Abstract
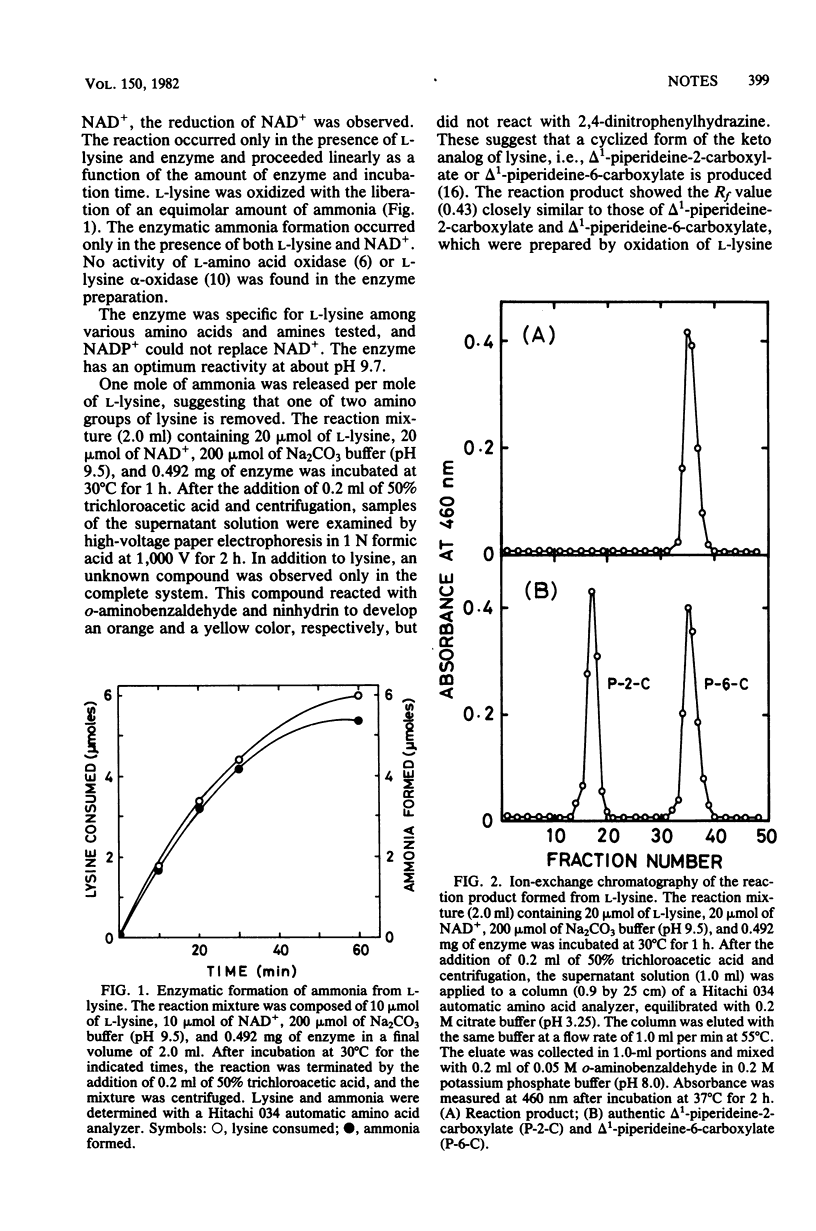
A novel amino acid dehydrogenase catalyzing the oxidative deamination of the ε-amino group of l-lysine was found in the crude extract of Agrobacterium tumefaciens ICR 1660. The enzyme required NAD+ and was specific for l-lysine. The enzyme was optimally active at about pH 9.7.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bürgi W., Richterich R., Colombo J. P. L-Lysine dehydrogenase deficiency in a patient with congenital lysine intolerance. Nature. 1966 Aug 20;211(5051):854–855. doi: 10.1038/211854a0. [DOI] [PubMed] [Google Scholar]
- Chang Y. F., Adams E. D-lysine catabolic pathway in Pseudomonas putida: interrelations with L-lysine catabolism. J Bacteriol. 1974 Feb;117(2):753–764. doi: 10.1128/jb.117.2.753-764.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirpich T. P., Zappia V., Costilow R. N., Barker H. A. Lysine 2,3-aminomutase. Purification and properties of a pyridoxal phosphate and S-adenosylmethionine-activated enzyme. J Biol Chem. 1970 Apr 10;245(7):1778–1789. [PubMed] [Google Scholar]
- Costilow R. N., Rochovansky O. M., Barker H. A. Isolation and identification of beta-lysine as an intermediate in lysine fermentation. J Biol Chem. 1966 Apr 10;241(7):1573–1580. [PubMed] [Google Scholar]
- Duerre J. A., Chakrabarty S. l-amino acid oxidases of Proteus rettgeri. J Bacteriol. 1975 Feb;121(2):656–663. doi: 10.1128/jb.121.2.656-663.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich F. P., Broquist H. P. Lysine catabolism in Rhizoctonia leguminicola and related fungi. J Bacteriol. 1976 Apr;126(1):338–347. doi: 10.1128/jb.126.1.338-347.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashino K., Lieberman I. Lysine catabolism by liver after partial hepatectomy. Biochim Biophys Acta. 1965 Nov 15;111(1):346–348. doi: 10.1016/0304-4165(65)90508-8. [DOI] [PubMed] [Google Scholar]
- Higashino K., Tsukada K., Lieberman I. Saccharopine, a product of lysine breakdown by mammalian liver. Biochem Biophys Res Commun. 1965 Jul 26;20(3):285–290. doi: 10.1016/0006-291x(65)90361-x. [DOI] [PubMed] [Google Scholar]
- Kusakabe H., Kodama K., Kuninaka A., Yoshino H., Misono H., Soda K. A new antitumor enzyme, L-lysine alpha-oxidase from Trichoderma viride. Purification and enzymological properties. J Biol Chem. 1980 Feb 10;255(3):976–981. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Rothstein M. Intermediates of lysine dissimilation in the yeast, Hansenula saturnus. Arch Biochem Biophys. 1965 Aug;111(2):467–476. doi: 10.1016/0003-9861(65)90210-9. [DOI] [PubMed] [Google Scholar]
- Sabo D. L., Boeker E. A., Byers B., Waron H., Fischer E. H. Purification and physical properties of inducible Escherichia coli lysine decarboxylase. Biochemistry. 1974 Feb 12;13(4):662–670. doi: 10.1021/bi00701a005. [DOI] [PubMed] [Google Scholar]
- Soda K., Misono H. L-Lysine:alpha-ketoglutarate aminotransferase. II. Purification, crystallization, and properties. Biochemistry. 1968 Nov;7(11):4110–4119. doi: 10.1021/bi00851a046. [DOI] [PubMed] [Google Scholar]
- Soda K., Misono H., Yamamoto T. L-Lysine:alpha-ketoglutarate aminotransferase. I. Identification of a product, delta-1-piperideine-6-carboxylic acid. Biochemistry. 1968 Nov;7(11):4102–4109. doi: 10.1021/bi00851a045. [DOI] [PubMed] [Google Scholar]
- Takeda H., Yamamoto S., Kojima Y., Hayaishi O. Studies on monooxygenases. I. General properties of crystalline L-lysine monooxygenase. J Biol Chem. 1969 Jun 10;244(11):2935–2941. [PubMed] [Google Scholar]



