Abstract
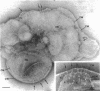
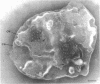
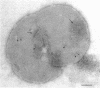
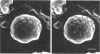
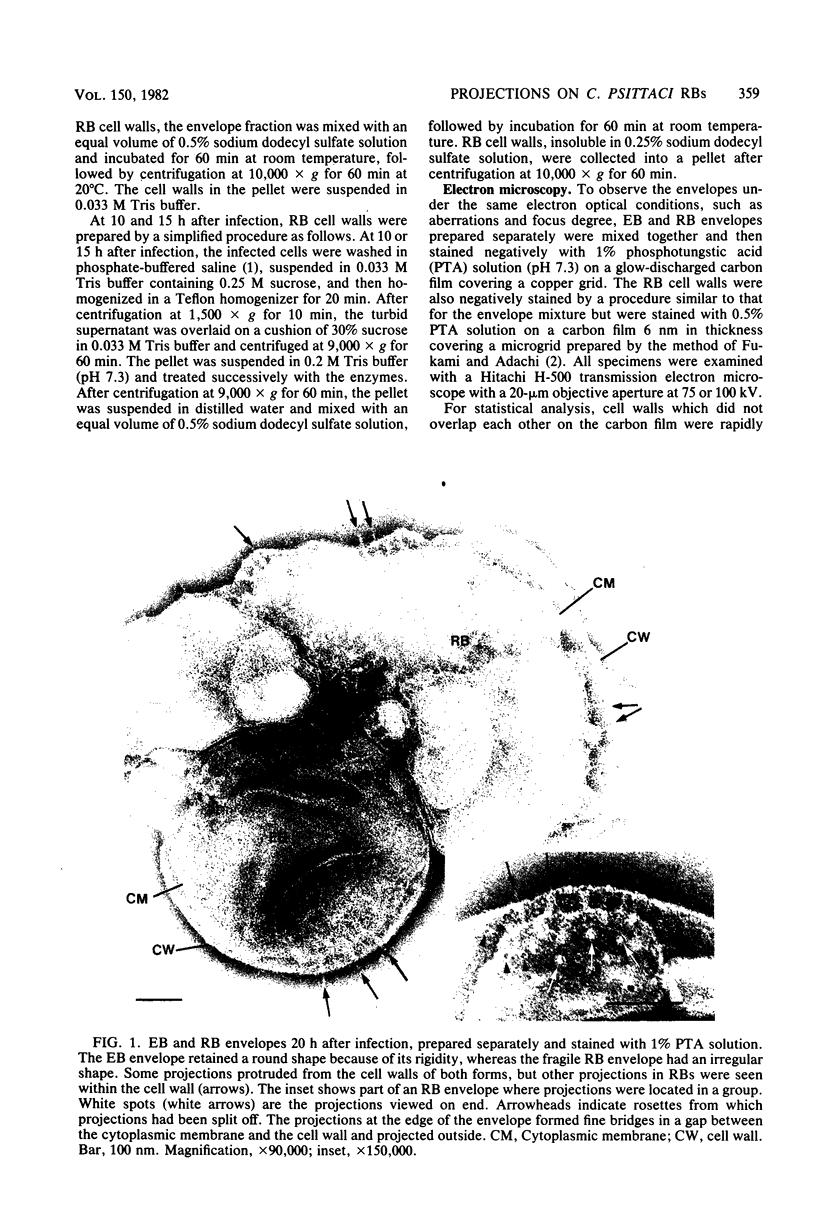
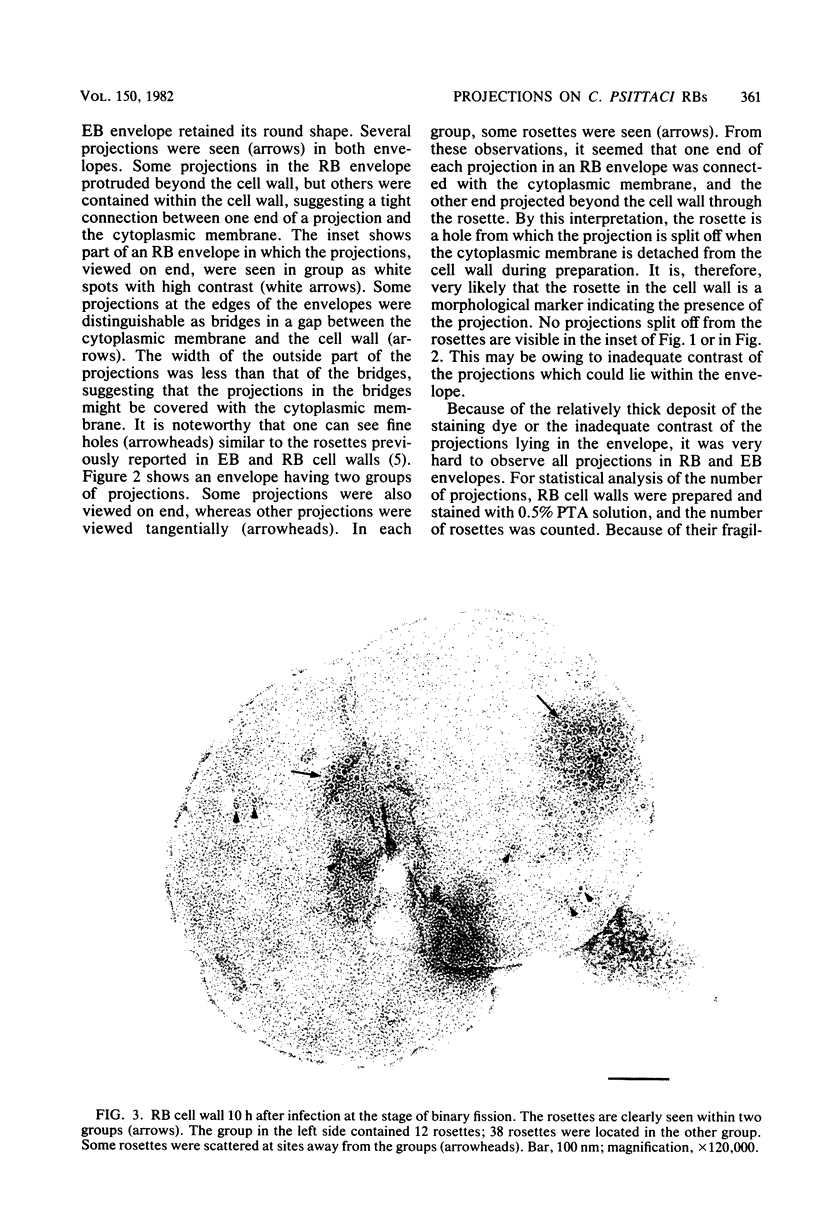
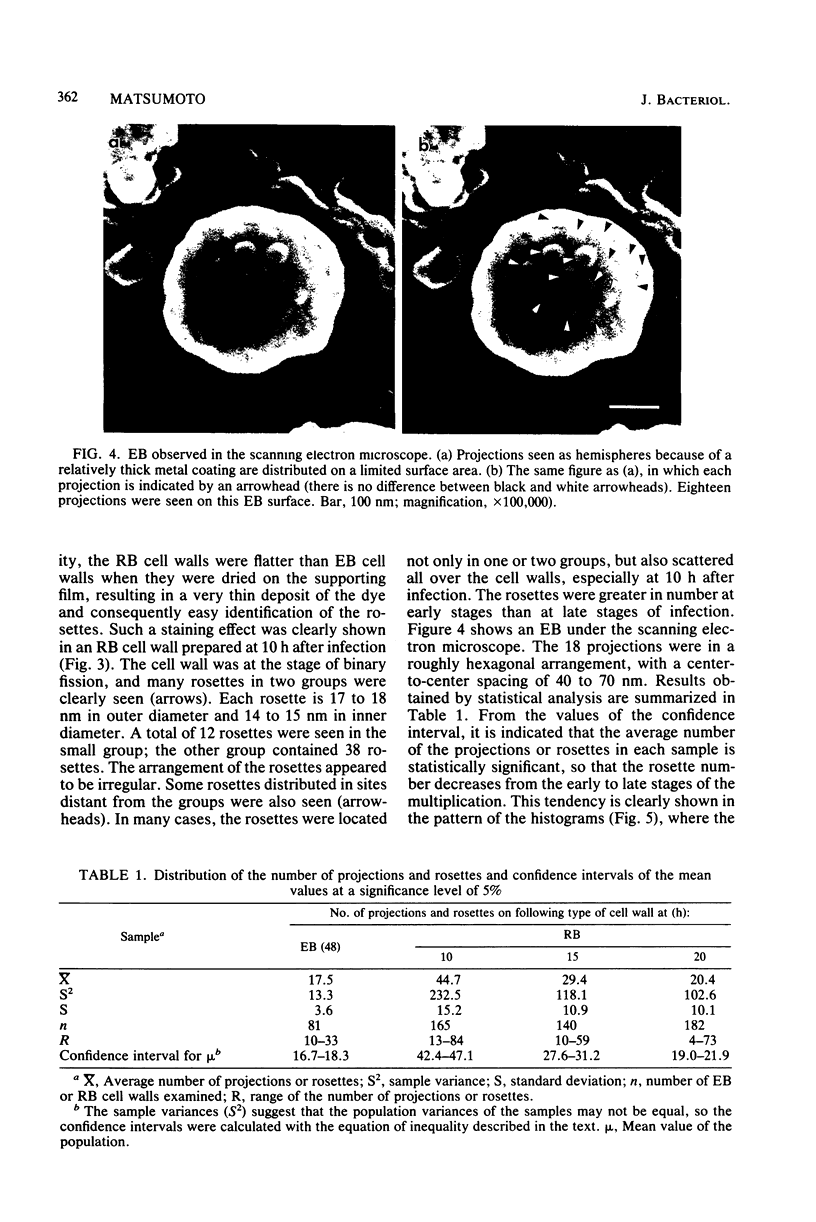
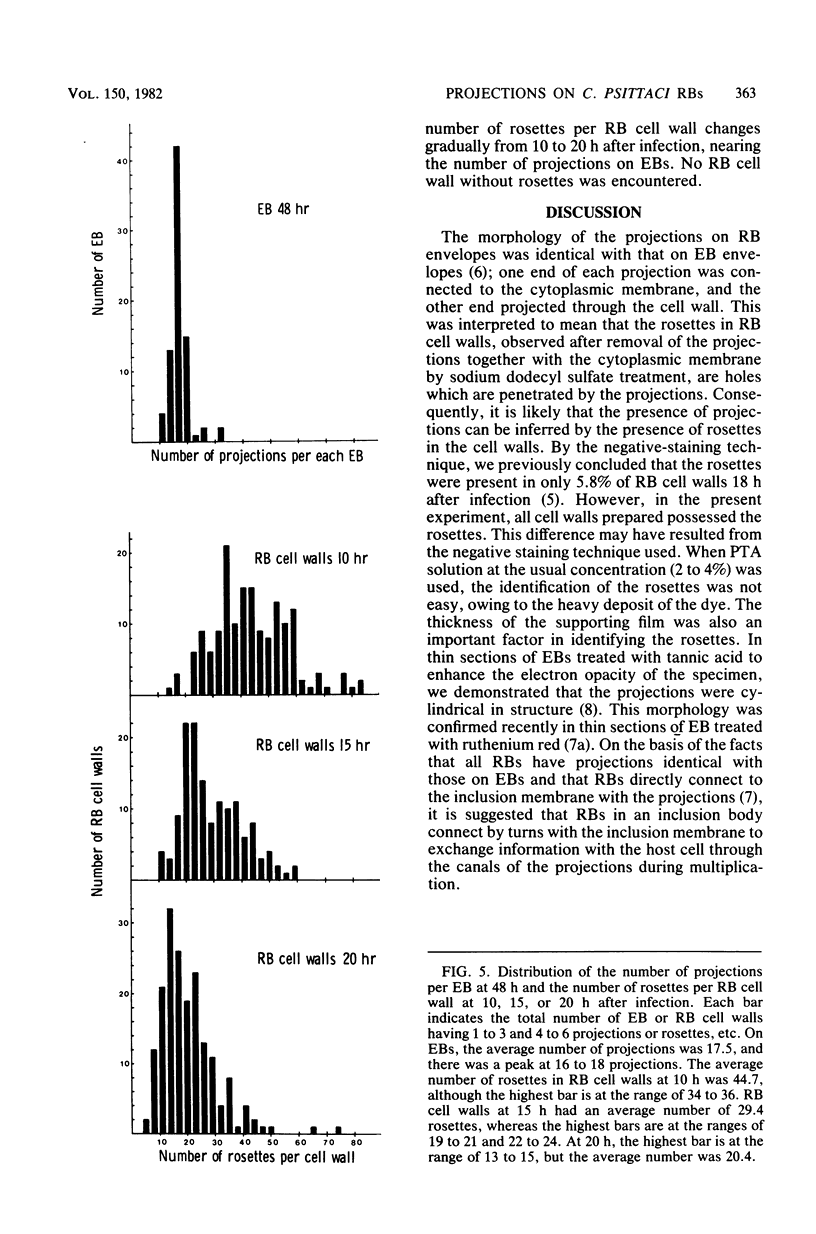
Electron microscopic observations were carried out to confirm the presence of surface projections on Chlamydia psittaci reticulate bodies (RBs). The morphology of the projections on RBs was identical with that on elementary bodies (EBs); one end of each projection was connected with the cytoplasmic membrane, but the other end of the projection protruded beyond the cell wall through a fine hole or rosette in the cell wall. The results demonstrated that the rosettes seen in RB cell walls were morphological markers indicating the presence of the surface projections. A statistical anaylsis of the number of projections on EBs and the number of rosettes in RB cell walls prepared at 10, 15, and 20 h after infection demonstrated that all RBs had the projections and that the number of projections was maximal by 10 h after infection and then decreased gradually to approximately the same number of projections on EBs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DULBECCO R., VOGT M. One-step growth curve of Western equine encephalomyelitis virus on chicken embryo cells grown in vitro and analysis of virus yields from single cells. J Exp Med. 1954 Feb;99(2):183–199. doi: 10.1084/jem.99.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami A., Adachi K. A new method of preparation of a self-perforated micro plastic grid and its application. J Electron Microsc (Tokyo) 1965;14(2):112–118. [PubMed] [Google Scholar]
- HIGASHI N. ELECTRON MICROSCOPIC STUDIES ON THE MODE OF REPRODUCTION OF TRACHOMA VIRUS AND PSITTACOSIS VIRUS IN CELL CULTURES. Exp Mol Pathol. 1965 Feb;76:24–39. doi: 10.1016/0014-4800(65)90021-3. [DOI] [PubMed] [Google Scholar]
- Matsumoto A. Electron microscopic observations of surface projections and related intracellular structures of Chlamydia organisms. J Electron Microsc (Tokyo) 1981;30(4):315–320. [PubMed] [Google Scholar]
- Matsumoto A. Fine structures of cell envelopes of Chlamydia organisms as revealed by freeze-etching and negative staining techniques. J Bacteriol. 1973 Dec;116(3):1355–1363. doi: 10.1128/jb.116.3.1355-1363.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A., Fujiwara E., Higashi N. Observations of the surface projections of infectious small cell of Chlamydia psittaci in thin sections. J Electron Microsc (Tokyo) 1976;25(3):169–170. [PubMed] [Google Scholar]
- Matsumoto A. Isolation and electron microscopic observations of intracytoplasmic inclusions containing Chlamydia psittaci. J Bacteriol. 1981 Jan;145(1):605–612. doi: 10.1128/jb.145.1.605-612.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMURA A., HIGASHI N. PURIFICATION AND CHEMICAL COMPOSITION OF MENINGOPNEUMONITIS VIRUS. Virology. 1963 Aug;20:596–604. doi: 10.1016/0042-6822(63)90284-8. [DOI] [PubMed] [Google Scholar]
- Tamura A., Matsumoto A., Higashi N. Purification and chemical composition of reticulate bodies of the meningopneumonitis organisms. J Bacteriol. 1967 Jun;93(6):2003–2008. doi: 10.1128/jb.93.6.2003-2008.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A., Matsumoto A., Manire G. P., Higashi N. Electron microscopic observations on the structure of the envelopes of mature elementary bodies and developmental reticulate forms of Chlamydia psittaci. J Bacteriol. 1971 Jan;105(1):355–360. doi: 10.1128/jb.105.1.355-360.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]