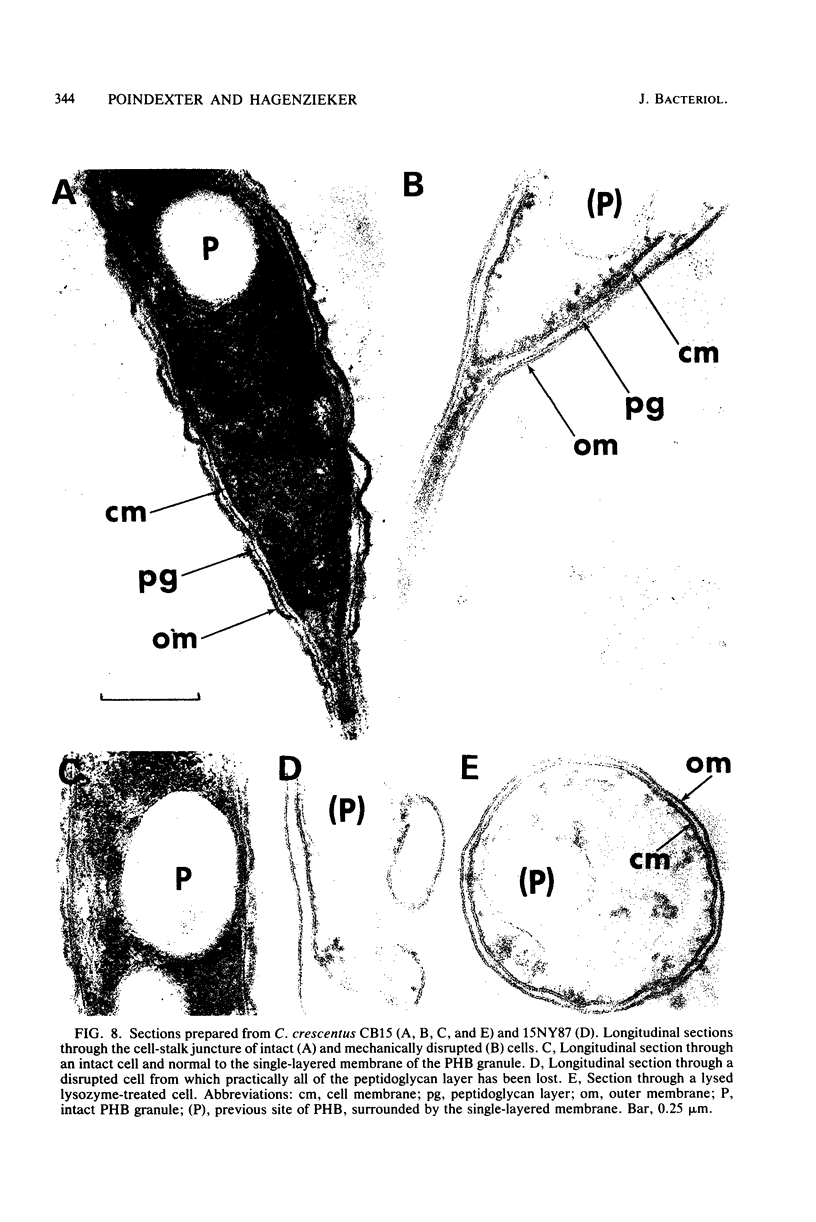
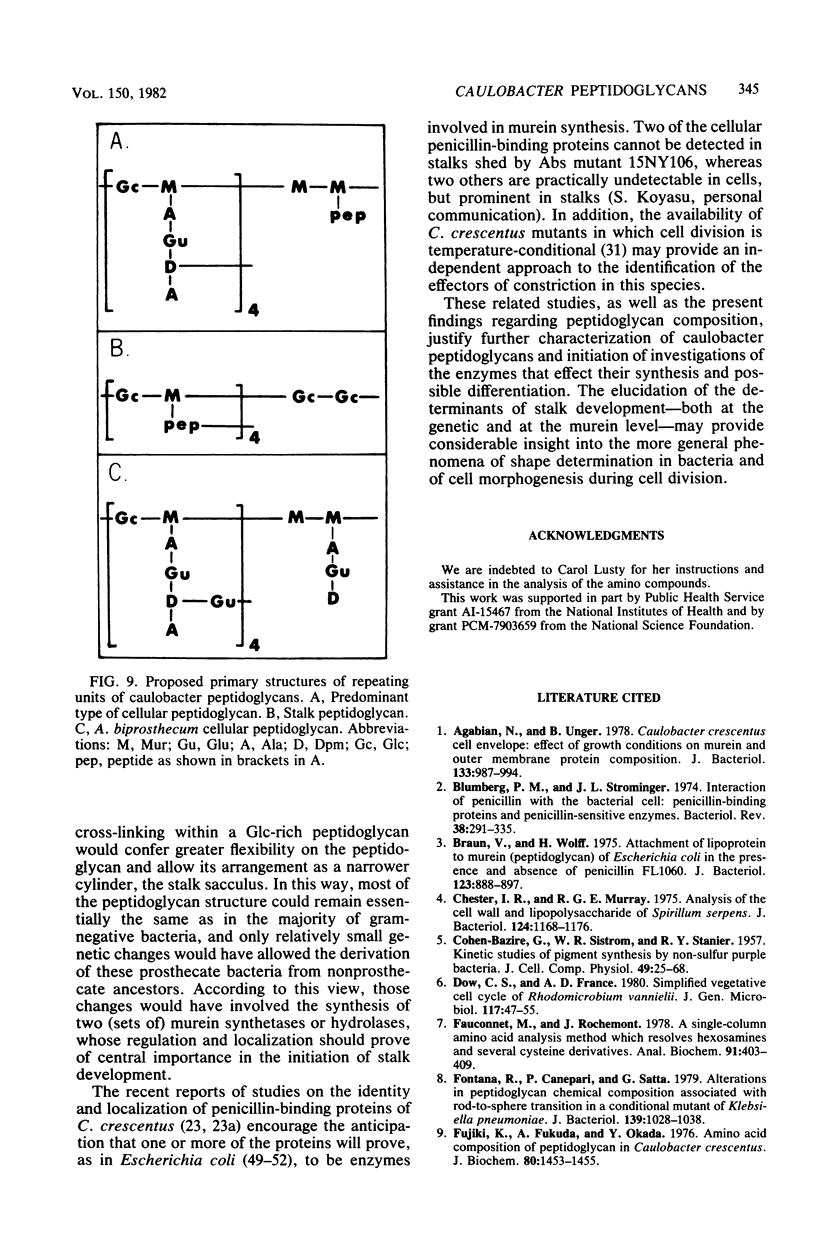


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agabian N., Unger B. Caulobacter crescentus cell envelope: effect of growth conditions on murein and outer membrane protein composition. J Bacteriol. 1978 Feb;133(2):987–994. doi: 10.1128/jb.133.2.987-994.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974 Sep;38(3):291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Wolff H. Attachment of lipoprotein to murein (peptidoglycan) of Escherichia coli in the presence and absence of penicillin FL 1060. J Bacteriol. 1975 Sep;123(3):888–897. doi: 10.1128/jb.123.3.888-897.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Chester I. R., Murray R. G. Analysis of the cell wall and lipopolysaccharide of Spirillum serpens. J Bacteriol. 1975 Dec;124(3):1168–1176. doi: 10.1128/jb.124.3.1168-1176.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauconnet M., Rochemont J. A single-column amino acid analysis method which resolves hexosamines and several cysteine derivatives. Anal Biochem. 1978 Dec;91(2):403–409. doi: 10.1016/0003-2697(78)90525-0. [DOI] [PubMed] [Google Scholar]
- Fontana R., Canepari P., Satta G. Alterations in peptidoglycan chemical composition associated with rod-to-sphere transition in a conditional mutant of Klebsiella pneumoniae. J Bacteriol. 1979 Sep;139(3):1028–1038. doi: 10.1128/jb.139.3.1028-1038.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki K., Fukuda A., Okada Y. Amino acid composition of peptidoglycan in Caulobacter crescentus. J Biochem. 1976 Dec;80(6):1453–1455. doi: 10.1093/oxfordjournals.jbchem.a131421. [DOI] [PubMed] [Google Scholar]
- Goodell W., Tomasz A. Alteration of Escherichia coli murein during amino acid starvation. J Bacteriol. 1980 Dec;144(3):1009–1016. doi: 10.1128/jb.144.3.1009-1016.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin S. D., Shedlarski J. G., Jr Purification of cell wall peptidoglycan of the dimorphic bacterium Caulobacter crescentus. Arch Biochem Biophys. 1975 Sep;170(1):23–36. doi: 10.1016/0003-9861(75)90094-6. [DOI] [PubMed] [Google Scholar]
- HIRSCH P., CONTI S. F. BIOLOGY OF BUDDING BACTERIA. II. GROWTH AND NUTRITION OF HYPHOMICROBIUM SPP. Arch Mikrobiol. 1964 Jun 26;48:358–367. doi: 10.1007/BF00405979. [DOI] [PubMed] [Google Scholar]
- Hakenbeck R., Messer W. Activity of murein hydrolases in synchronized cultures of Escherichia coli. J Bacteriol. 1977 Mar;129(3):1239–1244. doi: 10.1128/jb.129.3.1239-1244.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder W., Attwood M. M. Biology, physiology and biochemistry of hyphomicrobia. Adv Microb Physiol. 1978;17:303–359. doi: 10.1016/s0065-2911(08)60060-0. [DOI] [PubMed] [Google Scholar]
- Henning U., Rehn K., Braun V., Höhn B. Cell envelope and shape of Escherichia coli K12. Properties of a temperature-sensitive rod mutant. Eur J Biochem. 1972 Apr 24;26(4):570–586. doi: 10.1111/j.1432-1033.1972.tb01800.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann B., Messer W., Schwarz U. Regulation of polar cap formation in the life cycle of Escherichia coli. J Supramol Struct. 1972;1(1):29–37. doi: 10.1002/jss.400010105. [DOI] [PubMed] [Google Scholar]
- Iwaya M., Goldman R., Tipper D. J., Feingold B., Strominger J. L. Morphology of an Escherichia coli mutant with a temperature-dependent round cell shape. J Bacteriol. 1978 Dec;136(3):1143–1158. doi: 10.1128/jb.136.3.1143-1158.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. Y., White D. Myxospore formation in Myxococcus xanthus: chemical changes in the cell wall during cellular morphogenesis. J Bacteriol. 1972 Nov;112(2):849–855. doi: 10.1128/jb.112.2.849-855.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. C., Schmidt J. M. Ultrastructural study of crossbands occurring in the stalks of Caulobacter crescentus. J Bacteriol. 1973 Oct;116(1):466–470. doi: 10.1128/jb.116.1.466-470.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. E., Hirsch P. Cell wall composition of Hypomicrobium species. J Bacteriol. 1968 Oct;96(4):1037–1041. doi: 10.1128/jb.96.4.1037-1041.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Ensign J. C. Isolation and chemical structure of the peptidoglycan of Spirillum serpens cell walls. J Bacteriol. 1968 Jan;95(1):201–210. doi: 10.1128/jb.95.1.201-210.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyasu S., Fukuda A., Okada Y. The penicillin-binding proteins of Caulobacter crescentus. J Biochem. 1980 Jan;87(1):363–366. doi: 10.1093/oxfordjournals.jbchem.a132749. [DOI] [PubMed] [Google Scholar]
- Krulwich T. A., Ensign J. C. Activity of an autolytic N-acetylmuramidase during sphere-rod morphogenesis in Arthrobacter crystallopoietes. J Bacteriol. 1968 Sep;96(3):857–859. doi: 10.1128/jb.96.3.857-859.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Ensign J. C., Tipper D. J., Strominger J. L. Sphere-rod morphogenesis in Arthrobacter crystallopoietes. I. Cell wall composition and polysaccharides of the peptidoglycan. J Bacteriol. 1967 Sep;94(3):734–740. doi: 10.1128/jb.94.3.734-740.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Ensign J. C., Tipper D. J., Strominger J. L. Sphere-rod morphogenesis in Arthrobacter crystallopoietes. II. Peptides of the cell wall peptidoglycan. J Bacteriol. 1967 Sep;94(3):741–750. doi: 10.1128/jb.94.3.741-750.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAW J. H., SLEPECKY R. A. Assay of poly-beta-hydroxybutyric acid. J Bacteriol. 1961 Jul;82:33–36. doi: 10.1128/jb.82.1.33-36.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larson R. J., Pate J. L. Growth and morphology of Asticcacaulis biprosthecum in defined media. Arch Microbiol. 1975 Dec 31;106(3):147–157. doi: 10.1007/BF00446517. [DOI] [PubMed] [Google Scholar]
- Osley M. A., Newton A. Mutational analysis of developmental control in Caulobacter crescentus. Proc Natl Acad Sci U S A. 1977 Jan;74(1):124–128. doi: 10.1073/pnas.74.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POINDEXTER J. S. BIOLOGICAL PROPERTIES AND CLASSIFICATION OF THE CAULOBACTER GROUP. Bacteriol Rev. 1964 Sep;28:231–295. doi: 10.1128/br.28.3.231-295.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poindexter J. S., Hagenzieker J. G. Constriction and septation during cell division in caulobacters. Can J Microbiol. 1981 Jul;27(7):704–719. doi: 10.1139/m81-109. [DOI] [PubMed] [Google Scholar]
- Poindexter J. S. Selection for nonbuoyant morphological mutants of Caulobacter crescentus. J Bacteriol. 1978 Sep;135(3):1141–1145. doi: 10.1128/jb.135.3.1141-1145.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poindexter J. S. The caulobacters: ubiquitous unusual bacteria. Microbiol Rev. 1981 Mar;45(1):123–179. doi: 10.1128/mr.45.1.123-179.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- Rogers H. J. Biogenesis of the wall in bacterial morphogenesis. Adv Microb Physiol. 1979;19:1–62. doi: 10.1016/s0065-2911(08)60197-6. [DOI] [PubMed] [Google Scholar]
- Rogers H. J., Taylor C. Autolysins and shape change in rodA mutants of Bacillus subtilis. J Bacteriol. 1978 Sep;135(3):1032–1042. doi: 10.1128/jb.135.3.1032-1042.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A., Hirota Y., Schwarz U. Process of cellular division in Escherichia coli growth pattern of E. coli murein. J Mol Biol. 1973 Jun 25;78(1):185–195. doi: 10.1016/0022-2836(73)90437-3. [DOI] [PubMed] [Google Scholar]
- STOVEPOINDEXTER J. L., COHEN-BAZIRE G. THE FINE STRUCTURE OF STALKED BACTERIA BELONGING TO THE FAMILY CAULOBACTERACEAE. J Cell Biol. 1964 Dec;23:587–607. doi: 10.1083/jcb.23.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satta G., Fontana R., Canepari P., Botta G. Peptidoglycan synthesis in cocci and rods of a pH-dependent, morphologically conditional mutant of Klebsiella pneumoniae. J Bacteriol. 1979 Feb;137(2):727–734. doi: 10.1128/jb.137.2.727-734.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. M., Stanier R. Y. The development of cellular stalks in bacteria. J Cell Biol. 1966 Mar;28(3):423–436. doi: 10.1083/jcb.28.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Schwarz U., Ryter A., Rambach A., Hellio R., Hirota Y. Process of cellular division in Escherichia coli: differention of growth zones in the Sacculus. J Mol Biol. 1975 Nov 15;98(4):749–759. doi: 10.1016/s0022-2836(75)80008-8. [DOI] [PubMed] [Google Scholar]
- Shapiro L. Differentiation in the Caulobacter cell cycle. Annu Rev Microbiol. 1976;30:377–407. doi: 10.1146/annurev.mi.30.100176.002113. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Pardee A. B. Penicillin-binding proteins and cell shape in E. coli. Nature. 1975 Apr 10;254(5500):516–517. doi: 10.1038/254516a0. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Strominger J. L. Identification of the major penicillin-binding proteins of Escherichia coli as D-alanine carboxypeptidase IA. J Bacteriol. 1976 Jul;127(1):660–663. doi: 10.1128/jb.127.1.660-663.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley J. T. Prosthecomicrobium and Ancalomicrobium: new prosthecate freshwater bacteria. J Bacteriol. 1968 May;95(5):1921–1942. doi: 10.1128/jb.95.5.1921-1942.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwer R. W., Nanninga N. Pattern of meso-dl-2,6-diaminopimelic acid incorporation during the division cycle of Escherichia coli. J Bacteriol. 1980 Oct;144(1):327–336. doi: 10.1128/jb.144.1.327-336.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- Wang W. S., Lundgren D. G. Peptidoglycan of a chemolithotrophic bacterium, Ferrobacillus ferrooxidans. J Bacteriol. 1968 May;95(5):1851–1856. doi: 10.1128/jb.95.5.1851-1856.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittenbury R., Dow C. S. Morphogenesis and differentiation in Rhodomicrobium vannielii and other budding and prosthecate bacteria. Bacteriol Rev. 1977 Sep;41(3):754–808. doi: 10.1128/br.41.3.754-808.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]