Abstract
The protein kinase Akt/PKB is a crucial regulator of cell survival in response to mitogenic signals. The increased kinase activity of v-akt, an oncogenic form of Akt/PKB, causes mouse T cell lymphoma, and overexpression of Akt/PKB is associated with progression of several tumor types in human. In this study, we demonstrate that ligation of B cell antigen receptor (BCR) leads to activation of Akt/PKB in B lymphocytes. BCR-induced activation of Akt/PKB required the tyrosine kinase Syk, which was not previously known to regulate Akt/PKB. In contrast, BCR crosslinking of Lyn-deficient B cells resulted in markedly enhanced hyperphosphorylation and activation of Akt/PKB compared with wild-type B cells, indicating that this Src-family kinase acts as an endogenous antagonist of BCR-induced Akt/PKB activation. Lyn inhibited Akt/PKB additively with an okadaic acid-sensitive endogenous phosphatase(s). Expression of exogenous Lyn in mutant cells restored normal BCR-induced phosphorylation of Akt/PKB. Negative regulation of Akt/PKB by Lyn was not dependent on the protein phosphatases SHP-1, SHP-2, or SHIP. Our results show that Lyn provides a mechanism for negative regulation and opposes the effect of Syk on BCR-mediated activation of Akt/PKB. Deregulation of Akt/PKB correlates with the hyperresponsiveness of B cells from Lyn-deficient mice stimulated by BCR crosslinking and may contribute to the autoimmune syndrome that develops in Lyn-deficient animals.
Ligation of B cell antigen receptor (BCR) induces signaling pathways that, together with costimulatory signals, lead to clonal expansion, differentiation, or apoptosis of B lymphocytes. The activation of protein tyrosine kinase cascades is an essential BCR proximal signaling event. Activation of Src-family kinases (including Lyn, Fyn, and Blk), Syk, and Btk through BCR has been extensively studied (1–5). However, the regulation of the downstream serine/threonine kinases in B cells that may mediate cell survival, reorganization of the actin cytoskeleton, cell cycle progression, gene transcription, and protein translation is poorly understood. Several serine/threonine kinases are activated in response to ligation of B cell receptor including mitogen-activated protein kinases (MAPK) (6, 7), two ribosomal S6 kinases, p90Rsk and p70S6k (8), and various isoforms of protein kinase C. We recently established that the signaling pathways leading to the activation of p90Rsk, p70S6k and MAPK could be distinguished based on their requirements for the activation of upstream protein tyrosine kinases (8, 9).
BCR crosslinking also induces activation of phosphoinositide-3 kinase (PI3-kinase) (10, 11). The protooncogene Akt/PKB is an important target of the phosphoinositides generated by the activation of PI3-kinase (12, 13). Phospholipids generated by the activation of PI3-kinase recruit Akt/PKB to the plasma membrane, resulting in partial activation of Akt/PKB. Membrane-anchored Akt/PKB is phosphorylated by PDK1 and an as-yet-unidentified kinase to be fully activated (14, 15). Akt/PKB can be regulated by a variety of mechanisms in different cell types. However, the regulation of antigen receptor-mediated activation of Akt/PKB in B cells has not been determined.
Akt/PKB was identified as a protooncogene from rodent T cells (16). Furthermore, deregulation of Akt/PKB is highly associated with tumorigenesis in human (17–19). In addition, Akt/PKB can deliver antiapoptotic signals in response to growth factor and cytokine stimulation (20–23). Akt/PKB exerts its functions via several mechanisms. IL-2-induced activation of Akt/PKB leads to up-regulation of Bcl-2 and prevented apoptosis of BAF-3 cells (20). In contrast, serum-induced activation of Akt/PKB in Rat1 fibroblasts did not alter the expression of Bcl-2 but inhibited Ced3/ICE-like activity that ultimately blocks apoptosis (21). Moreover, Akt/PKB was shown to induce phosphorylation of Bad protein that prevents its association with Bcl-2 and thereby blocked Bad-induced cell death of primary neurons (22, 23).
In this study, we investigated the regulation of Akt/PKB after crosslinking of BCR. We demonstrate that crosslinking of the BCR leads to hyperphosphorylation and activation of Akt/PKB. Furthermore, we show that BCR-induced activation of Akt/PKB requires both PI3-kinase and Syk, which was not previously known to regulate Akt/PKB. Surprisingly, in contrast to previous reports that Src can activate Akt/PKB, we found that Lyn, a major Src-family tyrosine kinase expressed in hematopoietic cells, acts as a potent endogenous inhibitor of BCR-mediated activation of Akt/PKB.
MATERIALS AND METHODS
Cell Culture and DNA Transfection.
The chicken B cell lines, DT40, DT40Syk−, DT40Lyn−, and DT40Syk−Lyn− cells were cultured in RPMI medium 1640 supplemented with 10% heat-inactivated fetal bovine serum, 100 μg/ml penicillin, 100 μg/ml streptomycin, and 2 mM glutamine. Wild-type human Lyn and Syk and a catalytically inactive mutant (SykK402R) cDNA were cloned into the expression vector pApuro (8) and transfected into DT40Lyn− or DT40Syk− cells by electroporation using a Gene Pulser apparatus (Bio-Rad) at 330 V and 250 μF and selected in the presence of 0.3 μg/ml puromycin. All results represent at least three independent experiments.
Isolation of Primary B Cells.
Splenic B cells were isolated from 6- to 8-week-old wild-type C57BL/6 and Lyn null mice (5, 24, 25) as described (26). Freshly isolated splenocytes were depleted of red blood cells by using hypotonic red blood cell lysis buffer (Sigma). T cells were depleted by using antibody [monoclonal anti-CD4 Ab (GK1.5), anti-CD8 Ab (3.168.8) and anti-Thy1.2 (J1j)]-dependent complement-mediated cytolysis with Low-Tox guinea pig sera as a source of complement (Accurate Scientific, Westbury, NY). The enrichment of B cells was >85% as measured by reactivity with anti-B220 and fluorescence-activated flow cytometry. The purified B cells were incubated in serum-free medium for 3 hours before stimulation.
Immunoprecipitation and Immunoblotting.
Primary B cells or DT40 cells in exponential growth phase were serum-starved for 3 hours and 8 hours, respectively. Cells (3 × 107) were stimulated with 10 μg/ml polyclonal anti-mouse Ig or monoclonal mouse anti-chicken IgM Ab (M4) for 30 minutes at 37°C. Cells were quickly chilled on ice and pelleted by using centrifugation immediately after stimulation. Cell pellets were lysed in 500 μl of ice-cold lysis buffer (PBS containing 1 mM PMSF/100 μg/ml soy bean trypsin inhibitor/20 μg/ml aprotinin/100 μg/ml leupeptin/1% NP40/0.1% deoxycholate/10 mM NaF/10 mM sodium pyrophosphate/100 mM sodium orthovanadate) for 15 minutes on ice. Cell lysates were clarified by centrifuging at 12,000 × g for 10 minutes. Clarified cell lysates were normalized based on protein concentration as determined by using BCA kit (Pierce), and equal amounts of protein were subject to immunoprecipitation with 2 μl of polyclonal goat anti-Akt Ab (Santa Cruz Biotechnology) at 4°C overnight. Immune complexes were precipitated with 30 μl of protein A/G agarose beads (Santa Cruz Biotechnology) for an additional 3-hour incubation at 4°C. The beads were washed twice with lysis buffer and twice with PBS. Bound proteins were eluted by boiling in Laemmli buffer containing 0.4% DTT and resolved by using SDS/8% PAGE. The proteins were transferred to poly(vinylidene difluoride) membrane and subjected to immunoblotting. The blots were blocked with TNB buffer (30 mM Tris, pH 7.6/75 mM NaCl/3% BSA) overnight at 4°C. The blots were then incubated with polyclonal goat anti-Akt (Santa Cruz Biotechnology, Chatsworth, CA) or rabbit anti-phosphoSer-473–Akt Ab (Biolabs, Northbrook, IL) for 1 hour at 4°C. The blots were further incubated with 15 μg/ml rabbit anti-goat Ab for 1 hour at 4°C. The blots were then incubated with 1 μCi/ml (1 Ci = 37 GBq) iodinated protein A for 45 minutes at room temperature followed by autoradiography.
Akt Kinase Assay.
Cells were stimulated and lysed as described above. Normalized protein was subjected to immunoprecipitation with anti-Akt2, and the immune complexes were recovered by using protein A agarose. The anti-Akt2 antibody was generated in rabbits immunized with a carboxyl-terminal peptide of human and rat akt2 (NH2–CDQTHFPQFSYSASIRE), coupled to carrier protein, and affinity-purified on peptide. The immune complexes were washed three times with lysis buffer and three times with kinase buffer and then resuspended in kinase buffer [1 mM DTT/10 μM MgATP/0.2 mM EGTA/2 μg PKI (Sigma)/25 μg of histone 2B/5 μCi [γ-32P]ATP]. The reaction was allowed to proceed for 15 minutes at 30°C. The reaction was stopped by boiling in Laemmli sample buffer, and the reaction products were resolved by using SDS/12.5% PAGE. Proteins were transferred to nitrocellulose and quantitated by using PhosphorImager analysis.
RESULTS
BCR Crosslinking Results in Hyperphosphorylation of Akt/PKB.
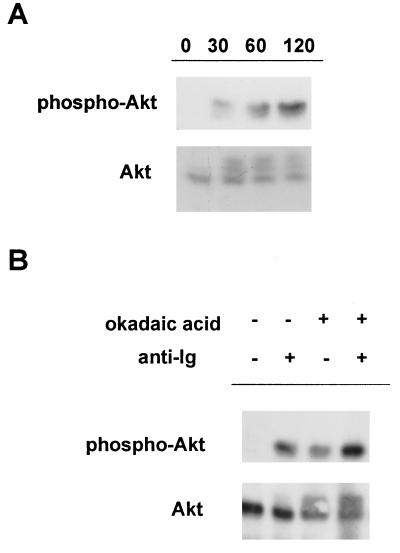
We initially investigated the regulation of Akt/PKB after crosslinking of BCR in an avian B cell line, DT40. Crosslinking of BCR on parental DT40 cells with anti-Ig induced a modest shift in the mobility of Akt/PKB at the various time points tested, indicative of hyperphosphorylation of the enzyme (Fig. 1A Lower). This shift in mobility was associated with increased reactivity of Akt/PKB with an antibody against Ser-473-phosphorylated Akt (anti-phosphoSer-473–Akt), which recognizes the activated form of the enzyme (14, 27) (Fig. 1A Upper).
Figure 1.
BCR crosslinking results in hyperphosphorylation of Akt/PKB in DT40 cells. (A) DT40 cells were stimulated with 10 μg/ml mouse anti-chicken IgM (M4) mAb for the indicated times. Equal amounts of cell lysates were immunoprecipitated with anti-Akt Ab (Santa Cruz Biotechnology) and immunoblotted with anti-Akt (Lower) or anti-phosphoSer-473-Akt specific Ab (Upper). (B) DT40 cells were stimulated with 10 μg/ml M4 after preincubation in the presence or absence of okadaic acid. The phosphorylation state of Akt was measured as described in A.
The activation of Akt/PKB can be regulated by endogenous phosphatase(s) (28). Thus, the modest accumulation of phosphoSer-473–Akt could reflect the degree of activation of the enzyme or be due to rapid dephosphorylation of activated enzyme. Pretreating DT40 cells with the phosphatase inhibitor okadaic acid resulted in an increased accumulation of the hyperphosphorylated form, indicating that Akt/PKB is activated and then rapidly dephosphorylated by an endogenous phosphatase after BCR ligation (Fig. 1B).
BCR-Mediated Activation of Akt/PKB Is Syk-Dependent but Inhibited by Lyn in DT40 cells.
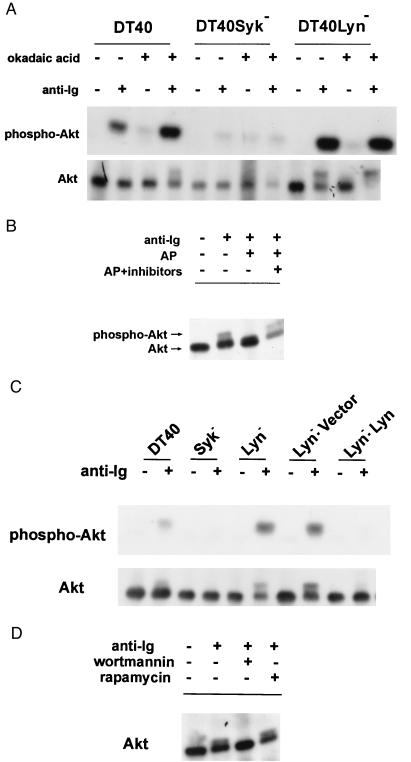
Syk and Lyn are the sole members of the Syk- and Src-family tyrosine kinases, respectively, expressed in DT40 cells (29). To determine the role of these protein tyrosine kinases in the regulation of BCR-induced Akt/PKB, the phosphorylation state of Akt/PKB in DT40 cells deficient in Syk or Lyn in the presence and absence of okadaic acid was determined. Surprisingly, we detected a markedly enhanced accumulation of the slower migrating form of Akt/PKB in anti-Ig stimulated Lyn-deficient DT40 (DT40Lyn−) cells compared with the parental cells even in the absence of okadaic acid. Furthermore, virtually all of the Akt/PKB in DT40Lyn− cells stimulated in the presence of okadaic acid accumulated as the slower migrating form (Fig. 2A Lower). This shift in mobility was attributed to hyperphosphorylation of Akt/PKB because it could be abolished by alkaline phosphatase (Fig. 2B). Furthermore, Akt/PKB immunoprecipitated from anti-Ig stimulated DT40 Lyn− cells showed markedly enhanced reactivity with anti-phosphoSer-473–Akt antibody (Fig. 2A, Upper) and augmented kinase activity in the in vitro kinase (Fig. 3A). To demonstrate that BCR-induced hyperphosphorylation of Akt/PKB in the mutant cells is caused by loss of Lyn per se, we transfected DT40Lyn− cells with human Lyn. BCR-induced hyperphosphorylation of Akt was abolished by the expression of exogenous Lyn but was not affected in DT40Lyn− cells transfected with vector alone (Fig. 2C). Taken together, these data establish that Lyn is a potent endogenous inhibitor of BCR-mediated activation of Akt/PKB. Moreover, Lyn and an okadaic acid-sensitive phosphatase(s) additively blocked BCR-induced Akt/PKB activation.
Figure 2.
BCR-mediated activation of Akt/PKB is Syk-dependent but inhibited by Lyn. (A) Okadaic acid-sensitive phosphatase(s) and Lyn inhibit anti-Ig induced phosphorylation of Akt. DT40, DT40Syk−, and DT40Lyn− cells were incubated in the presence or absence of okadaic acid before stimulation with M4 mAb. The phosphorylation state of Akt was analyzed by immunoblotting with anti-Akt (Lower) and immunoreactivity with anti-phosphoSer-473-Akt antibody (Upper). (B) Cell lysates were subjected to immunoprecipitation with anti-Akt Ab. The immune complexes were treated with alkaline phosphatase in the presence or absence of phosphatase inhibitors. The immune complexes were immunoblotted with anti-Akt. (C) Expression of human Lyn inhibited anti-Ig induced activation of Akt in DT40Lyn− cells. DT40, DT40Lyn−, and DT40Lyn− cells stably transfected with empty pApuro vector or a recombinant vector encoding human Lyn were stimulated with M4. Phosphorylation of Akt was measured as described in A (Upper, immunoblotted with anti-Akt; Lower, immunoblotted with anti-phosphoSer-473–Akt). (D) Cells were treated with 50 nM wortmannin or 10 nM rapamycin for 15 minutes before stimulation with M4 Ab. The level of phosphorylated Akt was measured as described in B.
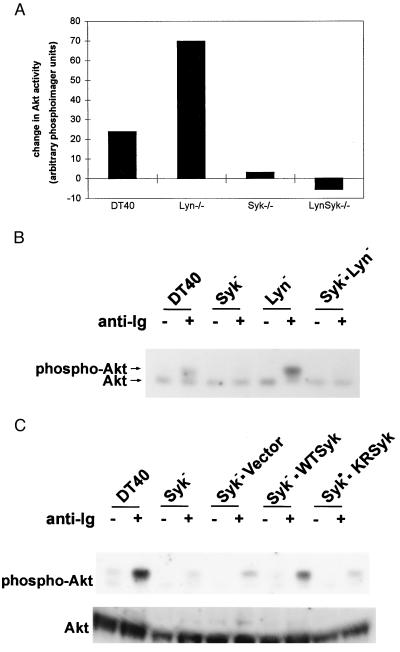
Figure 3.
The enzymatic activity of Syk is required for BCR-induced activation of Akt/PKB. (A) Wild-type and mutant DT40 cells were stimulated with mouse anti-chicken IgM Ab for 30 minutes. Akt/PKB was immunoprecipitated and subjected to an in vitro kinase assay. The change in Akt kinase activity in anti-Ig stimulated vs. unstimulated wild-type, and the indicated mutant DT40 cells is presented as the change in arbitrary PhosphorImager units. (B) Cells were stimulated as in A, and the phosphorylation state of Akt/PKB was determined by immunoprecipitation with anti-Akt Ab followed by immunoblotting with anti-Akt Ab. (C) DT40 Syk− cells transfected with wild-type and catalytically inactive mutant of Syk were stimulated with M4. The phosphorylation states of Akt/PKB were determined as described in A.
In contrast to Lyn-deficient DT40 cells, no or very low levels of Akt phosphorylation (Fig. 2A) or Akt kinase activity (Fig. 3A) was observed in DT40Syk− cells after anti-Ig stimulation, even in the presence of okadaic acid, suggesting that Syk might play a positive role in anti-Ig induced phosphorylation of Akt. To better understand the hierarchy of Lyn and Syk in the regulation of Akt, we compared the activation of Akt/PKB in double-knockout cells lacking both Syk and Lyn (DT40Syk−Lyn−). No induction of Akt kinase activity (Fig. 3A) or phosphorylation of Akt/PKB (Fig. 3B) was detected in anti-Ig-stimulated DT40Syk−Lyn− cells. Expression of wild-type human Syk, but not an enzymatically inactive mutant of Syk, SykK402R, restored anti-Ig-dependent Akt phosphorylation in the double-knockout cells (Fig. 3C). These data demonstrate that Syk is an important upstream mediator of Akt/PKB activation. Furthermore, anti-Ig-induced hyperphosphorylation of Akt/PKB was ablated by pretreating DT40 (data not shown) and Lyn-deficient DT40 cells with wortmannin (Fig. 2D) and Ly294002 (data not shown), indicating that Syk-dependent activation of Akt/PKB was mediated through a PI3-kinase-dependent pathway. In contrast, rapamycin, which inhibits activation of p70S6 kinase, had no effect on the activation of Akt.
Lyn-Mediated Inhibition of Akt/PKB Does Not Require the Phosphatases SHP1, SHP2, and SHIP.
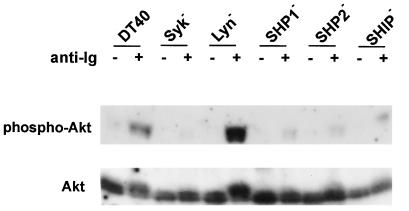
Another mechanism by which Lyn mediates down-regulation of B cell responses was recently established in which Lyn is required for the phosphorylation of receptors that contain immune tyrosine-based inhibition motifs (ITIMs), such as CD22 and Fc receptor (30–32). Phosphorylation of ITIM motifs leads to recruitment of SH2 domain-containing phosphatases such as SHP-1 in the case of CD22 (33, 34) and SHIP in the case of Fc receptor (34). Even though we used a monoclonal IgM antibody to stimulate DT40 cells that avoids concomitant signaling via FcγR, we further investigated whether the inhibition of Akt/PKB by Lyn depended on these phosphatases. If Akt/PKB lies on the same pathway, we would expect SHP-1-deficient cells to exhibit Akt/PKB hyperphosphorylation, like Lyn-deficient cells. A similar argument holds for SHP-2 or SHIP if they are limiting components of the same negative regulatory pathway of Akt/PKB as Lyn. Therefore, Akt/PKB phosphorylation in unstimulated and anti-Ig-stimulated DT40 cells deficient in SHP-1, SHP-2, and SHIP was compared with parental DT40 cells and DT40Lyn− cells. Akt/PKB immunoprecipitated from cells deficient in these phosphatases showed only a modest mobility shift and modest increase in reactivity with the anti-phosphoSer-473–Akt antibody after receptor cross-linking, similar to the parental DT40 cells; as expected, stimulation of DT40Lyn− cells resulted in a much greater accumulation of the hyperphosphorylated form of Akt/PKB (Fig. 4). Thus, Lyn can negatively regulate BCR signaling via at least two independent pathways. CD22 and Fc receptors inhibit BCR responses via the phosphatase SHP-1 and SHIP, whereas Lyn-mediated inhibition of Akt/PKB in B cells does not require these phosphatases.
Figure 4.
The protein tyrosine phosphatases SHP-1, SHP-2, and SHIP are not required for Lyn-mediated inhibition of Akt/PKB in B cells. DT40 cells, DT40Syk−, and DT40Lyn− cells and DT40 cells deficient in SHP-1 (DT40SHP-1−), SHP-2 (DT40SHP-2−), SHIP (DT40SHIP−) were stimulated with M4 and hyperphosphorylation of Akt was analyzed as described in Fig. 3.
Enhanced BCR-Mediated Activation of Akt/PKB in Primary B Cells from Lyn-Deficient Mice.
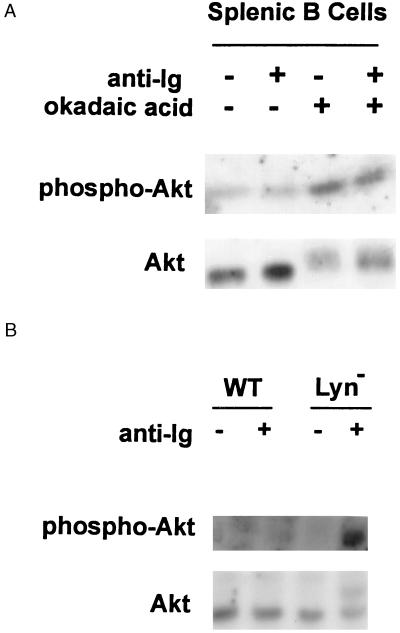
To determine whether Lyn acts as an inhibitor of Akt/PKB activation in primary cells as well, we compared the phosphorylation of Akt/PKB in splenic B cells isolated from wild-type mice and Lyn-deficient mice. A F(ab′)2 fragment of anti-mouse Ig was used to stimulate primary B cells to prevent concomitant signaling via FcγR. Consistent with results obtained in parental DT40 cells, we detected only a modest increase in reactivity of Akt/PKB with the anti-phosphoSer-473–Akt antibody after anti-Ig stimulation of wild-type primary B cells that was only evident with prolonged exposure of the blot to film. In comparison, the majority of Akt/PKB immunoprecipitated from anti-Ig-stimulated Lyn-deficient B cells was hyperphosphorylated (Fig. 5). Importantly, these results establish that Lyn acts as an antagonist of BCR-mediated Akt/PKB activation in primary cells as well.
Figure 5.
Enhanced BCR-mediated activation of Akt/PKB in primary B cells from Lyn-deficient mice. (A) Splenic B cells were isolated from wild-type C57BL/6 mice as described (26). B cells (2 × 107) were treated with okadaic acid for 2 hours before stimulation with 10 μg/ml F(ab′)2 goat anti-mouse Ig for 30 minutes. Equal amounts of cell lysates were immunoprecipitated with anti-Akt Ab and immunoblotted with anti-Akt (Lower) or anti-phosphoSer473-Akt (Upper). (B) Splenic B cells were isolated from both wild type and Lyn-deficient mice. Cells are stimulated with 10 μg/ml F(ab′)2 goat-anti-mouse Ig for 30 minutes. The phosphorylation state of Akt/PKB was measured as described in A.
Like Akt, p70S6 kinase is a potential downstream target of PI3-kinase and PDK1, and activation of p70S6k is closely associated with BCR-induced DNA synthesis (9). If hyperactivation of Akt is caused by enhanced PDK activity in Lyn−/− cells, then it might be predicted that p70S6k activity would also be enhanced. We compared the mobility shift and in vitro kinase activity of p70S6k immunoprecipitated from lysates of Lyn-deficient DT40 cells and primary B cells, respectively. No consistent difference in the kinetics and degree of activation of p70S6k was apparent (data not shown). Thus, the increased activation of Akt/PKB was not associated with a concomitant enhancement of p70S6k activity in vivo. These data suggest that PDK1 is unlikely to be a major target by which Lyn mediates regulation of Akt.
DISCUSSION
The data presented in this study establish that ligation of antigen receptor on B cells results in the hyperphosphorylation and activation of Akt/PKB. Furthermore, we demonstrated that the cytoplasmic tyrosine kinase Syk, which is expressed in all hematopoietic cells, is an important mediator of Akt/PKB activation. Whereas previous studies demonstrated that Src can induce the activation of Akt/PKB, we demonstrated in this study that Lyn, a major Src-family tyrosine kinase expressed in hematopoietic cells, on the other hand, is a potent antagonist of BCR-mediated Akt/PKB activation. Because primary B cells express significant levels of other members of the Src family that are also activated after BCR crosslinking, including Blk and Fyn, the enhanced phosphorylation of Akt/PKB in Lyn-deficient primary cells suggests that even if other Src-family members can contribute to the regulation of Akt/PKB, Lyn plays a significant role in the negative regulation of Akt/PKB in B cells. Interestingly, however, overexpression of Src in Lyn-deficient DT40 cells also partially inhibited BCR-mediated Akt/PKB activation (data not shown), raising the possibility that other Src kinases may play a similar negative regulatory role in mitogen-induced activation of Akt/PKB in other cell types.
BCR-induced activation of Akt/PKB is mediated through a pathway that involves Syk, PI3-kinase, and presumably the recently identified kinase PDK1, which phosphorylates Akt/PKB on Thr-308. Another as-yet-unidentified kinase is presumably responsible for the further phosphorylation of Akt/PKB on Ser-473. Lyn may regulate activation of Akt/PKB by inhibiting any of these steps along the signal-transduction pathway. Interestingly, it was recently reported that three of six tyrosine residues in Syk that are phosphorylated in BCR-stimulated cells are targets of Lyn and that phosphorylation of one of these, Tyr-317, inhibited Syk mediated activation of nuclear factor of activated T cells (NF-AT) activity (35). It is also possible that Lyn up-regulates the activity of a phosphatase responsible for the dephosphorylation of Akt/PKB or negatively regulates the signals upstream of Akt/PKB. In this report, we demonstrate that the tyrosine phosphatases SHP-1, SHP-2, and the phosphoinositide phosphatase SHIP are not required to mediate the inhibition of Akt/PKB by Lyn. Interestingly, similar to our results with Lyn-deficient B cells, it was recently reported that the activation of Akt/PKB is augmented in fibroblasts deficient in the phosphatase PTEN, which is the product of a tumor-suppressor gene (36). The enhanced activation of Akt/PKB resulted in enhanced survival of PTEN-deficient fibroblasts. Furthermore, evidence was provided to suggest that PTEN negatively regulates Akt/PKB by inhibiting intracellular levels of phosphatidylinositol 3,4,5-triphosphate and dephosphorylating phosphatidylinositol 3,4,5-triphosphate (36). Because we found that BCR-induced activation of Akt/PKB is PI3-kinase-dependent, it is tempting to speculate that Lyn may also inhibit Akt/PKB activation by regulating the levels of phosphatidylinositol 3,4,5-triphosphate.
Studies from several investigators have demonstrated that Lyn also down-regulates B cell responses by mediating the phosphorylation of receptors, such as CD22, that contain ITIMs (32–34, 37). Phosphorylation of ITIM motifs leads to the recruitment of SH2 domain-containing phosphatases that down-regulate protein tyrosine kinase-dependent signal transduction (32–34, 37). However, to date there is no direct evidence that this pathway is responsible for the phenotypic defects evident in Lyn-deficient mice. The Lyn-mediated negative regulatory pathway we defined in this study appears to be independent of the phosphatases SHP-1, SHP-2, and SHIP. Thus, the conditions used to stimulate B cells in this study prevent concomitant negative signaling via the FcγR/SHIP pathway. Furthermore, the accumulation of hyperphosphorylated Akt/PKB was comparable or slightly decreased in SHP-1-, SHP-2-, and SHIP-deficient DT40 cells and B cells from motheaten viable mice (data not shown) to that observed in parental DT40 cells and wild-type primary B cells, suggesting that the effect of Lyn on Akt/PKB occurs independently of this previously defined mechanism.
Similar to Akt/PKB, the activation of p70S6 kinase requires Syk and can be mediated through a PI3-kinase-dependent pathway involving PDK. Furthermore, we recently demonstrated that the activation of p70S6k may play a role in BCR-induced protein and DNA synthesis (9). We found that the activation of p70S6k was not enhanced in Lyn-deficient B cells as might have been expected. This may be explained by the complex regulation of p70S6k through multiple pathways, but also suggests that PDK1, which acts upstream of both Akt and p70S6k, may not be a major target of negative regulation by Lyn responsible for the effect on Akt (9).
The hyperphosphorylation of Akt/PKB in B cells from Lyn-deficient mice is particularly intriguing in light of previous reports that the mutant mice develop an autoimmune syndrome. In addition, B cells from several lines of Lyn-deficient mice exhibited enhanced proliferation in response to stimulation with suboptimal doses of anti-Ig in vitro compared with B cells from wild-type animals (5, 25). In previous studies, it was suggested that hyperproliferation of Lyn-deficient B cells correlated with hyperactivation of MAPK. In experiments performed in parallel with those described above, we confirmed previous reports (5, 25) that stimulation with anti-Ig resulted in hyperproliferation of Lyn-deficient B cells relative to B cells from wild-type mice (data not shown). Our studies demonstrate that the hyperproliferation also correlates with hyperactivation of Akt/PKB. The B cell compartment of these mutant mice is also characterized by increased levels of circulating Ig and an accumulation of plasma cells (5, 24, 25). With age, autoantibodies develop in these animals, and autoimmune pathology is observed (5, 24, 25). Further studies will be required to determine how the hyperactivation of MAPK and Akt/PKB each contribute to the functional hyperactivation of Lyn-deficient B cells in vitro or to the development of autoimmunity in the mutant mice.
Acknowledgments
The authors thank Drs. Paul Stein and Robert Ryan for many helpful discussions; Dr. Clifford Lowell for providing breeders for generating the Lyn-deficient mice used in these studies; Drs. Tadashi Yamamoto and Charles Abrams for Lyn cDNA; and Drs. Jeffrey Ravetch and Tomohiro Kurosaki for providing mutant DT40 cell lines. This work was supported by Public Health Service Grant AI25185 and the Ludwig Institute for Cancer Research. E.L.W. is the recipient of a Howard Hughes Predoctoral Fellowship for the Biological Sciences.
ABBREVIATIONS
- BCR
B cell receptor
- PKB
protein kinase B
- MAPK
mitogen-activated protein kinase
- PI3-kinase
phosphoinositide 3-kinase
- ITIM
immune tyrosine-based inhibition motif
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Burkhardt A L, Brunswick M, Bolen J B, Mond J J. Proc Natl Acad Sci USA. 1991;88:7410–7414. doi: 10.1073/pnas.88.16.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng A M, Rowley B, Pao W, Hayday A, Bolen J, Pawson T. Nature (London) 1995;378:303–306. doi: 10.1038/378303a0. [DOI] [PubMed] [Google Scholar]
- 3.Tsukada S, Saffran D C, Rawlings D J, Parolini O, Allen R C, Klisak I, Sparkes R S, Kubagawa H, Mohandas T, Quan S, et al. Cell. 1993;72:279–290. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- 4.Yamanashi Y, Kakiuchi T, Mizuguchi J, Yamamoto T, Toyoshima K. Science. 1991;251:192–194. doi: 10.1126/science.1702903. [DOI] [PubMed] [Google Scholar]
- 5.Chan V W-F, Meng F, Soriano P, DeFranco A L, Lowell C A. Immunity. 1997;7:69–81. doi: 10.1016/s1074-7613(00)80511-7. [DOI] [PubMed] [Google Scholar]
- 6.Purkerson J M, Parker D C. J Immunol. 1998;160:2121–2129. [PubMed] [Google Scholar]
- 7.Sutherland C L, Heath A W, Pelech S L, Young P R, Gold M R. J Immunol. 1996;157:3381–3390. [PubMed] [Google Scholar]
- 8.Li H-L, Forman M S, Kurosaki T, Puré E. J Biol Chem. 1997;272:18200–18208. doi: 10.1074/jbc.272.29.18200. [DOI] [PubMed] [Google Scholar]
- 9.Li H-L, Davis W, Puré E. J Biol Chem. 1999;274:9812–9820. doi: 10.1074/jbc.274.14.9812. [DOI] [PubMed] [Google Scholar]
- 10.Gold M R, Chen V W-F, Turck C W, DeFranco A L. J Immunol. 1992;148:2012. [PubMed] [Google Scholar]
- 11.Aagard-Tillery K M, Jelinek D F. J Immunol. 1996;156:4543–4554. [PubMed] [Google Scholar]
- 12.Stokoe D, Stephens L R, Copeland T, Gaffney P R, Reese C B, Painter G F, Holmes A B, McCormick F, Hawkins P T. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 13.King W G, Mattaliano M D, Chan T O, Tsichlis P N, Brugge J S. Mol Cell Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alessi D R, James S R, Downes C P, Holmes A B, Gaffney P R, Reese C B, Cohen P. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 15.Alessi D R, Deak M, Casamayor A, Caudwell F B, Morrice N, Norman D G, Gaffney P, Reese C B, MacDougall C N, Harbison D, et al. Curr Biol. 1997;7:776–789. doi: 10.1016/s0960-9822(06)00336-8. [DOI] [PubMed] [Google Scholar]
- 16.Bellacosa A, Testa J R, Staal S P, Tsichlis P N. Science. 1991;254:274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 17.Staal, S. P. & Hartley, J. W. (1988) 167, 1259–1264. [DOI] [PMC free article] [PubMed]
- 18.Bellacosa A, de Feo D, Godwin A K, Bell D W, Cheng J Q, Altomare D A, Wan M, Dubeau L, Scambia G, Masciullo V, et al. Int J Cancer. 1995;64:280–285. doi: 10.1002/ijc.2910640412. [DOI] [PubMed] [Google Scholar]
- 19.Staal S P. Proc Natl Acad Sci USA. 1987;84:5034–5037. doi: 10.1073/pnas.84.14.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed N N, Grimes H L, Bellacosa A, Chan T O, Tsichlis P N. Proc Natl Acad Sci. 1997;94:3627–3632. doi: 10.1073/pnas.94.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy S G, Wagner A J, Conzen S D, Jordan J, Bellacosa A, Tsichlis P N, Hay N. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 22.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Science. 1997;278:687–688. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 23.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 24.Hibbs M L, Tarlinton D M, Armes J, Grail D, Hodgson G, Maglitto R, Stacker S A, Dunn A R. Cell. 1995;83:310–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 25.Nishizumi H, Taniuchi I, Yamanashi Y, Kitamura D, Ilic D, Mori S, Watanabe T, Yamamoto T. Immunity. 1995;3:549–560. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 26.Mishell B B, Shiigi S M. Selected Methods in Cellular Immunology. San Francisco: Freeman; 1980. [Google Scholar]
- 27.Bellacosa A, Chan T O, Ahmed N N, Datta K, Malstrom S, Stokoe D, McCormick F, Feng J, Tsichlis P. Oncogene. 1998;17:313–325. doi: 10.1038/sj.onc.1201947. [DOI] [PubMed] [Google Scholar]
- 28.Downes C P. Curr Opin Cell Biol. 1998;10:262–267. [Google Scholar]
- 29.Takata M, Sabe H, Hata A, Inazu T, Homma Y, Nukada T, Yamamura H, Kurosaki T. EMBO J. 1994;13:1341–1349. doi: 10.1002/j.1460-2075.1994.tb06387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isakov N. Immunol Res. 1997;16:85–100. doi: 10.1007/BF02786325. [DOI] [PubMed] [Google Scholar]
- 31.Unkeless J C, Jin J. Curr Opin Immunol. 1997;9:338–343. doi: 10.1016/s0952-7915(97)80079-9. [DOI] [PubMed] [Google Scholar]
- 32.Smith K G C, Tarlinton D M, Doody G M, Hibbs M L, Fearon D T. J Exp Med. 1998;187:807–811. doi: 10.1084/jem.187.5.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cornall R J, Cyster J G, Hibbs M L, Dunn A R, Otipoby K L, Clark E A, Goodnow C C. Immunity. 1998;8:497–508. doi: 10.1016/s1074-7613(00)80554-3. [DOI] [PubMed] [Google Scholar]
- 34.Ono M, Okada H, Bolland S, Yanagi S, Kurosaki T, Ravetch J V. Cell. 1997;90:293–301. doi: 10.1016/s0092-8674(00)80337-2. [DOI] [PubMed] [Google Scholar]
- 35.Keshvara L M, Isaacson C C, Yankee T M, Sarac R, Harrison M L, Geahlen R L. J Immunol. 1998;161:5276–5283. [PubMed] [Google Scholar]
- 36.Stambolic V, Suzuki A, de la Pompa J L, Brothers G M, Mirtsos C, Sasaki T, Ruland J, Penninger J M, Siderovski D P, Mak T W. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 37.Nishizumi H, Horikawa K, Mlinaric-Rascan I, Yamamoto T. J Exp Med. 1998;187:1343–1348. doi: 10.1084/jem.187.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]